Abstract
Using flow-mediated vasodilation (FMD), reactive hyperemia (RH), and an acute oral antioxidant cocktail (AOC [Vitamin C, E and α-lipoic acid]), this study aimed to provide greater insight into altered vascular function and the role of oxidative stress in chronic heart failure patients with reduced ejection fraction (HFrEF) and at several time points beyond heart transplantation (HTx). A total of 61 age-matched subjects (12 healthy controls, 14 NYHA Class II and III HFrEF patients, and 35 HTx recipients (< 3 yrs post-HTx, 5-10 yrs post-HTx, and > 14 yrs post-HTx)) ingested either placebo (PL) or an AOC prior to FMD and RH testing of the brachial artery. Vascular function, as measured by FMD, was not different between the controls (6.8 ± 1.9 %), recent < 3 yrs post-HTx group (8.1 ± 1.2%), and the 5-10 yrs post-HTx group (5.5 ± 1.0%). However, PL FMD was lower in the HFrEF patients (4.5 ± 0.7%) and in the > 14 yrs post-HTx group (2.9 ± 0.8%). The AOC increased plasma ascorbate levels in all groups, but only increased FMD in the controls (PL 6.8 ± 1.9%; AOC 9.2 ± 1.0%) and > 14 yrs post-HTx recipients (PL 2.9 ± 0.8%; AOC 4.5 ± 1.3%). There were no differences in RH in any of the groups with PL or AOC. This cross-sectional study reveals that, compared to controls, vascular function is blunted in HFrEF patients, is similar soon after HTx, but is decreased with greater time post-HTx with free radicals implicated in this progression.
Keywords: antioxidants, flow-mediated vasodilation, vascular health, blood flow
INTRODUCTION
Vascular endothelial dysfunction is a systemic pathology that impairs the health of both conduit and resistance vessels. Impaired endothelium-dependent vasodilation has been associated with various cardiovascular diseases including hypertension,1 coronary artery disease, 2 and heart failure. 3, 4 Importantly, impaired endothelium-dependent vasodilation may also precede the development of these cardiovascular diseases. 5, 6 Endothelial dysfunction has therefore been proposed as a prognostic marker in both healthy individuals and patients with cardiovascular disease. Non-invasive tests such as flow-mediated dilation (FMD) 5, 6 and the measurement of reactive hyperemia (RH) 7, 8 have become common research tools used to assess and quantify disturbances in vascular function. However, the real impact of disease progression and surgical intervention on vascular function across the continuum from health, heart failure (HF), and heart transplantation (HTx) has not been well characterized.
HF patients have chronically elevated peripheral vasoconstriction, the result of elevated sympathetic nervous system 9 and renin-angiotensin system 10 activity, as well as a concomitant dysfunction of the peripheral vasculature. 3 The latter appears to be the consequence of an attenuated L-arginine-nitric oxide pathway 11 and has been, at least partially, attributed to the increased destruction of nitric oxide (NO) by free radicals. 12 Indeed, previous studies have revealed that elevated levels of free radicals, particularly superoxide (O2−), contribute to decreased NO bioavailability 13, 14 and the subsequent attenuation in endothelial function. 15 Antioxidant supplementation, has previously restored endothelial function in healthy aged individuals 16-18 as well as HF patients, 19 presumably by improving NO bioavailability. Heart transplantation restores many of the hemodynamic abnormalities characteristic of advanced HF. However, whether HTx also results in normalization of endothelial function remains controversial. 20-24
Accordingly, using FMD and RH to assess vascular function and an acute oral AOC to examine the role of free radicals, this cross-sectional study sought to provide greater insight into vascular function in heart failure patients with reduced ejection fraction (HFrEF) and at several time points beyond HTx. We hypothesized that 1) when compared to controls, vascular function will be reduced in HFrEF patients, will be comparable within the initial years following HTx, and will progressively decline thereafter and 2) by attenuating the levels of free radicals, the administration of the AOC will improve vascular function in all groups.
METHODS
Subjects
A total of 61 subjects (12 healthy age-matched controls, 14 NYHA Class II and III HFrEF patients, and 35 HTx recipients (< 3 yrs post-HTx, 5-10 yrs post-HTx, and > 14 yrs post- HTx)) were recruited in the HF and HTx clinics at the University of Utah and the Salt Lake City VA Medical Center, and the controls by word of mouth. The protocol was approved by and written informed consent was obtained according to the Institutional Review Board of the University of Utah and the Salt Lake City Veterans Affairs Medical Center. All studies were performed in a thermoneutral environment at least three days apart to allow for washout of the oral antioxidants. Subjects reported to the laboratory in the fasted state, and without caffeine or past 24 hours and if antioxidants and/or a multivitamin were part of a subject’s daily routine they were asked to refrain from such use for at least three days prior to testing days.
Flow-mediated Dilation (FMD) and Reactive Hyperemia (RH) Measurements
Details of the FMD procedure have been described previously 25 and were performed in accordance with current recommendations 26 Briefly, a blood pressure cuff was placed on the right arm proximal to the elbow and distal to the placement of the ultrasound Doppler probe on the brachial artery. The brachial artery was insonated approximately midway between the antecubital and axillary regions, and measurements of brachial artery diameter and blood velocity (Vmean) measurements were obtained continuously at rest and for two minutes after cuff deflation (Logiq 7, GE Medical Systems, Milwaukee, WI).
Analyses
Vmean was automatically calculated using commercially available software (Logiq 7). End-diastolic, ECG R-wave gated images were collected via video output from the Logiq 7 for offline analysis of brachial artery vasodilation using automated edge-detection software (Medical Imaging Applications, Coralville, IA). FMD was quantified as the maximal percentage of change in brachial artery diameter after cuff release. Shear rate was calculated as follows: Shear rate (s−1) = 8Vmean /arterial diameter. Blood flow was calculated as follows: Blood flow = Vmeanπ (arterial diameter/2)2 × 60. For both shear rate and blood flow, cumulative area under the curve values were integrated with the trapezoidal rule and calculated as follows: Σ(yi(x(i+1) - xi) + (1/2)(y(i+1) - yi)(x(i+1) - xi)). Reactive hyperemia was quantified as cumulative brachial artery blood flow for two minutes (area under the curve) after cuff occlusion. Normalized FMD was calculated by dividing FMD (percentage) by the cumulative shear rate area under the curve until the time of peak brachial artery vasodilation.
Antioxidant supplementation
Subjects received either the AOC or placebo in a balanced, single blind design for the subject’s two visits. The supplements were administered 90 and 60 minutes prior to the FMD protocol. A split dosing was used to improve absorbance and distribution of the antioxidants. The first antioxidant dose included Vitamin E (200 IU), Vitamin C (500 mg), Alpha-lipoic Acid (300 mg), and the subsequent dose included Vitamin E (400 IU), Vitamin C (500 mg), and Alpha-lipoic Acid (300 mg). The placebo microcrystalline cellulose capsules, of similar taste, color and appearance, were also consumed in two equivalently timed doses. The efficacy of this AOC to reduce plasma free radical concentration has previously been established using ex-vivo spin trapping and electron paramagnetic resonance (EPR) spectroscopy. 27
Assays
In both the placebo and antioxidant trials blood samples were obtained from the antecubital vein prior to FMD testing (1 hr following ingestion of the second dose of either AOC or placebo. Quantitative determination of thiobarbituric acid reactive substances (TBARS) was performed to assess lipid peroxidation 28 (Bioassays Systems, Hayward, CA). Endogenous plasma antioxidant activity, assessed by superoxide dismutase (SOD) and catalase (CAT) activity, was assessed 29 (Cayman Chemical Company, Ann Arbor, MI) as well as ascorbic acid levels 30 (CosmoBio, Carlsbad, CA). Resting plasma endothelin (ET-1) (Cayman Chemical Company, Ann Arbor, MI) and nitrite and nitrate levels were measured using a standard fluorometric assay kit (Cayman Chemical Company, AnnArbor, MI). A lipid panel and complete blood count were assessed by standard clinical techniques.
Statistical Analyses
Statistical analyses were performed using commercially available software (SPSS 17.0, < 0.05) was used to Chicago, IL). A repeated measures analysis of variance 2×5 (ANOVA) ( determine if the oxidative stress/antioxidant assays and vascular responses to placebo and antioxidant supplementation for FMD and RH differed between healthy controls, HFrEF patients, and the HTx groups. Tukey’s Honestly Significant Difference post hoc test was conducted to evaluate pairwise differences among the means. A one-way ANOVA was used to determine differences in subject characteristics. All data are expressed as means ± standard error (SE).
RESULTS
Subject Characteristics
The healthy controls, HFrEF patients, and HTx recipients were well matched for age and most other physical characteristics (Table 1). The healthy controls were not currently taking any medications and the relevant medications used by the patients with HFrEF and the HTx recipients are listed in Table 2.
Table 1. Subject Characteristics.
| Variable | Controls | HFrEF | HTx (< 3 yrs) |
HTx (5-10 yrs) |
HTx (>14 yrs) |
|---|---|---|---|---|---|
| N = 12 | N = 14 | N = 12 | N = 12 | N = 11 | |
| Male/Female | 10/2 | 13/1 | 11/1 | 10/2 | 9/2 |
| Age (yrs) | 58 ± 4 | 62 ± 2 | 58 ± 3 | 58 ± 4 | 67 ± 2 |
| Weight (kg) | 94 ± 4 | 97 ± 5 | 94 ± 9 | 93 ± 5 | 78 ± 5 |
| Height (cm) | 176 ± 3 | 177 ± 2 | 174 ± 4 | 180 ± 2 | 172 ± 2 |
| Body mass index (kg/m2) | 26 ± 1 | 31 ± 1 | 34 ± 7 | 29 ± 1 | 26 ± 1 |
| Systolic blood pressure (mmHg) | 127 ± 4 | 114 ± 2 | 124 ± 5 | 125 ± 3 | 120 ± 6 |
| Diastolic blood pressure (mmHg) | 79 ± 2 | 72 ± 2 | 77 ± 4 | 83 ± 3 | 73 ± 5 |
| Glucose (mg/dL) | 94 ± 4 | 137±20 * | 120 ± 15 | 93 ± 5 t | 113 ± 14 |
| Cholesterol (mg/dL) | 175 ± 14 | 149 ± 12 | 146 ± 12 | 138 ±11 | 143 ± 6 |
| HDL (mg/dL) | 50 ± 4 | 39 ± 2 | 40 ± 2 | 42 ± 3 | 46 ± 5 |
| LDL (mg/dL) | 113 ± 13 | 91 ± 9 | 91 ± 10 | 75 ± 9 * | 76 ± 5 * |
| Triglycerides (mg/dL) | 101 ± 19 | 134 ± 18 | 116 ± 13 | 129 ± 21 | 154 ± 20 |
| Hemoglobin (g/dL) | 15 ± 0.6 | 15 ± 0.5 | 13 ± 0.4 *†‡ | 15 ± 0.4 | 13 ± 0.3 *‡ |
| Hematocrit (%) | 45 ± 1.6 | 44 ± 1.6 | 39 ± 1.6 *‡ | 45 ± 1.2 | 40 ± 0.8 ‡ |
| RBC (M/ uL) | 5.1 ± 0.2 | 4.8 ± 0.2 | 4.3 ± 0.2 *‡ | 5.1 ± 0.2 | 4.3 ± 0.1 *‡ |
| WBC (K/uL) | 5.3 ± 0.4 | 7.5 ± 0.6 | 6.1 ± 0.7 | 6.5 ± 0.6 | 5.5 ± 0.5 |
Mean ± standard error; HDL, high density lipoprotein; LDL, low density lipoprotein; RBC, red blood cells; WBC, white blood cells.
Significantly different from control
Significantly different from HFrEF
Significantly different from HTx (5-10 yrs).
Table 2. Characteristics pertinent to the HF and HTx recipient groups.
| Variable | HFrEF | HTx (< 3 yrs) |
HTx (5-10 yrs) | HTx (> 14 yrs) |
|---|---|---|---|---|
| N = 14 | N = 12 | N = 12 | N = 11 | |
| Diagnosis (ischemic cardiomyopathy) | 7/14 | 7/12 | 6/12 | 6/11 |
| Diagnosis (non-ischemic cardiomyopathy) | 7/14 | 5/12 | 6/12 | 5/11 |
| Time post-HTx (months ± SE) | NA | 13 ± 4 (≈ 1 yr) | 85 ± 7 (≈ 7 yrs) | 226 ± 12 (≈ 19 yrs) |
| History of Rejection (# of all cases) | NA | 1/12 | 1/12 | 2/12 |
| Left Ventricular Ejection fraction (%) | 29 ± 4 | 66 ± 2* | 61 ± 2* | 61 ± 2* |
| Diabetic (# of all cases) | 6/14 | 7/12 | 3/12 | 6/11 |
| Medications: | ||||
| Cyclosporine (# of all cases) | 0/14 | 2/12 | 4/12 | 8/11 |
| Tacrolimus (# of all cases) | 0/14 | 10/12 | 7/12 | 2/11 |
| Azathioprine (# of all cases) | 0/14 | 2/12 | 2/12 | 0/11 |
| Mycophenolic acid (# of all cases) | 0/14 | 9/12 | 7/12 | 5/11 |
| Sirolimus (# of all cases) | 0/14 | 0/12 | 4/12 | 2/11 |
| Prednisone (# of all cases) | 0/14 | 6/12 | 2/12 | 4/11 |
| Beta-blocker (# of all cases) | 14/14 | 1/12 | 4/12 | 6/11 |
| ACE-Inhibitor (# of all cases) | 10/14 | 6/12 | 5/12 | 2/11 |
| Angiotensin Receptor Blocker (# of all cases) |
3/14 | 2/12 | 4/12 | 2/11 |
| Statin (# of all cases) | 11/14 | 10/12 | 9/12 | 5/11 |
| Diuretic (# of all cases) | 9/14 | 3/12 | 5/12 | 3/11 |
| Calcium channel-blocker (# of all cases) | 1/14 | 5/12 | 3/12 | 4/11 |
Mean ± SE; Significantly different from HFrEF
Flow-mediated Dilation (FMD)
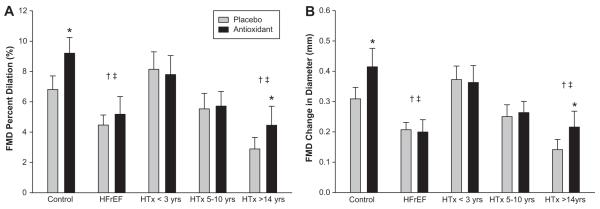
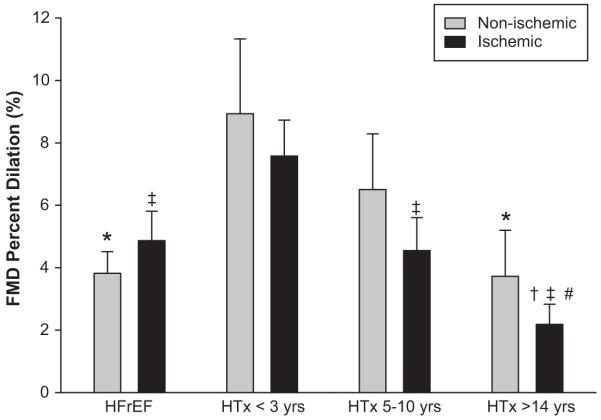
Baseline vascular function, as measured by PL FMD, was not different between the healthy age-matched controls (6.8 ± 1.9 %), recent, < 3 yrs post-HTx group (8.1 ± 1.2 %), and the 5-10 yrs post-HTx group (5.5 ± 1.0%) (Figure 1). In contrast, PL FMD was lower in the HFrEF patients (4.5 ± 0.7%) and in the > 14 yrs post-HTx recipients (2.9 ± 0.8%) compared to the controls. The HFrEF PL FMD was also significantly different from the <3 yrs post-HTx recipients. There was a significant negative correlation between time post HTx and PL FMD (r = -0.52) (Figure 2). When the HFrEF and HTx patient groups were broken down according to either current (HFrEF) or pre-HTx ischemic or non-ischemic disease etiology, there were no significant within group differences for PL FMD, however, the overall trends in vascular function remained the same (Figure 3). The antioxidant intervention increased FMD by 35% in the controls (PL 6.8 ± 1.9%; AOC 9.2 ± 1.0%) and by 55% in the > 14 yrs post-HTx recipients (PL 2.9 ± 0.8%; AOC 4.5 ± 1.3%). The AOC had no measurable vascular effect in any of the other patient groups. When the patient groups were separated by disease etiology, the > 14 yrs post-HTx ischemic group was the only group that exhibited a significant increase in FMD following the AOC (PL 2.2 ± 0.6%; AOC 3.4 ± 0.7%) (Table 3). Shear rate was not different between groups and therefore was not utilized to normalize FMD.
Figure 1.
(A) Brachial artery FMD with placebo and antioxidant supplementation following 5-min cuff occlusion expressed as a percent change from pre-cuff baseline in healthy controls, HFrEF patients, and 3 groups of HTx recipients. (*) Significantly different from Placebo; (†) Significantly different from Controls; (‡) Significantly different from HTx (< 3 yrs). (B) Brachial artery FMD expressed as absolute change in diameter from pre-cuff baseline in healthy controls, HFrEF patients, and 3 groups of HTx recipients. (*) Significantly different from Placebo; (†) Significantly different from Controls; (‡) Significantly different from HTx (< 3 yrs). Values are means ± SE.
Figure 2.
Time post-HTx and its influence on FMD (% diameter change).
Figure 3.
Placebo FMD broken down by current (HFrEF) or prior (HTx) disease etiology. (*) Significantly different from HTx < 3 yrs non-ischemic; (†) Significantly different from HFrEF ischemic; (‡) Significantly different from HTx < 3 yrs ischemic; (#) Significantly different from HTx 5-10 yrs ischemic. Values are means ± SE.
Table 3. Brachial artery FMD results based on current or pre-HTx etiology.
| Group | Etiology | PL FMD (%) | AO FMD (%) |
|---|---|---|---|
| HFrEF | Non-ischemic | 3.8 ± 0.7 | 4.1± 1.4 |
| Ischemic | 4.8 ± 0.9 | 5.2 ± 1.7 | |
| < 3 yrs post-HTx | Non-ischemic | 8.9 ± 2.4 | 9.8 ± 2.2 |
| Ischemic | 7.6 ± 1.2 | 6.4 ± 1.3 | |
| 5-10 yrs post-HTx | Non-ischemic | 6.5 ± 1.8 | 5.7 ± 1.6 |
| Ischemic | 4.5 ± 1.1 | 5.7 ± 1.2 (p = 0.06) | |
| >14 HTx yrs post-HTx | Non-ischemic | 3.7 ± 1.5 | 5.7 ± 2.7 |
| Ischemic | 2.2 ± 0.6 | 3.4 ± 0.7 * (p = 0.03) |
Mean ± SE; = significantly different from PL.
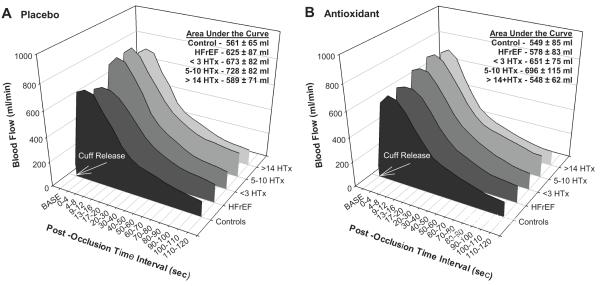
Resting Blood Flow and Reactive Hyperemia (RH)
Resting blood flow did not differ between healthy controls, HFrEF, and HTx recipients (Figure 4A). Similarly, RH, both when examined as peak flow (Control 742 ± 77 ml/min, HFrEF 707 ± 104 ml/min, < 3 yrs post-HTx 845 ± 97 ml/min, 5-10 yrs-post HTx 858 ± 93 ml/min, and > 14 yrs post-HTx 936 ± 170 ml/min) and AUC (Control 561 ± 65 ml, HFrEF 625 ± 87 ml, < 3 yrs post-HTx 673 ± 82 ml, 5-10 yrs post-HTx 728 ± 82 ml, and > 14 yrs post-HTx 589 ± 71 ml), was not different between groups (Figure 4A). After antioxidant administration, there was no significant change in RH evaluated either as peak flow (Control 724 ± 89 ml/min, HFrEF 705 ± 98 ml/min, < 3 yrs post-HTx 781 ± 88 ml/min, 5-10 yrs post-HTx 796 ± 92 ml/min, and > 14 yrs post-HTx 760 ± 72 ml/min) or AUC (Control 549 ± 85 ml, HFrEF 578 ± 83 ml/min, < 3yrs post-HTx 651 ± 75 ml, 5-10 yrs post-HTx 696 ± 115 ml, and > 14 yrs post- HTx 548 ± 62 ml) (Figure 3B).
Figure 4.
Resting brachial artery blood flow and RH with placebo and antioxidant supplementation following 5-min cuff occlusion in healthy controls, HFrEF patients, and 3 groups of HTx recipients. There were no significant differences at baseline, peak RH, or AUC for either Placebo (A) or Antioxidant (B).
Assays
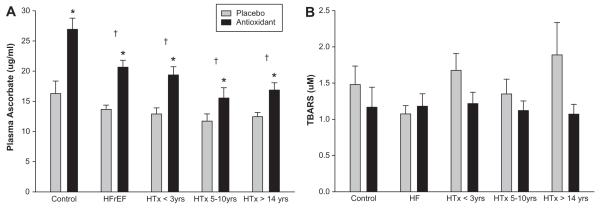
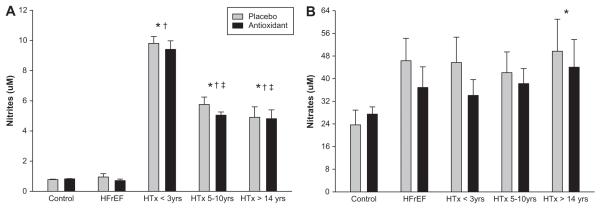
At baseline (PL) plasma ascorbate levels were significantly higher in the healthy controls compared to all of the other patient groups (Figure 5A). Two hours after AOC administration, there was a significant increase in plasma ascorbate concentration in all subject groups (Figure 5A). Baseline (PL) TBARS did not differ significantly between groups (Figure 5B), but after administration of the AOC, there was a trend for a reduction in TBARS in the healthy controls and in the HTx recipients. There were no significant differences in superoxide dismutase and catalase activity between the study groups, and no significant changes in these values were observed after AOC administration. There were no significant differences in baseline ET-1 values between groups although levels tended to be lower in the control group compared to the patient groups and there was evidence of an increased concentration in the >14yrs post-HTx group (Control 1.5 ± 0.2 pg/ml, HFrEF 1.8 ± 0.1 pg/ml, < 3 yrs post-HTx 1.9 ± 0.2 pg/ml, 5-10 yrs post-HTx 1.7 ± 0.2 pg/ml, and > 14 yrs post-HTx 2.1 ± 0.2 pg/ml). Baseline nitrite values were significantly higher in the HTx recipient groups compared to both the controls and the HFrEF patients (Figure 6A), with no measurable effect of the AOC. Baseline nitrate values were significantly higher in all the patient groups compared to the controls (Figure 6B), which tended to be reduced by the AOC.
Figure 5.
Quantitative assessment of antioxidant efficacy in healthy controls, HFrEF patients, and 3 groups of HTx recipients. (A) plasma ascorbate; (B) Thiobartituric Acid Reactive Substances (TBARS). Values are means ± SE. (*) Significantly different from Controls; (†) Significantly different from Placebo.
Figure 6.
Plasma nitrite (A) and plasma nitrate (B) with placebo and antioxidant supplementation in healthy controls, HFrEF patients, and 3 groups of HTx recipients. Values are means ± SE. (*) Significantly different from Controls; (†) Significantly different from HFrEF; (‡) Significantly different from < 3 yrs post-HTx.
DISCUSSION
This study, with a cross-sectional design, sought to determine vascular function and the role of oxidative stress in healthy controls, patients with HFrEF, and comprehensively with time beyond HTx. Utilizing FMD to assess endothelium-dependent vascular function, compared to controls, we documented reduced vasodilatory capacity in the HFrEF patients and comparable vascular function in the early HTx recipients. However, vascular function was lower in the other HTx recipient groups to such a point where those who were the furthest time beyond transplantation (> 14 yrs post HTx) exhibited vascular function similar to, if not more compromised than the HFrEF patients. Interestingly, unlike the other patient groups, the acute ingestion of the AOC was able to significantly increase FMD by 55% in these > 14 yrs post-HTx recipients suggesting that free radicals, and the associated decrease in NO bioavailability, are largely responsible for endothelial dysfunction in this group. RH, an index of microvascular function, was not different across the groups and did not change after AOC administration, highlighting the differing physiology and pathophysiology assessed by FMD and RH. Also of significant importance to the interpretation of these data is the fact that these cross-sectional observations were not confounded by aging as the controls and all patient groups were of similar age. These findings not only highlight the transient nature of vascular function across the continuum from HF to HTx, but also reveal the significant deterioration in endothelium- dependent vasodilation in HTx recipients, regardless of the initial disease etiology, who are one to two decades beyond transplantation. This ultimate decline, as with the controls, appears to be a consequence of a free radically-mediated reduction in NO bioavailability.
Flow-mediated Vasodilation in HFrEF and HTx Recipients
It is well accepted that peripheral endothelial function is impaired in patients with HF, 3, 4 as a consequence of an attenuated cardiac output, reduced levels of physical activity, elevated peripheral vasoconstriction, and neurohormonal activation. Data from the current study are in agreement with this dogma as FMD, a reliable non-invasive measurement of endothelial function, was significantly blunted in HFrEF patients compared to the healthy, age-matched controls (Figure 1). Vascular function appears to be relatively normal in the first few years following HTx as the < 3yrs post-HTx recipients were similar to the controls. Both Kubo et al. 20 and Roig et al. 21 documented a normalization of endothelial function within the first year following HTx, which is most likely a result of an improved cardiac output with the donor heart in place and a reduction in sympathetic nervous system activity 31 which would increase nitric oxide bioavailability. However, endothelial function in HTx recipients, as a whole, is still somewhat controversial. 32, 33
To our knowledge, this is the first vascular function study to include healthy age-matched controls, HFrEF patients, and a comprehensive cross-sectional group of HTx recipients at three discrete time points following transplantation, particularly a group that was > 14 yrs post-HTx. With this approach, utilizing FMD, we clearly documented relatively normal endothelial- dependent vasodilation following HTx that gradually declines to a level similar to that of HFrEF patients. This took place despite normal left ventricular ejection fraction in all three HTx groups examined. It is important to note that this decrease in FMD is not a consequence of age-related changes in vascular function 17, as all groups were similar in terms of age.
Although certainly not the main goal of the current study due to limited numbers of available subjects in the groups long after HTx, these data do afford the opportunity to examine vascular function in HFrEF patients and HTx recipients over time according to ischemic and non-ischemic disease etiology. Previous work has suggested that endothelium-dependent vasodilation, as measured by FMD, is attenuated to a greater degree in HF patients 34 and HTx recipients 32 with ischemic cardiomyopathy compared to those with non-ischemic cardiomyopathy. However, the study by Klosinska et al. 34 also documented that although the vascular function in the non-ischemic HF patients was significantly higher than those with ischemic cardiomyopathy, it was still significantly lower than the age-matched healthy controls. In the current study, although the ischemic HTx recipients in each group tended to have lower FMD values in comparison to those with non-ischemic cardiomyopathy, there were no significant differences in PL FMD between disease etiologies in any of the patient groups (Figure 3). Interestingly, when only examining the patients with ischemic cardiomyopathy, FMD in both the 5-10 yrs post-HTx and > 14 yrs post-HTx groups was significantly lower than that in the < 3 yrs post-HTx, and FMD in the 5-10 yrs post-HTx was comparable to the ischemic HFrEF patients. These data suggest the decrease in vascular function with time following surgery occurs more rapidly in those with ischemic cardiomyopathy. However, despite this additional group delineation based on disease etiology, the overall trend for declining vascular function in all HTx recipients with the passage of time remained essentially unchanged.
While HTx improves central hemodynamics, an event likely to be favorable to vascular function, there are also a number of factors that may actually exert negative effects on the vasculature. These include ischemia, 35 preservation and reperfusion effects at the time of transplant, 36, 37 impaired cardiovascular and pulmonary responses to physical activity, 38, 39 infections such as cytomegalovirus in the immunosuppressed patient, 40 as well as other effects of chronic immunosuppression and complex long-term pharmacological therapy after transplant. 41 Specifically, most HTx recipients are treated with calcineurine inhibitors, cyclosporine or tacrolimus. 42 Previous studies have revealed that cyclosporine may result in endothelial dysfunction, 41 as it has direct cytotoxic effects on the endothelium, 43 impairs endothelium- derived relaxing factor release, 44 and increases ET-1 production. 45 In addition, cyclosporine has been linked to increased sympathetic tone, new-onset hypertension, and peripheral vasoconstriction in HTx recipients. 46 These effects may, at least in part, be mediated by free radicals. 22 Our data provide further support for the idea that long-term cyclosporine exposure contributes to endothelial dysfunction as ~75% of the > 14 yrs post-HTx recipients were currently taking cyclosporine (Table 2) and there was a tendency for ET-1 levels to be increased in this group.
Reactive Hyperemia in HFrEF and HTx Recipients
In contrast to FMD, the RH response represents both endothelium-dependent and independent vasodilation of the microvasculature. 47, 48 We hypothesized that RH would follow a similar pattern to the FMD measurements, such that when compared to controls, RH would be reduced in HFrEF patients, improve immediately following HTx, and progressively decline thereafter. In fact, resting limb blood flow and RH, both in terms of peak, and AUC were not different between groups (Figure 4). Thus, in this study, there was no evidence of microvascular dysfunction in the controls, HFrEF patients, or HTx recipients. These results are in contrast to many of the HF animal model studies that suggest microcirculation is impaired 49, 50, but the overall body of literature related to endothelial-independent vascular function in HF patients is equivocal. 34, 37, 51 Further, there is convincing evidence that endothelial-independent vascular function is not attenuated in HTx recipients. 20, 21 Again, it should be noted that microvascular function (i.e. RH) does not necessarily track conduit vessel endothelial function which makes the divergent FMD and RH results not so surprising and suggests that further studies are necessary to better examine the changes in blood flow distribution within and between different muscles in patients with HF.
Free Radicals and Vascular Function in HFrEF and HTx Recipients
We hypothesized that the administration of an acute AOC would attenuate the circulating levels of free radicals and improve vascular function. In this study, administration of the AOC did not alter FMD or RH response compared to placebo in the HFrEF patients, or the HTx recipients that were < 3 yrs and 5-10 yrs after HTx. However, the AOC increased FMD by 35% in the controls and by 55% in the group of HTx recipients furthest beyond transplant (> 14 yrs) (Figure 1). This finding suggests that in these patients with markedly decreased FMD and the longest immunosuppressant use, the attenuation in vascular function can be improved by decreasing free radical concentration.
As direct measurement of NO bioavailability in humans is often not feasible, vasomotor function (e.g. FMD) or NO-related compounds are often measured as surrogates. Recognizing that such NO-related compounds vary in their biological activity, concentration, and compartmentalization between plasma, blood, and other tissues 52 we measured plasma nitrites and nitrates in the current study. Interestingly, plasma nitrites were not different between the controls and patients with heart failure, but were significantly increased in all the HTx recipients (Figure 6A). Also, despite the nitrites being elevated in all the HTx groups there was a significant decline in those that were 5-10 yrs, and > 14 yrs post-HTx in a pattern similar to the FMD results. Plasma nitrates tended to be elevated in all the patient groups compared to the controls (Figure 6B) with the > 14 yrs post-HTx group exhibiting significantly greater levels than the controls. There was also a tendency for a reduction in nitrates following AOC consumption in all of the patient groups (Figure 6B). Elevated plasma nitrates have previously been reported in HF patients 53 and the higher values in the HTx recipients could be the result of poor renal function and greater oxidative stress in these individuals. 52, 54
Despite the confirmation of an AOC-induced increase in circulating antioxidant capacity in all subject groups, by documenting an increase in plasma vitamin C levels, (Figure 5A), there were no significant changes in TBARS, a marker of total oxidative stress (Figure 5C). However, there was an overall trend for TBARS to decrease in the control and HTx groups, but it is likely that the sensitivity of this assay was not adequate to detect acute changes, in oxidative stress in the current study. Such conclusions about the TBARS assay are supported by Silvestro et al. 55 who reported that in patients with intermittent claudication, there was no relationship between TBARS and FMD following vitamin C infusion, suggesting that TBARS is unable to accurately reflect acute changes occurring within the vasculature.
Experimental Considerations
It should be noted that the present study employed a cross-sectional experimental design, however, although this approach comes with limitations, it was necessary in order to include a wide range of HTx recipients some of whom were over 20 years post-HTx. We also acknowledge that although we matched the groups for age and many other factors, including etiology of heart failure, we did not control for variations in pharmacological therapies across these groups which may have influenced our findings. Additionally, HTx recipients that were 3 to 5 years post-HTx and 10 to 14 years post-HTx were specifically not recruited and therefore not studied, however, given our hypothesis that vascular function gradually declines as time post-HTx increases this was necessary to differentiate our groups.
Perspectives
Heart transplantation is an accepted therapy that results in improved quality of life and dramatically better survival in patients with severe HF. While survival after HTx has been improving over the past two decades, most of this has been a result of decreased mortality in the first post-transplant year. 42 Approaches to improve long-term survival after HTx are therefore needed. This study extends the current understanding of the long-term effects of HTx on vascular function. These findings may help guide new approaches aimed at maintaining vascular health after HTx.
Conclusion
This study has documented that endothelial-dependent vasodilation, determined by FMD, is reduced in HFrEF patients and by using a comprehensive, cross-sectional approach this study has revealed normal vascular function soon after HTx followed by a gradual decline to a level similar to HFrEF patients in the years beyond transplant regardless of previous disease etiology. Interestingly, the attenuated vascular function in HTx recipients one to two decades following transplantation is most likely related to decreased nitric oxide bioavailability, as an acute dosage of oral antioxidants, and a likely decrease in free radicals, significantly improves FMD in these subjects.
Novelty and Significance.
What is New?
-
-
There is relatively little information regarding peripheral artery endothelial function in HFrEF patients and HTx recipients at various time points following surgery.
-
-
Endothelial-dependent vasodilation, determined by FMD, is reduced in HFrEF patients, is restored to normal soon after HTx, followed by a gradual decline to a level similar to HFrEF patients in the years beyond transplant regardless of previous disease etiology.
-
-
The attenuated vascular function in HTx recipients one to two decades following transplantation is most likely related to decreased nitric oxide bioavailability, as an acute dosage of oral antioxidants, and a subsequent decrease in free radicals, significantly improves FMD in these subjects.
What is Relevant?
-
-
Impaired endothelium-dependent vasodilation has been associated with various cardiovascular diseases including hypertension, coronary artery disease, and even heart failure.
-
-
Importantly, impaired endothelium-dependent vasodilation may also precede the development of these cardiovascular diseases.
-
-
Hypertension is common in HTx recipients therefore determining levels of vascular function and the role of nitric oxide may be beneficial in better understanding the mechanisms contributing to the abnormal pressures often seen in this patient population.
Summary
-
-
This cross-sectional study reveals that, compared to controls, vascular function is blunted in HFrEF patients, is similar soon after HTx, but decreases with greater time beyond HTx, with free radicals implicated in this progression.
ACKNOWLEDGEMENTS
The authors thank the subjects for their time and effort in participating in this research study. We would also like to thank Mary Beth Hagan, FNP and Robin Waxman, APRN from the Salt Lake City VA Medical Center Heart Failure and Heart Transplant clinic and Le Ann Stamos, RN, MS, Shirley Belleville, RN, BSN, and Kirk Volkman, NP, from the University of Utah Heart Failure and Heart Transplant clinic for their invaluable help with subject recruitment.
GRANTS
This study was supported in part by grants from the NIH (P01 HL091830 - Richardson) and AHA (0835209N - Wray).
Footnotes
DISCLOSURES
The authors of this manuscript have no conflicts of interest to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Panza JA, Quyyumi AA, Brush JE, Jr., Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 2.Monnink SH, van Haelst PL, van Boven AJ, Stroes ES, Tio RA, Plokker TW, Smit AJ, Veeger NJ, Crijns HJ, van Gilst WH. Endothelial dysfunction in patients with coronary artery disease: A comparison of three frequently reported tests. J Investig Med. 2002;50:19–24. doi: 10.2310/6650.2002.33513. [DOI] [PubMed] [Google Scholar]
- 3.Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvao M, Solomon S, Nikolic SD, Forman R, LeJemtel TH. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol. 1992;19:918–925. doi: 10.1016/0735-1097(92)90271-n. [DOI] [PubMed] [Google Scholar]
- 4.Drexler H, Hayoz D, Munzel T, Hornig B, Just H, Brunner HR, Zelis R. Endothelial function in chronic congestive heart failure. Am J Cardiol. 1992;69:1596–1601. doi: 10.1016/0002-9149(92)90710-g. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer D, Sorensen K, Spiegelhalter D, Georgakopoulos D, Robinson J, Deanfield J. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 7.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF, Jr., Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr., Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: The framingham heart study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 9.Hasking GJ, Esler MD, Jennings GL, Dewar E, Lambert G. Norepinephrine spillover to plasma during steady-state supine bicycle exercise. Comparison of patients with congestive heart failure and normal subjects. Circulation. 1988;78:516–521. doi: 10.1161/01.cir.78.3.516. [DOI] [PubMed] [Google Scholar]
- 10.Francis GS, Rector TS, Cohn JN. Sequential neurohumoral measurements in patients with congestive heart failure. Am Heart J. 1988;116:1464–1468. doi: 10.1016/0002-8703(88)90729-6. [DOI] [PubMed] [Google Scholar]
- 11.Katz SD, Khan T, Zeballos GA, Mathew L, Potharlanka P, Knecht M, Whelan J. Decreased activity of the l-arginine-nitric oxide metabolic pathway in patients with congestive heart failure. Circulation. 1999;99:2113–2117. doi: 10.1161/01.cir.99.16.2113. [DOI] [PubMed] [Google Scholar]
- 12.Bauersachs J, Bouloumie A, Fraccarollo D, Hu K, Busse R, Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: Role of enhanced vascular superoxide production. Circulation. 1999;100:292–298. doi: 10.1161/01.cir.100.3.292. [DOI] [PubMed] [Google Scholar]
- 13.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 14.Wei EP, Kontos HA, Christman CW, DeWitt DS, Povlishock JT. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res. 1985;57:781–787. doi: 10.1161/01.res.57.5.781. [DOI] [PubMed] [Google Scholar]
- 15.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 16.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: Effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol. 2010;298:H671–678. doi: 10.1152/ajpheart.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O’Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension. 2012;59:818–824. doi: 10.1161/HYPERTENSIONAHA.111.189456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornig B, Arakawa N, Kohler C, Drexler H. Vitamin c improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation. 1998;97:363–368. doi: 10.1161/01.cir.97.4.363. [DOI] [PubMed] [Google Scholar]
- 20.Kubo SH, Rector TS, Bank AJ, Tschumperlin LK, Raij L, Brunsvold N, Kraemer MD. Effects of cardiac transplantation on endothelium-dependent dilation of the peripheral vasculature in congestive heart failure. Am J Cardiol. 1993;71:88–93. doi: 10.1016/0002-9149(93)90716-p. [DOI] [PubMed] [Google Scholar]
- 21.Roig E, Cuppoletti A, Masotti M, Kianco R, Vallejos I, Sitges M, Ortiz J, Perez-Villa F. Assessment of peripheral endothelial-dependent vasodilatation within the first year after heart transplantation. J Heart Lung Transplant. 2009;28:299–304. doi: 10.1016/j.healun.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Diederich D, Skopec J, Diederich A, Dai FX. Cyclosporine produces endothelial dysfunction by increased production of superoxide. Hypertension. 1994;23:957–961. doi: 10.1161/01.hyp.23.6.957. [DOI] [PubMed] [Google Scholar]
- 23.Mugge A, Brandes RP, Heublein B, Nolte C, Haverich A, Lichtlen PR. Endothelial dysfunction in heart transplanted patients with graft vasculopathy. European heart journal. 1995;16(Suppl J):78–83. doi: 10.1093/eurheartj/16.suppl_j.78. [DOI] [PubMed] [Google Scholar]
- 24.Sinoway LI, Minotti JR, Davis D, Pennock JL, Burg JE, Musch TI, Zelis R. Delayed reversal of impaired vasodilation in congestive heart failure after heart transplantation. Am J Cardiol. 1988;61:1076–1079. doi: 10.1016/0002-9149(88)90129-4. [DOI] [PubMed] [Google Scholar]
- 25.Harris RA, Nishiyama SK, Wray DW, Tedjasaputra V, Bailey DM, Richardson RS. The effect of oral antioxidants on brachial artery flow-mediated dilation following 5 and 10 min of ischemia. Eur J Appl Physiol. 2009;107:445–453. doi: 10.1007/s00421-009-1147-x. [DOI] [PubMed] [Google Scholar]
- 26.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: A radically different outcome. Clin Sci (Lond) 2009;116:433–441. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- 28.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem. Med. 1976;15:212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler CR, Salzman JA, Elsayed NM, Omaye ST, Korte DW., Jr. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Analytical biochemistry. 1990;184:193–199. doi: 10.1016/0003-2697(90)90668-y. [DOI] [PubMed] [Google Scholar]
- 30.Bradley DW, Emery G, Maynard JE. Vitamin c in plasma: A comparative study of the vitamin stabilized with trichloroacetic acid or metaphosphoric acid and the effects of storage at-70,-20, 4, and 25 on the stabilized vitamin. Clinica Chimica Acta. 1973;44:47–52. doi: 10.1016/0009-8981(73)90158-7. [DOI] [PubMed] [Google Scholar]
- 31.Levine TB, Olivari MT, Cohn JN. Effects of orthotopic heart transplantation on sympathetic control mechanisms in congestive heart failure. The American journal of cardiology. 1986;58:1035–1040. doi: 10.1016/s0002-9149(86)80034-0. [DOI] [PubMed] [Google Scholar]
- 32.Patel AR, Kuvin JT, Pandian NG, Smith JJ, Udelson JE, Mendelsohn ME, Konstam MA, Karas RH. Heart failure etiology affects peripheral vascular endothelial function after cardiac transplantation. J Am Coll Cardiol. 2001;37:195–200. doi: 10.1016/s0735-1097(00)01057-3. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt A, Pleiner J, Bayerle-Eder M, Wiesinger GF, Rodler S, Quittan M, Mayer G, Wolzt M. Regular physical exercise improves endothelial function in heart transplant recipients. Clin Transplant. 2002;16:137–143. doi: 10.1034/j.1399-0012.2002.1o100.x. [DOI] [PubMed] [Google Scholar]
- 34.Klosinska M, Rudzinski T, Grzelak P, Stefanczyk L, Drozdz J, Krzeminska-Pakula M. Endothelium-dependent and -independent vasodilation is more attenuated in ischaemic than in non-ischaemic heart failure. Eur J Heart Fail. 2009;11:765–770. doi: 10.1093/eurjhf/hfp091. [DOI] [PubMed] [Google Scholar]
- 35.Furchgott RF. The 1989 ulf von euler lecture. Studies on endothelium-dependent vasodilation and the endothelium-derived relaxing factor. Acta Physiologica Scandinavica. 1990;139:257–270. doi: 10.1111/j.1748-1716.1990.tb08923.x. [DOI] [PubMed] [Google Scholar]
- 36.Lindenfeld J, Miller GG, Shakar SF, Zolty R, Lowes BD, Wolfel EE, Mestroni L, Page RL, 2nd, Kobashigawa J. Drug therapy in the heart transplant recipient: Part i: Cardiac rejection and immunosuppressive drugs. Circulation. 2004;110:3734–3740. doi: 10.1161/01.CIR.0000149745.83186.89. [DOI] [PubMed] [Google Scholar]
- 37.Morgan DR, Dixon LJ, Hanratty CG, Hughes SM, Leahey WJ, Rooney KP, Johnston GD, McVeigh GE. Impaired endothelium-dependent and -independent vasodilation in elderly patients with chronic heart failure. European journal of heart failure. 2004;6:901–908. doi: 10.1016/j.ejheart.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Scott JM, Esch BT, Haykowsky MJ, Warburton DE, Toma M, Jelani A, Taylor D, Paterson I, Poppe D, Liang Y, Thompson R. Cardiovascular responses to incremental and sustained submaximal exercise in heart transplant recipients. American journal of physiology. Heart and circulatory physiology. 2009;296:H350–358. doi: 10.1152/ajpheart.01100.2008. [DOI] [PubMed] [Google Scholar]
- 39.Kao A, Van Trigt P, 3rd, Shaeffer-McCall G, Shaw J, Kuzil B, Page R, Higginbotham M. Central and peripheral limitations to upright exercise in untrained cardiac transplant recipients. Circulation. 1994;89:2605. doi: 10.1161/01.cir.89.6.2605. [DOI] [PubMed] [Google Scholar]
- 40.Petrakopoulou P, Kubrich M, Pehlivanli S, Meiser B, Reichart B, von Scheidt W, Weis M. Cytomegalovirus infection in heart transplant recipients is associated with impaired endothelial function. Circulation. 2004;110:II207–212. doi: 10.1161/01.CIR.0000138393.99310.1c. [DOI] [PubMed] [Google Scholar]
- 41.Jeanmart H, Malo O, Carrier M, Nickner C, Desjardins N, Perrault LP. Comparative study of cyclosporine and tacrolimus vs newer immunosuppressants mycophenolate mofetil and rapamycin on coronary endothelial function. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2002;21:990–998. doi: 10.1016/s1053-2498(02)00429-1. [DOI] [PubMed] [Google Scholar]
- 42.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The registry of the international society for heart and lung transplantation: Twenty-eighth adult heart transplant report-2011. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Zoja C, Furci L, Ghilardi F, Zilio P, Benigni A, Remuzzi G. Cyclosporin-induced endothelial cell injury. Laboratory investigation; a journal of technical methods and pathology. 1986;55:455–462. [PubMed] [Google Scholar]
- 44.Voss BL, Hamilton KK, Samara EN, McKee PA. Cyclosporine suppression of endothelial prostacyclin generation. A possible mechanism for nephrotoxicity. Transplantation. 1988;45:793–796. doi: 10.1097/00007890-198804000-00025. [DOI] [PubMed] [Google Scholar]
- 45.Bunchman TE, Brookshire CA. Cyclosporine-induced synthesis of endothelin by cultured human endothelial cells. The Journal of clinical investigation. 1991;88:310–314. doi: 10.1172/JCI115293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrama YC, van Dam T, Fijnheer R, Hene RJ, de Groot P, Rabelink TJ. Cyclosporine is associated with endothelial dysfunction but not with platelet activation in renal transplantation. Neth J Med. 2001;59:6–15. doi: 10.1016/s0300-2977(01)00127-9. [DOI] [PubMed] [Google Scholar]
- 47.Loscalzo J, Vita JA. Ischemia, hyperemia, exercise, and nitric oxide. Complex physiology and complex molecular adaptations. Circulation. 1994;90:2556–2559. doi: 10.1161/01.cir.90.5.2556. [DOI] [PubMed] [Google Scholar]
- 48.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. The American journal of physiology. 1996;270:H1435–1440. doi: 10.1152/ajpheart.1996.270.4.H1435. [DOI] [PubMed] [Google Scholar]
- 49.Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: Rest and exercise. The American journal of physiology. 1992;262:H411–419. doi: 10.1152/ajpheart.1992.262.2.H411. [DOI] [PubMed] [Google Scholar]
- 50.McAllister RM, Laughlin MH, Musch TI. Effects of chronic heart failure on skeletal muscle vascular transport capacity of rats. The American journal of physiology. 1993;264:H689–691. doi: 10.1152/ajpheart.1993.264.3.H686. [DOI] [PubMed] [Google Scholar]
- 51.Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 52.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M, Kelm M. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40:295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 53.Winlaw DS, Smythe GA, Keogh AM, Schyvens CG, Spratt PM, Macdonald PS. Increased nitric oxide production in heart failure. Lancet. 1994;344:373–374. doi: 10.1016/s0140-6736(94)91403-6. [DOI] [PubMed] [Google Scholar]
- 54.Szabo C, Day BJ, Salzman AL. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. FEBS letters. 1996;381:82–86. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- 55.Silvestro A, Scopacasa F, Oliva G, de Cristofaro T, Iuliano L, Brevetti G. Vitamin c prevents endothelial dysfunction induced by acute exercise in patients with intermittent claudication. Atherosclerosis. 2002;165:277–283. doi: 10.1016/s0021-9150(02)00235-6. [DOI] [PubMed] [Google Scholar]