Summary
Estrogen receptor α (ERα) plays a key role in the adaptation of increased uterine blood flow in pregnancy. Chronic hypoxia is a common stress to maternal cardiovascular homeostasis and causes increased risk of preeclampsia. Studies in pregnant sheep demonstrated that hypoxia during gestation downregulated ERα gene expression in uterine arteries. The present study tested the hypothesis that hypoxia causes epigenetic repression of the ERα gene in uterine arteries via heightened promoter methylation. Ovine ERα promoter of 2035 bp spanning from −2000 to +35 of the transcription start site was cloned. No estrogen or HIF response elements were found at the promoter. Two transcription factor binding sites USF-15 and Sp1-520 containing CpG dinucleotides were identified, which had significant effects on the promoter activity. The USF element binds transcription factors USF1 and USF2, and the Sp1 element binds Sp1, as well as ERα through Sp1. Deletion of the Sp1 site abrogated 17β-estradiol-induced increase in the promoter activity. In normoxic control sheep, CpG methylation at the Sp1, but not USF, site was significantly decreased in uterine arteries of pregnant, as compared with nonpregnant animals. In pregnant sheep exposed to long-term high altitude hypoxia, CpG methylation at both Sp1 and USF sites in uterine arteries was significantly increased. Methylation inhibited transcription factor binding and the promoter activity. The results provide evidence of hypoxia causing heightened promoter methylation and resultant ERα gene repression in uterine arteries, and suggest new insights of molecular mechanisms linking gestational hypoxia to aberrant uteroplacental circulation and increased risk of preeclampsia.
Keywords: Hypoxia, estrogen receptor, methylation, epigenetic modulation, uterine artery
Introduction
The striking increase in uterine blood flow during pregnancy is a key adaptation to maintain normal fetal development as well as maternal cardiovascular well being. Animal studies have demonstrated that a chronic reduction of uteroplacental perfusion leads to a hypertension state that closely resembles preeclampsia in women.1,2 Chronic hypoxia during gestation is a common stress to maternal cardiovascular homeostasis and has profound adverse effects on uteroplacental circulation, leading to attenuated uterine blood flow and increased risk of preeclampsia and fetal intrauterine growth restriction.3–8 It has been shown that both endogenous or exogenous estrogen plays a pivotal role in regulating uterine blood flow during pregnancy.9–14 We have recently demonstrated in sheep a direct genomic effect of exogenous estrogen in down-regulating the protein kinase C signaling pathway and up-regulating the large-conductance Ca2+-activated K (BKCa) channel activity, resulting in a reduced myogenic tone of the uterine artery in pregnancy.15,16 Further studies demonstrated that pregnancy or exogenous estrogen induced up-regulation of BKCa channel activity and a reduction of pressure-induced myogenic tone of the uterine artery were abrogated in pregnant sheep acclimatized to long-term high altitude hypoxia.17,18 Chronic hypoxia during gestation did not alter maternal plasma estrogen levels, but significantly suppressed estrogen receptor α (ERα) expression in the uterine artery.18
The molecular mechanisms underlying gestational hypoxia-mediated ERα gene repression in the uterine artery remain undetermined. Epigenetic mechanisms are essential for the homeostasis in response to the environment through changes in gene expression patterns.19–21 DNA methylation is a chief mechanism in epigenetic modification of gene expression patterns, and occurs at cytosines of the dinucleotide sequence CpG.21–23 While cytosine is methylated in 70% of CpGs of mammalian DNA, CpGs in the promoter/enhancer regions of many mammalian genes are not methylated. Increased methylation in promoter regions is generally associated with transcription repression of the associated genes. Although methylation of the ERα promoter has been reported to occur as a direct function of physiological regulation in several tissue types and as part of a pathological progression of numerous types of cancerous tissues,24–31 little is known about the epigenetic regulation of ERα gene expression patterns in vascular smooth muscle and its functional consequences. Herein, we present novel evidence that chronic hypoxia during gestation causes repression of the ERα gene in the uterine artery via heightened promoter methylation, providing a molecular mechanism linking hypoxia and maladaptation of uteroplacental circulation and increased risk of preeclampsia in pregnancy.
Materials and Methods
An expanded Materials and Methods section is available in the online data supplement at http://hyper.ahajournals.org.
Tissue Preparation and Treatment
Uterine arteries were harvested from nonpregnant sheep regardless of stages of the estrous cycle and near-term pregnant (~140 days’ gestation) ewes maintained at sea level (~300m, arterial PaO2~102 mmHg) or exposed to high-altitude (3801m, arterial PaO2~60 mmHg) hypoxia for 110 days.18 To investigate the direct effect of hypoxia, some arteries obtained from normoxic control nonpregnant and pregnant animals were treated in a humidified incubator with either 21.0% or 10.5% O2 for 48 hours, as described previously.18 All procedures and protocols were approved by the Institutional Animal Care and Use Committee and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Reporter Gene Assay
Genomic DNA isolated in uterine arteries from control nonpregnant animals was used as a PCR template. Using primers designed based on the bovine ERα gene promoter sequence (Gene ID: 407238), a 2035 bp ovine genomic fragment spanning −2000 bp to +35 bp relative to the transcription start site was cloned. The activities of wild-type or site specific deletion of USF-15, Sp1-520, and PRA/B-563, respectively, promoter constructs were determined in uterine arterial smooth muscle cells of control nonpregnant sheep, as described previously.32,33
Quantitative Methylation-Specific PCR
Genomic DNA was isolated from uterine arteries of all four group animals. Bisulfite-treated DNA was used as a template for real-time fluorogenic methylation-specific PCR (MSP) using specific primers designed to amplify the regions of interest with unmethylated CpG dinucleotides or methylated CpG dinucleotides (CmG), respectively (Table S1, available in the online data supplement), as previously described.33,34
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts were collected in uterine arteries of control nonpregnant animals. The oligonucleotide probes with CpG and CmG in the three putative transcription factor binding sites, USF-15, Sp1-520, and PRA/B-563 at the ovine ERα promoter region were labeled and subjected to gel shift assays using the Biotin 3′ end labeling kit and Light-Shift Chemiluminescent EMSA Kit (Pierce Biotechnology, Rockford, IL), as previously described.33,34
Chromatin Immunoprecipitation (ChIP)
Chromatin extracts were prepared from uterine arteries of all four group animals. ChIP assays were performed using the ChIP-IT kit (Active Motif, Carlsbad, CA), as previously described.33,34 Primers flanking USF-15 and Sp1-520 binding sites are shown in Table S2 (available in the online data supplement).
Site-Directed CpG Methylation Mutagenesis and Reporter Gene Assay
The effect of site-directed CpG methylation on the ERα promoter activity was determined in uterine arterial smooth muscle cells of control nonpregnant sheep, as described previously.33 Table S3 (available in the online data supplement) lists the oligonucleotide probes used in site-directed CpG methylation mutagenesis and reporter gene assay.
Data Analysis
Results are expressed as means ± SEM obtained from the number of experimental animals given. Differences were evaluated for statistical significance (P<0.05) by ANOVA or t test, where appropriate.
Results
Sequencing of Ovine ERα Gene Proximal Regulatory Region
The sequence of the regulatory region consisting of 2035 bp ovine genomic fragment spanning −2000 bp to +35 bp relative to the transcription start site of ERα gene is shown in Figure S1 (available in the online data supplement), which has 96% homology to the bovine ERα proximal promoter obtained from GenBank (Gene ID: 407238). Similar to the bovine ERα promoter, the ovine ERα promoter lacks a TATA-like element. In silico analysis of the ovine ERα promoter sequence identified three putative transcription factor binding sites that contained CpG dinucleotides in their core binding sequences: USF-15, Sp1-520, and PRA/B-563 (Figure S1, available in the online data supplement).
Deletion of USF-15, Sp1-520 and PRA/B-563 Inhibits ERα Promoter Activity
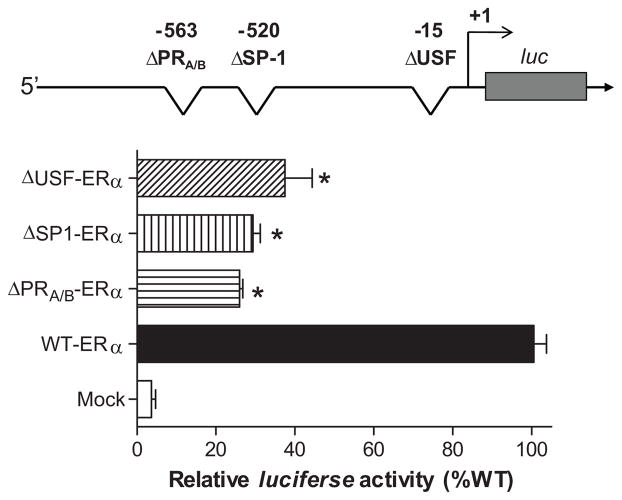
The 2035 bp ovine ERα promoter was first cloned into pCR4-TOPO vector and subsequently cloned in Xho I-Hind III orientation into pGL3 basic vector to drive the transcription of the luciferase reporter gene transfected in smooth muscle cells of uterine arteries from control nonpregnant sheep. Site specific deletion of USF-15, Sp1-520, or PRA/B-563, respectively, caused a significant inhibition of the promoter activity (Figure 1), indicating a strong stimulatory role of these promoter elements in driving the transcription of ovine ERα promoter.
Figure 1. Deletion of USF-15, Sp1-520 and PRA/B-563 inhibits ERα promoter activity.
A 2035 bp fragment of ovine ERα promoter region spanning −2000 to +35 bp relative to the transcription start site was amplified by PCR and inserted into pGL3 to yield the full-length promoter–reporter plasmid (WT-ERα). Three site-specific deletion mutations at USF-15 (ΔUSF-ERα), Sp1-520 (ΔSp1-ERα), and PRA/B-563 (ΔPRA/B-ERα) were constructed. Constructs were then transiently transfected to uterine arterial smooth muscle cells. Firefly and Renilla reniformis luciferase activities were measured after 48 hours in a luminometer using a dual-luciferase reporter assay system. Data are means ± SEM, * P < 0.05, versus WT-ERα. n = 6–10
Chronic Hypoxia Increases CpG Methyaltion at ERα Promoter
Our previous studies in sheep demonstrated that pregnancy upregulated ERα gene expression and significantly increased ERα mRNA and protein abundance in the uterine artery, which was abrogated in animals acclimatized to long-term high altitude hypoxia.18 The effects of pregnancy and hypoxia on methylation patterns of CpG dinucleotides in the three putative binding sites at ovine ERα promoter were determined in uterine arteries by quantitative methylation-specific PCR. In normoxic control animals, there were no significant changes in CpG methylation status at the USF-15 and PRA/B-563 sites, but a significant decrease in CpG methylation at the Sp1-520 site in uterine arteries of pregnant, as compared with nonpregnant sheep (Figure 2A). In animals acclimatized to long-term high altitude hypoxia, there were significant increases in CpG methylation at USF-15 and Sp1-520, but not at PRA/B-563, sites in uterine arteries of pregnant sheep (Figure 2A). Although it appeared trend of increased methylation at the Sp1-520 site, chronic hypoxia had no significant effect on ERα promoter methylation in uterine arteries of nonpregnant animals (Figure 2A), suggesting that it is the decrease in methylation in pregnancy that is abrogated by hypoxia. In the previous study, we demonstrated that ex vivo hypoxic treatment of uterine arteries under 10.5% O2 for 48 hours produced the results similar to those found in high-altitude hypoxic animals and caused a significant decrease in ERα mRNA and protein abundance.18 To determine the direct effect of hypoxia on CpG methylation patterns at ERα promoter, uterine arteries from normoxic control nonpregnant and pregnant sheep were treated ex vivo with 21.0% or 10.5% O2 for 48 hours. Figure 2B shows that the ex vivo hypoxic treatment of uterine arteries produced the results similar to those found in uterine arteries from animals exposed to high-altitude hypoxia.
Figure 2. Hypoxia increases CpG methylation at ERα promoter.
CpG methylation of USF-15, Sp1-520 and PRA/B-563 binding sites at ERα promoter region was determined in uterine arteries isolated from nonpregnant and pregnant sheep maintained at sea level (normoxic control, C) or exposed to high-altitude hypoxia (H) for 110 days (A), and in uterine arteries of normoxic animals treated ex vivo with 21.0% O2 (normoxic control, C) or 10.5% O2 (H) for 48 hours (B). Data are means ± SEM. * P < 0.05, versus nonpregnant; + P < 0.05, versus normoxic control. n = 5–10
CpG Methylation Inhibits Transcription Factor Binding to the Promoter
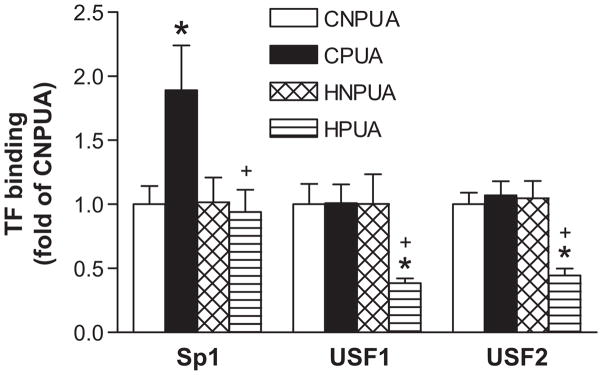
Given the finding that pregnancy and hypoxia had no significant effects on methylation status at the putative PRA/B-563 site, our further investigation was focused on USF-15, and Sp1-520 binding sites. To evaluate the nuclear protein binding to the putative USF-15, and Sp1-520 elements at the ERα promoter, electrophoretic mobility shift assays (EMSA) were performed. Incubation of nuclear extracts from uterine arteries of control nonpregnant animals with double-stranded oligonucleotide probes encompassing the putative Sp1-520 binding site resulted in the appearance of a major DNA-protein complex (Figure 3A). Super-shift analyses showed that Sp1 antibody caused super-shifting of the DNA-protein complex (Figure 3A). Unlike the Sp1 binding site, incubation of nuclear extracts with double-stranded oligonucleotide probes encompassing the putative USF-15 binding site resulted in the formation of gel retarded oligo-protein complexes that contained two close but distinct species with different electrophorectic mobilities (Figure 3B). This is in agreement with the previous report demonstrating two species of USF binding proteins that are close in their molecular weights.35 In the presence of USF1 or USF2 antibodies, the formation of both S1 and S2 gel retarded complexes were strongly inhibited (Figure 3B), indicating that the USF-15 binding site binds to both USF1 and USF2, and the binding domains of USF1 and USF2 to the USF-15 site are blocked by the antibodies. Similar findings were reported previously in several cell types.36 To determine if methylation of the Sp1-520 and USF-15 sites inhibits the transcription factor binding to ERα promoter, EMSA was performed with nuclear extracts isolated from uterine arteries with methylated and unmethylated CpG of oligonucleotide probes containing the Sp1-520 and USF-15 sites. As shown in Figure 3A and 3B, nuclear extracts from uterine arteries bound and shifted the double-stranded unmethylated Sp1 and USF oligonucleotide probes, respectively, but failed to cause a shift of methylated Sp1 and USF oligonucleotides. To further determine whether hypoxia-mediated increase in CpG methylation of the Sp1-520 and USF-15 binding sites inhibits Sp1 or USF1/USF2 binding to ERα promoter in vivo in the context of intact chromatin, ChIP assays were performed. In normoxic control animals, there were no significant changes in USF1 or USF2 binding to the USF-15 site, but a significant increase in Sp1 binding at the Sp1-520 site in uterine arteries of pregnant, as compared with nonpregnant sheep (Figure 4). In animals acclimatized to long-term high altitude hypoxia, there were significant decreases in Sp1 and USF1/USF2 binding to Sp1-520 or USF-15 binding sites, respectively, in uterine arteries of pregnant sheep (Figure 4). Chronic hypoxia had no significant effects on the transcription factor binding to ERα promoter in uterine arteries of nonpregnant animals (Figure 4).
Figure 3. SP1 and USF binding to unmethylated and methylated binding elements at ERα promoter.
Nuclear extracts (NE) from uterine arteries were incubated with double-stranded oligonucleotide probes containing unmethylated (UM) or methylated (M) Sp1-520 binding site (A) or USF-15 binding site (B), in the absence or presence of antibodies (Ab) against Sp1, USF1, or USF2. Cold competition (CC) was performed with unlabeled competitor oligonucleotide at a 200-fold molar excess. Free oligo, no nuclear extracts were added. S, shift; SS, supershift.
Figure 4. Hypoxia decreases SP1 and USF binding to ERα promoter.
Sp1, USF1, and USF2 binding to ERα promoter in vivo in the context of intact chromatin was determined with ChIP assays in uterine arteries from nonpregnant (CNPUA) or pregnant (CPUA) sheep of normoxic control, and from nonpregnant (HNPUA) and pregnant (HPUA) sheep of long-term high altitude hypoxia. Data are means ± SEM. * P < 0.05, versus nonpregnant; + P < 0.05, versus normoxic control. n = 5
Methylation of Sp1 and USF Sites Reduces ERα Promoter Activity
The functional significance of hypoxia-mediated increase in CpG methylation rendering the inhibition of Sp1 and USF binding to the promoter was further investigated by determining the effect of site-specific CpG methylation of Sp1-520 and USF-15 sites on ERα promoter activity in smooth muscle cells of uterine arteries from control nonpregnant sheep. To create a constitutive site-directed CmG at the Sp1 site, a SacII site was engineered at 3′ downstream of the Sp1-520 site in ERα promoter-luciferase reporter gene constructs. As shown in Figure 5, the insertion of SacII site had no significant effect on the promoter activity compared to wild type ERα promoter. The mutation of CmG at the Sp1-520 site significantly reduced the promoter activity (Figure 5). Similarly, to create a constitutive site-directed CmG at the USF site, a SacII and an EcoRI sites were engineered at both 3′ downstream and 5′ upstream of USF-15 site in ERα promoter-luciferase reporter gene constructs. As shown in Figure 5, the insertion of SacII and EcoRI sites had no significant effect on the promoter activity, but the mutation of CmG at USF-15 site significantly reduced the promoter activity.
Figure 5. Site-directed mutation of CmG at Sp1-520 or USF-15 sites inhibits ERα promoter activity.
Full-length wild type (WT-ERα), an insertion of SacII site (SacIIΛ), CpG methylation at Sp1-520 (Sp1-M), unmethylation at Sp1-520 (Sp1-UM), a dual insertion of SacII site and EcoR1 site (SacII-EcoR1Λ), CpG methylation at USF-15 (USF-M), unmethylation at USF-15 (USF-UM) ERα promoter-reporter gene constructs were transiently co-transfected with pRL-SV40 driven R. reniformis luciferase in uterine arterial smooth muscle cells. After 48 hours, firefly and R. reniformis luciferase activities in cell extracts were measured using a dual-luciferase reporter assay system. The promoter activities were determined by normalizing the firefly luciferase activities to R. reniformis luciferase activity, and expressed as % wild type (%WT). Data are mean ± SEM. * P < 0.05, versus wild type. n = 5–8
Deletion of the Sp1 Site Inhibits Estrogen-Mediated ERα Promoter Activity
Our previous studies in sheep demonstrated that ex vivo treatment of uterine arteries with 17β-estradiol (E2β) significantly increased ERα expression, which was abrogated by chronic hypoxia.18 These findings suggest that estrogen regulates its own receptor expression in uterine arteries and this regulation is inhibited by chronic hypoxia. Nonetheless, the analysis of ovine ERα promoter sequence revealed no complete estrogen response element (ERE). Given that estrogen is known to stimulate transcription of many genes through its interaction with Sp1 promoter elements,37,38 we reasoned that E2β-induced ERα expression in uterine arteries was mediated via the Sp1 element at the ERα promoter. As shown in Figure 6A, chromatin immunoprecipitation assay demonstrated the PCR products of the Sp1-520 binding site in DNA sequences pulled down by both ERα and ERβ antibodies in ovine uterine arteries, suggesting an interaction between estrogen receptors and the Sp1-520 binding site at the ERα promoter. We then determined the effect of E2β on ERα promoter activity. Smooth muscle cells were isolated from uterine arteries of nonpregnant sheep and were transiently transfected with the ERα promoter-luciferase reporter gene constructs. As shown in Figure 6B, the treatment of cells with 10 nmol/L E2β for 24 hours significantly increased the promoter activity, which was blocked by a site-specific deletion of the Sp1 element at the ERα promoter.
Figure 6. Deletion of Sp1-520 site inhibits estrogen-mediated ERα promoter activity.
A. PCR products of the Sp1-520 binding site after ChIP with ERα and ERβ antibodies, respectively, in uterine arteries. M indicates markers. B. A 2035 bp fragment of ovine ERα promoter region spanning −2000 to +35 bp relative to the transcription start site was amplified by PCR and inserted into pGL3 to yield the full-length promoter–reporter plasmid (WT). A site-specific deletion mutation at Sp1-520 (ΔSp1) was constructed. Constructs were transiently transfected to uterine arterial smooth muscle cells. Cells were then treated with 10 nmol/L 17β-estradiol (E2β) for 24 hours. Firefly and Renilla reniformis luciferase activities were measured in a luminometer using a dual-luciferase reporter assay system, and promoter activity was expressed as % wild type (%WT). Data are means ± SEM, * P < 0.05, versus WT; + P < 0.05, versus WT+ E2β. n = 4
Discussion
Here for the first time we report cloning of a 2035 bp ovine ERα promoter sequence, relate the promoter activity to specific transcription factors that transcribe this region, and demonstrate that the transcription in uterine arteries is affected by pregnancy and chronic hypoxia. The DNA sequence of the 471 bp ovine ERα promoter region identified previously (GenBank: AF159145.1) is in complete agreement with the proximal DNA sequence of the ovine ERα promoter that we cloned in the present study (from −471 to −1). The ovine ERα promoter has 96% homology to the bovine ERα promoter sequence obtained from GenBank. The USF, Sp1, and PRA/B response elements are present in both bovine and ovine promoters, and are located almost in the same region. Although the PRA/B element in the ovine promoter is identical in sequence to the PRA/B site in the bovine promoter, unlike the bovine USF site (CACATG) that lacks CpG dinucleotide, the ovine USF site (CACGGG) is similar to the consensus USF binding sequence (CACGTG) and contains CpG in its core binding sequence. Similarly, unlike the bovine Sp1 site, the Sp1 element in the ovine ERα promoter (AGGGCGGGCT) contains CpG in its core binding sequence.
In the 5′ region of human ERα gene, besides protein coding exons, nine additional exons have been described.39 The two proximal exons A and B are functional in the majority of tissue type studied including breast, uterus, brain, heart and vascular smooth muscle cells.26,40–42 Many of these alternative exons do not code for proteins, but produce tissue specific splice variant mRNA. The structural resemblance at the 5′-region of bovine and human ERα genes has been described.43 Like the human ERα gene, the bovine ERα promoter also contains two similar proximal exons A and B. In both human and bovine ERα genes, the alternative exons are spliced to a single acceptor site. Following the transcription initiation site (+1), translation starts at +254 nucleotides for the human, and +153 nucleotides for the bovine sequence. In the sequence of ovine ERα promoter cloned in the present study, we have indicated the putative transcription initiation site using our sequencing data, based on the bovine ERα gene. This is in agreement with the ovine ERα mRNA reported previously (GenBank:Z49257.1).44 The ovine ERα transcription initiation site has almost the same nucleotide sequence as it is in the bovine ERα mRNA (GenBank, NM001001443.1). Comparison of the ovine ERα mRNA44 with that of the bovine ERα gene sequence indicates that like bovine, the ovine gene also contains the two proximal exons. Further, the short sequence of open reading frame (ORF) that could potentially code a short peptide of 18 amino acid,44 is also present in the bovine gene. The ovine ERα protein translation for a long ORF begins at +149 nucleotide downstream the transcription initiation site.44 Taken together, thus, there is a high degree of conservation of the ERα gene between bovine and ovine species. Figure S2 (available in the online data supplement) presents the comparison of human, bovine and ovine ERα promoter, transcription and translation initiation structures.
The functional significance of USF, Sp1, and PRA/B response elements in the regulation of the ovine ERα gene activity was demonstrated by the finding that deletion of these sites significantly decreased the ERα promoter activity. The finding that pregnancy and chronic hypoxia differentially regulated CpG methylation at the Sp1 and USF sites is intriguing and suggests an important epigenetic mechanism in regulating ERα gene expression patterns in uterine arteries. The present study demonstrated clearly that an antiserum to Sp1 caused super-shifting of the DNA-protein complex resulting from binding of nuclear extracts from uterine arteries with the double-stranded oligonucleotide probes containing the putative Sp1-520 element, indicating a true Sp1 binding site in the ovine ERα promoter. The finding of more than one band in the gel retarded complex formed between the USF oligonucleotide probes and nuclear extracts is in agreement with the previous studies showing a highly heterogeneous group of USF proteins that bind to the USF site with USF1 and USF2 being the major USF species.35,45–47 In the present study, USF1 and USF2 antibodies failed to produce the super-shifting of nuclear extracts binding to the putative USF-15 site but significantly inhibited the binding. This is possibly due to that the binding domains of USF1/2 to the USF-15 site are blocked by the antibodies. Similar findings were reported previously in several cell types.36
Our previous studies in sheep demonstrated that pregnancy caused a significantly increase in ERα mRNA and protein abundance in uterine arteries, which was abrogated in animals acclimatized to long-term high altitude hypoxia.18 Consistent with this finding, the present study found that CpG methylation at the Sp1 binding site was significantly decreased in uterine arteries of pregnant sheep, which was abolished by chronic hypoxia. Unlike the Sp1 site, CpG methylation at the USF binding site was not significantly changed in uterine arteries of pregnant animals, suggesting its minimal role of the USF site in pregnancy-induced upregulation of ERα expression. However, CpG methylation at the USF site was significantly increased in uterine arteries of pregnant sheep exposed to long-term high altitude hypoxia, suggesting its role in suppressing ERα gene expression in uterine arteries caused by chronic hypoxia during gestation. These effects observed in hypoxic animals are most likely because of the direct effect of chronic hypoxia, given the finding that the ex vivo prolonged hypoxic treatment of isolated uterine arteries from the normoxic ewes produced the similar effects on CpG methylation patterns as those found in uterine arteries from high-altitude hypoxic animals. This finding is consistent with the previous studies showing that chronic hypoxia had a direct effect in suppressing ERα expression in uterine arteries.18 Taken together, these findings provide evidence of a novel epigenetic mechanism of CpG methylation at sequence-specific transcription factor binding sites in regulating ERα gene expression patterns in the uterine artery.
Although the transcriptional regulation by DNA methylation is often observed in CpG islands located around the promoter region via the sequence-nonspecific and methylation-specific binding of inhibiting methylated CpG-binding proteins,48,49 DNA methylation of sequence-specific transcription factor binding sites can alter gene expression through changes in the binding affinity of transcription factors by altering the major groove structure of DNA to which the DNA-binding proteins bind.50–52 The finding that both Sp1 and USF probes with methylated CpG dinucleotides at the core of the consensus elements at ERα promoter inhibited the binding of Sp1 and USF1/2 in the present study indicates that CpG methylation in sequence-specific binding sites may directly inhibit the transcription factor binding, resulting in down-regulation of ERα gene expression in uterine arteries. Similar findings were demonstrated in the heart showing site-specific CpG methylation at Sp1 and Egr1 binding sites suppressed protein kinase C epsilon gene expression.32–34 The results of ChIP assays in the present study demonstrate further that the hypoxia-induced increase in methylation of the Sp1 and USF binding sites inhibits the transcription factor binding to the ERα promoter in vivo in uterine arteries in the context of intact chromatin. The functional significance of CpG methylation at sequence-specific transcription factor binding sites in regulating ERα gene expression was further addressed by site-directed methylation of ERα promoter selectively at Sp1-520 or USF-15 binding sites, showing that the mutation of CmG at either Sp1-520 or USF-15 sites significantly reduced the promoter activity.
In the previous study, we demonstrated the direct ex vivo effect of exogenous E2β in up-regulating ERα expression in uterine arteries of sheep.18 This is consistent with the premise that ovarian steroid estrogen maintains and regulates the expression of their own receptors, ERα and ERβ, and is largely in agreement with the finding of exogenous E2β treatments in vivo on steroid receptor expressions in uterine arteries of sheep.53 Of interest, in sheep acclimatized to long-term high altitude hypoxia, pregnancy or exogenous E2β-mediated upregulation of ERα was abrogated, leading to no significant difference in the ERα density in uterine arteries between nonpregnant and pregnant animals.18 Nonetheless, the ovine ERα promoter sequence contains no ERE. Several studies have indicated that hormone activated ERα often uses an alternative mode of activation, known as tethering, in which ERα does not directly bind to DNA but interacts with another DNA-binding transcription factor.54 Sp1 is a transcription factor, through which activated ERα drives transcription despite lack of ERE in the genome.37,38,55–57 In the present study, we demonstrated that both ERα and ERβ interacted with the Sp1 binding site at ovine ERα promoter in intact chromatin, indicating a mechanism of the Sp1 binding site in E2β-mediated regulation of ERα transcription in uterine arteries. This was further demonstrated by the finding that E2β-induced increase in ovine ERα promoter activity in uterine arterial smooth muscle cells was abrogated by the deletion of Sp1-520 binding site.
Perspectives
Hypoxia during gestation is a common stress to maternal cardiovascular homeostasis, causing a reduction of uteroplacental perfusion and an increased risk of preeclampsia. Estrogen via the activation of ERα and ERβ plays a pivotal role in regulating uterine blood flow during pregnancy. The present investigation provides evidence of a novel mechanism of promoter methylation at sequence-specific transcription factor binding sites in epigenetic modifications of ERα gene expression patterns in uterine arteries, linking heightened promoter methylation and ERα gene repression in hypoxia-mediated maladaptation of uteroplacental circulation. These findings will not only significantly advance our knowledge of the molecular mechanisms underlying aberrant uteroplacental circulation in response to hypoxia in pregnancy and hence improve our understanding of pathophysiology of preeclampsia and intrauterine growth restriction, but they also provide important original insights into epigenetic mechanisms in regulating ERα gene expression patterns in vascular smooth muscle in general and hence a comprehensive understanding of the role of ERα in the estrogen-mediated protective effect of vascular function in physiology and pathophysiology.
Novelty and Significance.
What Is New?
Gestation hypoxia increases promoter methylation of ERα gene in uterine arteries.
Heightened methylation inhibits transcription factor binding and ERα gene promoter activity.
Estrogen plays a key role in regulating ERα gene promoter activity in uterine arteries via its interaction with Sp1.
What Is Relevant?
Gestation hypoxia and reduced uteroplacental perfusion are major risk factors of preeclampsia.
Estrogen via activation of ERα and ERβ plays a pivotal role in regulating uterine blood flow in pregnancy and suppression of ERα leads to aberrant uteroplacental circulation.
Epigenetic regulation of ERα gene expression patterns provides a molecular mechanism linking gestation hypoxia and impaired uteroplacental perfusion and increased risk of preeclampsia, as well as estrogen-mediated protective effect of vascular function in physiology and pathophysiology in general.
Summary
The present study demonstrates a novel mechanism of promoter methylation in epigenetic regulation of ERα gene expression patterns in uterine arteries linking gestation hypoxia and impaired uteroplacental perfusion and increased risk of preeclampsia.
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health Grants HD031226 (LZ), HL089012 (LZ), HL110125 (LZ).
Footnotes
Disclosures: None.
References
- 1.Alexander BT, Bennett WA, Khalil RA, Granger JP. Preeclampsia: Linking placental ischemia with cardiovascular-renal dysfunction. News Physiol Sci. 2001;16:282–286. doi: 10.1152/physiologyonline.2001.16.6.282. [DOI] [PubMed] [Google Scholar]
- 2.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol. 2007;293:H2080–H2084. doi: 10.1152/ajpheart.00667.2007. [DOI] [PubMed] [Google Scholar]
- 3.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res. 2003;54:20–25. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- 4.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol. 1999;180:1161–1168. doi: 10.1016/s0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- 5.White MM, Zhang L. Effects of chronic hypoxia on maternal vasodilation and vascular reactivity in guinea pig and ovine pregnancy. High Alt Med Biol. 2003;4:157–169. doi: 10.1089/152702903322022776. [DOI] [PubMed] [Google Scholar]
- 6.Zamudio S, Palmer SK, Dahms TE, Berman JC, McCullough RG, McCullough RE, Moore LG. Blood volume expansion, preeclampsia, and infant birth weight at high altitude. J Appl Physiol. 1993;75:1566–1573. doi: 10.1152/jappl.1993.75.4.1566. [DOI] [PubMed] [Google Scholar]
- 7.Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA, Moore LG. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol. 1995;79:15–22. doi: 10.1152/jappl.1995.79.1.15. [DOI] [PubMed] [Google Scholar]
- 8.Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol. 1995;79:7–14. doi: 10.1152/jappl.1995.79.1.7. [DOI] [PubMed] [Google Scholar]
- 9.Killam AP, Rosenfeld CR, Battaglia FC, Makowski EL, Meschia G. Effect of estrogens on the uterine blood flow of oophorectomized ewes. Am J Obstet Gynecol. 1973;115:1045–1052. doi: 10.1016/0002-9378(73)90552-8. [DOI] [PubMed] [Google Scholar]
- 10.Magness RR, Parker CR, Jr, Rosenfeld CR. Systemic and uterine responses to chronic infusion of estradiol-17 beta. Am J Physiol. 1993;265:E690–698. doi: 10.1152/ajpendo.1993.265.5.E690. [DOI] [PubMed] [Google Scholar]
- 11.Magness RR, Phernetton TM, Gibson TC, Chen DB. Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J Physiol. 2005;565:71–83. doi: 10.1113/jphysiol.2005.086439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magness RR, Phernetton TM, Zheng J. Systemic and uterine blood flow distribution during prolonged infusion of 17beta-estradiol. Am J Physiol. 1998;275:H731–H743. doi: 10.1152/ajpheart.1998.275.3.H731. [DOI] [PubMed] [Google Scholar]
- 13.Magness RR, Rosenfeld CR. Local and systemic estradiol-17 beta: Effects on uterine and systemic vasodilation. Am J Physiol. 1989;256:E536–542. doi: 10.1152/ajpendo.1989.256.4.E536. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld CR, Cox BE, Roy T, Magness RR. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest. 1996;98:2158–2166. doi: 10.1172/JCI119022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson S, Zhang L. Pregnancy upregulates large-conductance ca(2+)-activated k(+) channel activity and attenuates myogenic tone in uterine arteries. Hypertension. 2011;58:1132–1139. doi: 10.1161/HYPERTENSIONAHA.111.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao D, Huang X, Yang S, Zhang L. Direct chronic effect of steroid hormones in attenuating uterine arterial myogenic tone: Role of protein kinase c/extracellular signal-regulated kinase 1/2. Hypertension. 2009;54:352–358. doi: 10.1161/HYPERTENSIONAHA.109.130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X, Xiao D, Zhu R, Huang X, Yang S, Wilson S, Zhang L. Chronic hypoxia suppresses pregnancy-induced upregulation of large conductance Ca+2 -activated K+ channel activity in uterine arteries. Hypertension. 2012;60:214–222. doi: 10.1161/HYPERTENSIONAHA.112.196097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang K, Xiao D, Huang X, Xue Z, Yang S, Longo LD, Zhang L. Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension. 2010;56:750–757. doi: 10.1161/HYPERTENSIONAHA.110.155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res. 2007;100:520–526. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5:401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- 21.Jaenisch R, Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33 (Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 22.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 23.Reik W, Dean W. DNA methylation and mammalian epigenetics. Electrophoresis. 2001;22:2838–2843. doi: 10.1002/1522-2683(200108)22:14<2838::AID-ELPS2838>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 25.Issa JP, Zehnbauer BA, Civin CI, Collector MI, Sharkis SJ, Davidson NE, Kaufmann SH, Baylin SB. The estrogen receptor cpg island is methylated in most hematopoietic neoplasms. Cancer Res. 1996;56:973–977. [PubMed] [Google Scholar]
- 26.Pinzone JJ, Stevenson H, Strobl JS, Berg PE. Molecular and cellular determinants of estrogen receptor alpha expression. Mol Cell Biol. 2004;24:4605–4612. doi: 10.1128/MCB.24.11.4605-4612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 151:4871–4881. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sogon T, Masamura S, Hayashi S, Santen RJ, Nakachi K, Eguchi H. Demethylation of promoter c region of estrogen receptor alpha gene is correlated with its enhanced expression in estrogen-ablation resistant MCF-7 cells. J Steroid Biochem Mol Biol. 2007;105:106–114. doi: 10.1016/j.jsbmb.2006.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010;151:731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson ME, Westberry JM, Prewitt AK. Dynamic regulation of estrogen receptor-alpha gene expression in the brain: A role for promoter methylation? Front Neuroendocrinol. 2008;29:375–385. doi: 10.1016/j.yfrne.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida T, Eguchi H, Nakachi K, Tanimoto K, Higashi Y, Suemasu K, Iino Y, Morishita Y, Hayashi S. Distinct mechanisms of loss of estrogen receptor alpha gene expression in human breast cancer: Methylation of the gene and alteration of trans-acting factors. Carcinogenesis. 2000;21:2193–2201. doi: 10.1093/carcin/21.12.2193. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence J, Chen M, Xiong F, Xiao D, Zhang H, Buchholz JN, Zhang L. Foetal nicotine exposure causes pkcepsilon gene repression by promoter methylation in rat hearts. Cardiovasc Res. 2011;89:89–97. doi: 10.1093/cvr/cvq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKCepsilon gene repression in the fetal rat heart. J Mol Cell Cardiol. 2009;47:504–511. doi: 10.1016/j.yjmcc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKC{epsilon} gene repression in rat hearts. Circ Res. 2010;107:365–373. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawadogo M, Van Dyke MW, Gregor PD, Roeder RG. Multiple forms of the human gene-specific transcription factor USF. I. Complete purification and identification of USF from hela cell nuclei. J Biol Chem. 1988;263:11985–11993. [PubMed] [Google Scholar]
- 36.Harris AN, Mellon PL. The basic helix-loop-helix, leucine zipper transcription factor, USF (upstream stimulatory factor), is a key regulator of SF-1 (steroidogenic factor-1) gene expression in pituitary gonadotrope and steroidogenic cells. Mol Endocrinol. 1998;12:714–726. doi: 10.1210/mend.12.5.0100. [DOI] [PubMed] [Google Scholar]
- 37.Fleming JG, Spencer TE, Safe SH, Bazer FW. Estrogen regulates transcription of the ovine oxytocin receptor gene through gc-rich sp1 promoter elements. Endocrinology. 2006;147:899–911. doi: 10.1210/en.2005-1120. [DOI] [PubMed] [Google Scholar]
- 38.Porter W, Saville B, Hoivik D, Safe S. Functional synergy between the transcription factor sp1 and the estrogen receptor. Mol Endocrinol. 1997;11:1569–1580. doi: 10.1210/mend.11.11.9916. [DOI] [PubMed] [Google Scholar]
- 39.Kos M, Reid G, Denger S, Gannon F. Minireview: Genomic organization of the human eralpha gene promoter region. Mol Endocrinol. 2001;15:2057–2063. doi: 10.1210/mend.15.12.0731. [DOI] [PubMed] [Google Scholar]
- 40.Grandien K. Determination of transcription start sites in the human estrogen receptor gene and identification of a novel, tissue-specific, estrogen receptor-mrna isoform. Mol Cell Endocrinol. 1996;116:207–212. doi: 10.1016/0303-7207(95)03716-0. [DOI] [PubMed] [Google Scholar]
- 41.Kos M, Denger S, Reid G, Gannon F. Upstream open reading frames regulate the translation of the multiple mrna variants of the estrogen receptor alpha. J Biol Chem. 2002;277:37131–37138. doi: 10.1074/jbc.M206325200. [DOI] [PubMed] [Google Scholar]
- 42.Tanimoto K, Eguchi H, Yoshida T, Hajiro-Nakanishi K, Hayashi S. Regulation of estrogen receptor alpha gene mediated by promoter B responsible for its enhanced expressionin human breast cancer. Nucleic Acids Res. 1999;27:903–909. doi: 10.1093/nar/27.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szreder T, Zwierzchowski L. Estrogen receptors and their genes--potential markers of functional and production traits of farm animals. Mol Biol Rep. 2007;34:207–211. doi: 10.1007/s11033-006-9030-x. [DOI] [PubMed] [Google Scholar]
- 44.Madigou T, Tiffoche C, Lazennec G, Pelletier J, Thieulant ML. The sheep estrogen receptor: Cloning and regulation of expression in the hypothalamo-pituitary axis. Mol Cell Endocrinol. 1996;121:153–163. doi: 10.1016/0303-7207(96)03860-9. [DOI] [PubMed] [Google Scholar]
- 45.Sirito M, Lin Q, Deng JM, Behringer RR, Sawadogo M. Overlapping roles and asymmetrical cross-regulation of the usf proteins in mice. Proc Natl Acad Sci U S A. 1998;95:3758–3763. doi: 10.1073/pnas.95.7.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi K, Nishiyama C, Okumura K, Ra C, Ohtake Y, Yokota T. Molecular cloning of rat usf2 cdna and characterization of splicing variants. Biosci Biotechnol Biochem. 2001;65:56–62. doi: 10.1271/bbb.65.56. [DOI] [PubMed] [Google Scholar]
- 47.Viollet B, Lefrancois-Martinez AM, Henrion A, Kahn A, Raymondjean M, Martinez A. Immunochemical characterization and transacting properties of upstream stimulatory factor isoforms. J Biol Chem. 1996;271:1405–1415. doi: 10.1074/jbc.271.3.1405. [DOI] [PubMed] [Google Scholar]
- 48.Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 49.Wade PA. Methyl cpg-binding proteins and transcriptional repression. Bioessays. 2001;23:1131–1137. doi: 10.1002/bies.10008. [DOI] [PubMed] [Google Scholar]
- 50.Campanero MR, Armstrong MI, Flemington EK. CpG methylation as a mechanism for the regulation of e2f activity. Proc Natl Acad Sci U S A. 2000;97:6481–6486. doi: 10.1073/pnas.100340697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujimoto M, Kitazawa R, Maeda S, Kitazawa S. Methylation adjacent to negatively regulating ap-1 site reactivates TrkA gene expression during cancer progression. Oncogene. 2005;24:5108–5118. doi: 10.1038/sj.onc.1208697. [DOI] [PubMed] [Google Scholar]
- 52.Zhu WG, Srinivasan K, Dai Z, Duan W, Druhan LJ, Ding H, Yee L, Villalona-Calero MA, Plass C, Otterson GA. Methylation of adjacent cpg sites affects sp1/sp3 binding and activity in the p21(cip1) promoter. Mol Cell Biol. 2003;23:4056–4065. doi: 10.1128/MCB.23.12.4056-4065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byers MJ, Zangl A, Phernetton TM, Lopez G, Chen DB, Magness RR. Endothelial vasodilator production by ovine uterine and systemic arteries: Ovarian steroid and pregnancy control of ERalpha and ERbeta levels. J Physiol. 2005;565:85–99. doi: 10.1113/jphysiol.2005.085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gruber CJ, Gruber DM, Gruber IM, Wieser F, Huber JC. Anatomy of the estrogen response element. Trends Endocrinol Metab. 2004;15:73–78. doi: 10.1016/j.tem.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Porter W, Wang F, Wang W, Duan R, Safe S. Role of estrogen receptor/sp1 complexes in estrogen-induced heat shock protein 27 gene expression. Mol Endocrinol. 1996;10:1371–1378. doi: 10.1210/mend.10.11.8923463. [DOI] [PubMed] [Google Scholar]
- 56.Safe S. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm. 2001;62:231–252. doi: 10.1016/s0083-6729(01)62006-5. [DOI] [PubMed] [Google Scholar]
- 57.Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson JA, Safe S. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC- rich (Sp1) promoter elements. J Biol Chem. 2000;275:5379–5387. doi: 10.1074/jbc.275.8.5379. [DOI] [PubMed] [Google Scholar]