Abstract
Gram-negative bacteria express a wide variety of organelles on their cell surface. These surface structures may be the end products of secretion systems, such as the hair-like fibers assembled by the chaperone/usher and type IV pilus pathways, which generally function in adhesion to surfaces and bacterial-bacterial and bacterial-host interactions. Alternatively, the surface organelles may be integral components of the secretion machinery itself, such as the needle complex and pilus extensions formed by the type III and type IV secretion systems, which function in the delivery of bacterial effectors inside host cells. Bacterial surface structures perform functions critical for pathogenesis and have evolved to withstand forces exerted by the external environment and cope with defenses mounted by the host immune system. Given their essential roles in pathogenesis and exposed nature, bacterial surface structures also make attractive targets for therapeutic intervention. This review will describe the structure and function of surface organelles assembled by four different Gram-negative bacterial secretion systems: the chaperone/usher pathway, the type IV pilus pathway, and the type III and type IV secretion systems.
Keywords: Pili, Fimbriae, chaperone/usher pathway, type IV pili, type III secretion, type IV secretion
INTRODUCTION
Bacteria assemble a range of different proteinaceous appendages on their cell surface. These surface organelles are traditionally grouped into two categories: flagella, which are long, whip-like structures required for motility; or pili or fimbriae, which are thinner, hair-like structures required for adhesion. Bacterial surface structures distinct from flagella were first described in the late 1940’s and early 1950’s, made possible by the introduction of the electron microscope (Ottow, 1975). Duguid and co-workers first used the term fimbriae, Latin for thread or fiber, in 1955 to describe surface appendages that allowed Escherichia coli to bind and agglutinate erythrocytes (Duguid et al., 1955). Brinton later used the term pili, Latin for hair, to describe the non-flagellar surface structures expressed by E. coli (Brinton, 1959). In an important 1975 review, Ottow suggested that the term pili be reserved for the F or conjugative pili involved in bacterial mating, and that the term fimbriae be used to describe surface fibers involved in adhesion (Ottow, 1975). However, this terminology scheme did not stick and today the terms pili and fimbriae are generally used interchangeably. We will use the term pili in this review.
Various schemes have been proposed over the years to classify the different types of pili (Duguid et al., 1966; Ottow, 1975; Orskov & Orskov, 1990; Low et al., 1996; Thanassi et al., 2007). Although most of these classification schemes are no longer commonly used, parts have entered the standard nomenclature, most prominently the term type 1 pili to refer to surface fibers that mediate adhesion in a mannose-sensitive manner (Duguid et al., 1966), and the term type IV pili to refer to polarly-localized appendages that mediate twitching motility (originally referred to by Ottow as Group 4) (Ottow, 1975). Unfortunately, this terminology causes confusion in that it has now become standard practice to refer to bacterial secretion systems by “type” names. Thus, type I secretion is not related to type 1 pili, which are assembled by the chaperone/usher secretion system, and type IV pili are not related to type IV secretion, but instead are closely related to the type II secretion pathway (readers are referred to the companion review in this issue by Dalbey and Kuhn for an overview of bacterial secretion pathways).
Organelles such as flagella and pili can be considered the functional end products of bacterial secretion systems; i.e., they are assembled by dedicated secretion systems to function in motility or adherence. However, bacterial secretion systems also assemble surface structures that function as integral components of the secretion machinery itself. For example, some secretion systems assemble pilus-like structures to serve as extensions of the secretion machinery outside the bacterial envelope, providing conduits for the delivery of substrate proteins and in some cases enabling the transfer of effector proteins into the cytoplasm of target host cells. Such surface structures may also provide sensory feedback to the secretion systems to detect target cell contact or function as adhesins to allow docking to host cell receptors.
This review will focus on surface organelles assembled by secretion systems found in Gram-negative bacteria. We will provide overviews of the structure and function of two types of canonical adhesive pili: pili assembled by the chaperone/usher pathway and type IV pili. We will also describe the type III and type IV secretion pathways and the surface structures assembled by these pathways for the delivery of bacterial molecules inside target cells. For the chaperone/usher and type IV pilus systems, we will focus on the structures of the surface fibers and their roles during pathogenesis. For the type III secretion system, we will focus on the structure and function of the tip complex that caps the surface-exposed needle structure of the type III secretion machinery. Finally, for the type IV system, we will describe recent developments in understanding the structural organization of the secretion machinery and the structure and function of surface-exposed elements, including the conjugative pilus.
PILI ASSEMLBED BY THE CHAPERONE/USHER PATHWAY
Introduction
The chaperone/usher (CU) pathway is used by Gram-negative bacteria to assemble a large family of virulence-associated surface fibers (for recent reviews, see (Nuccio & Bäumler, 2007; Waksman & Hultgren, 2009; Zav'yalov et al., 2010)). The CU pathway takes its name from the components of its secretion machinery, which consist of a dedicated periplasmic chaperone and an integral outer membrane assembly platform termed the usher. The CU pathway assembles peritrichous surface fibers that range from thin, flexible filaments to rigid, rod-like pili or fimbriae. These structures generally function as adhesins, mediating bacterial attachment to and interactions with host tissues. As such, organelles assembled by the CU pathway often function as critical virulence factors. The structures of CU pili are optimized to withstand the various forces encountered during colonization of surfaces, and each pilus is adapted to its particular function in its particular niche. In addition to colonization of host surfaces, CU pili are important for bacterial-bacterial interactions, biofilm formation, and adhesion to abiotic surfaces. The mechanism of fiber assembly and secretion by the CU pathway has been subject to extensive study and the CU pathway is one of the best understood bacterial secretion systems.
CU pathways are prevalent among enteric members of the γ-proteobacteria, including E. coli, Salmonella and Yersinia, and are also found among members of the β-proteobacteria as well as in Cyanobacteria and Deinococcus (Yen et al., 2002; Nuccio & Bäumler, 2007). A group of pili expressed primarily by enterotoxigenic E. coli (ETEC), exemplified by the CS1 and CFA/I pili, were thought to be evolutionarily distinct from the CU pathway and were termed the alternate CU pathway (also known as class 5 fimbriae) (Sakellaris & Scott, 1998; Anantha et al., 2004). However, recent genetic and structural studies have shown that these pili are in fact part of the canonical CU pathway family (Li et al., 2007; Nuccio & Bäumler, 2007; Poole et al., 2007). Genes for CU pathways are encoded on both chromosomal and plasmid locations, and are clustered together with similar organization among different bacteria: a 5’ region containing regulatory genes and promoters, and a single downstream operon containing the required structural and assembly components. In addition, multiple CU pathways may be present in a single bacterial genome, which presumably provides the ability to adhere to a variety of different receptors and surfaces (van der Velden et al., 1998; Felek et al., 2010; Korea et al., 2010). Expression of CU gene clusters is typically highly regulated, subject to phase variation, and responsive to environmental cues (Chen & Elberg, 1977; van der Woude et al., 1996; Blomfield, 2001). Regulatory cross-talk may occur among the multiple CU gene clusters present within a bacterium (Xia et al., 2000). This cross-talk likely ensures that each bacterium only expresses a single pilus at a given time, thus controlling adhesive specificity.
Pili assembled by the CU pathway range from 2–10 nm in diameter and generally 1–3 µm in length. These pili can be divided into two general architectural classes. One class comprises rigid, rod-like fibers of larger diameter and the second class comprises thin, flexible fibers or amorphous structures. CU pathways can also be divided into two subfamilies based on conserved sequence differences present in the chaperones. Chaperones with a short loop connecting their F1 and G1 β-strands belong to the FGS (F1-G1 short) subfamily and chaperones with a long F1-G1 loop belong to the FGL subfamily (Hung et al., 1996; Zavialov et al., 2007). Interestingly, FGL chaperones assemble only thin or amorphous pilus fibers composed of only one or two types of subunits. In contrast, FGS chaperones assemble both rod-like and thin pilus fibers, and the fibers are generally composed of multiple different types of subunits and may have composite architectures. The P and type 1 pili expressed by uropathogenic E. coli (UPEC) are prototypical rigid, hairlike pili belonging to the FGS subfamily and the F1 capsule of Yersinia pestis is a prototypical thin, “afimbrial” structure assembled by the FGL subfamily (Fig. 1). P pili bind to Galα(1–4)Gal moieties present in the globoseries of glycolipids on uroepithelial cells and are associated with the ability of UPEC to colonize the kidney and cause pyelonephritis (Bock et al., 1985; Roberts et al., 1994). Type 1 pili mediate binding to a number of host tissues in a mannose-sensitive manner. UPEC use type 1 pili to bind mannosylated glycoproteins present in the bladder, leading to bacterial invasion inside bladder epithelial cells and the development of cystitis (Abraham et al., 1988; Mulvey et al., 1998). The F1 capsule of Y. pestis forms a dense coating around the bacteria that, in contrast to the adhesive functions typically attributed to pili, acts as an “anti-adhesive” structure, preventing phagocytosis by macrophages and inhibiting internalization by respiratory tract epithelial cells (Du et al., 2002; Liu et al., 2006). The F1 capsule is also an important protective antigen of Y. pestis and has roles in transmission from the flea vector and in virulence (Titball & Williamson, 2001; Sebbane et al., 2009; Weening et al., 2011). The P and type 1 pili of UPEC and the Y. pestis F1 capsule will be used as examples in this section to highlight the structure and function of pili assembled by the CU pathway.
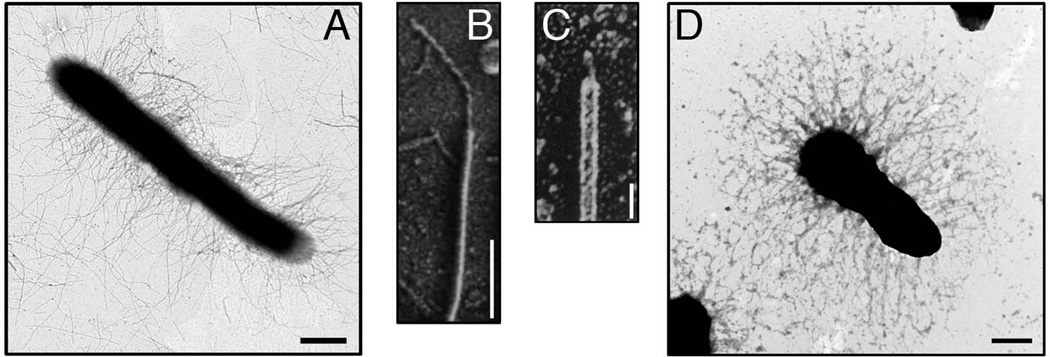
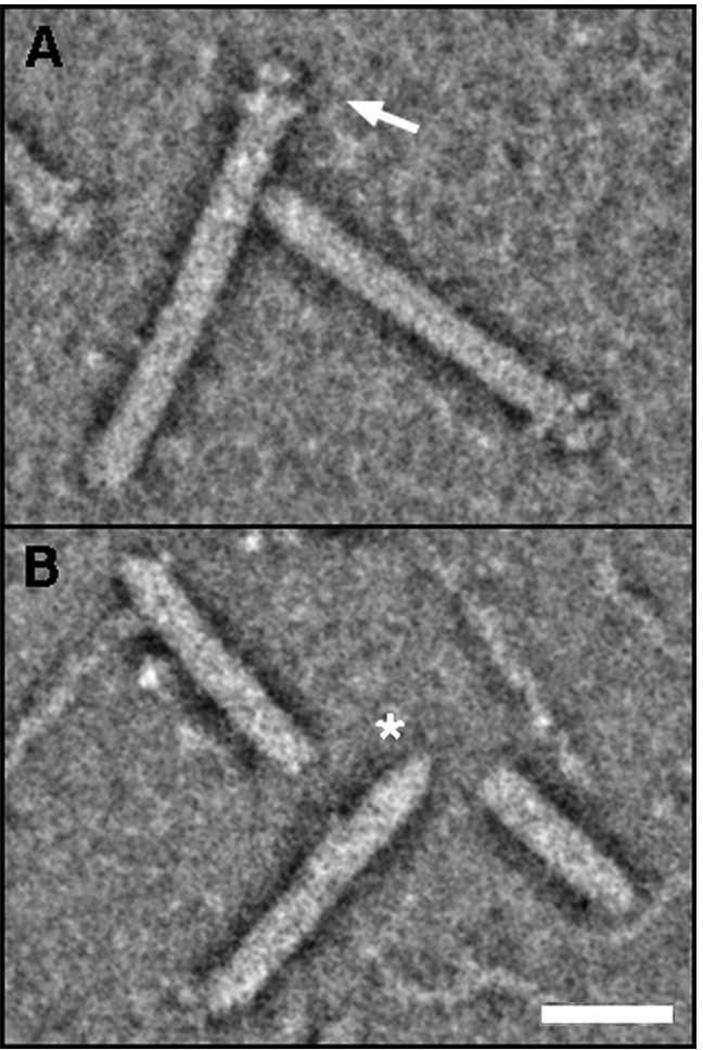
Fig. 1.
Electron micrographs of pili assembled by the CU pathway. (A) An E. coli bacterium expressing P pili. (B and C) High-resolution, freeze-etch images of individual E. coli P and type 1 pili, respectively, showing distal linear tip fibers and helical pilus rods. (D) A Y. pestis bacterium expressing F1 capsule. Scale bars = 500 nm (A), 100 nm (B), 20 nm (C), and 500 nm (D). Images in (A–D) reproduced with permission from (Kuehn et al., 1992; Jones et al., 1995; Runco et al., 2008; Li et al., 2010), respectively.
Structural details of CU pilus fibers
Many pili assembled by the CU pathway are composed of two distinct subassemblies: a rigid, helical rod that extends out from the cell surface, and a distal tip structure that contains the adhesive activity. Type 1 and P pili exemplify this structural arrangement (Fig. 1B and C). Type 1 and P pilus rods measure 7–8 nm in diameter and are built from a linear homopolymer of over 1000 copies of the major pilin subunit (PapA for P pili, FimA for type 1 pili), wound into a 1-start, right-handed helix (Fig. 2) (Hahn et al., 2002; Mu & Bullitt, 2006). The P pilus rod is terminated by the PapH minor pilin, which also plays a role in anchoring the pilus fiber in the OM (Baga et al., 1987; Verger et al., 2006). In contrast to the rigid helical rod, the pilus tip is a flexible, open helical fiber measuring 2 nm in diameter (Kuehn et al., 1992; Hahn et al., 2002). The P pilus tip fiber is approximately 40 nm long and composed mainly of PapE, which is present at approximately 5–10 copies per pilus. The P pilus adhesin PapG is present in single copy at the distal end of the tip fiber and is joined to PapE via the PapF adaptor subunit (Kuehn et al., 1992; Jacob-Dubuisson et al., 1993) (Fig. 2). Another adaptor subunit, PapK, links the tip fiber to the pilus rod (Jacob-Dubuisson et al., 1993). Type 1 pili have shorter tip fibers compared to P pili (Fig. 1B and C)(Jones et al., 1995; Hahn et al., 2002). Type 1 tips measure 10–19 nm in length and contain a single copy of the FimH adhesin at the distal end, followed by the FimG and FimF adaptor subunits, which are generally present in single copy (Fig. 2) (Jones et al., 1995; Hahn et al., 2002; Le Trong et al., 2010).
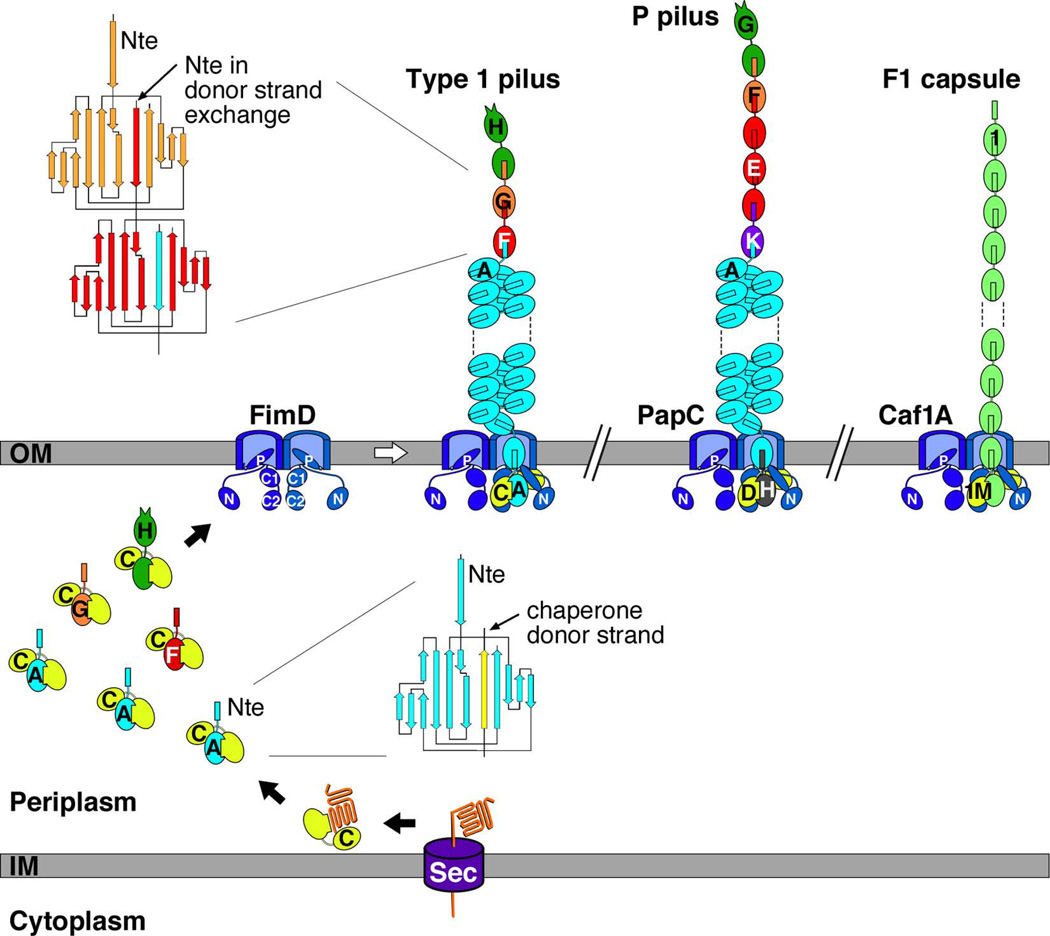
Fig. 2.
Model for pilus biogenesis by the CU pathway. The assembly steps for E. coli type 1 pili are shown, together with models for fully assembled E. coli type 1 and P pili, and the Y. pestis F1 capsule. The Fim, Pap, and Caf proteins are indicated by single letters (H, FimH; C, FimC; etc.). Pilus subunits enter the periplasm as unfolded polypeptides via the Sec pathway. The subunits fold upon interaction with the periplasmic chaperone, forming stable complexes via donor strand complementation. Assembly and secretion of the pilus fiber occurs at the OM usher, where chaperone-subunit interactions are replaced with subunit-subunit interactions via the donor strand exchange reaction. Topology diagrams are shown depicting the donor strand complementation and exchange reactions occurring in the periplasmic and pilus fiber, respectively. The dimeric ushers are depicted with the plug domain that gates the channel shut (P) and the periplasmic N and C domains indicated. The N domain forms the initial binding site for chaperone-subunit complexes and the C domains provide a second binding site for the assembling pilus fiber. Chaperone-adhesin complexes have highest affinity for the usher and initiate pilus assembly by binding to the usher N domain.
In contrast to type 1 and P pili, the F1 capsule is built from a single subunit protein, Caf1, which polymerizes into an extended linear fiber comprising over a thousand subunits (Fig. 2) (Galyov et al., 1990; Miller et al., 1998; Zavialov et al., 2003). Each F1 fiber is approximately 2 nm in diameter, with a flexible, open helical structure similar to the P and type 1 pilus tip fibers. Thus, although the F1 capsule and other so-called afimbrial structures may appear to lack distinct pilus-like architectures when viewed by electron microscopy (EM), these are in fact polymeric fibers similar to all CU pili. The individual F1 fibers likely coil randomly and aggregate together to form the dense, poorly defined coating typically attributed to the Y. pestis capsule (Chen & Elberg, 1977; Liu et al., 2006; Runco et al., 2008).
Assembly of pili by the CU pathway: structure dictates assembly and function
The physical and functional attributes of pili assembled by the CU pathway can be understood through the structures of the subunit proteins and how they are assembled into the pilus fiber. Fig. 2 provides an overview of pilus biogenesis by the CU pathway, as depicted for type 1 pili. See companion review by Dalbey and Kuhn in this issue for additional information on protein secretion mechanisms and recent reviews for detailed discussions of the CU assembly pathway (Waksman & Hultgren, 2009; Zav'yalov et al., 2010). Newly synthesized pilus subunit proteins enter the periplasm via the Sec general secretory pathway (Driessen & Nouwen, 2008). Once in the periplasm, the subunits form stable complexes with the periplasmic chaperone. The chaperone enables proper folding of the pilus subunits, prevents premature subunit-subunit interactions, and maintains the subunits in an assembly-competent state (Choudhury et al., 1999; Sauer et al., 1999; Sauer et al., 2002; Zavialov et al., 2003). Periplasmic chaperone-subunit complexes then must interact with the outer membrane usher for release of the chaperone, assembly of subunits into the pilus fiber, and secretion of the fiber to the cell surface through the usher channel (Remaut et al., 2008; Phan et al., 2011). The usher acts as a pilus assembly catalyst, accelerating the rate of subunit incorporation into the pilus fiber (Nishiyama et al., 2008). The usher forms a dimeric, gated secretion complex in the outer membrane, although only one usher channel is used for secretion of the pilus fiber and the function of the usher dimer remains to be determined (Remaut et al., 2008). Recent structural information shows that the usher monomer contains two binding sites for chaperone-subunit complexes, provided by the usher’s periplasmic N- and C-terminal domains (Fig. 2) (Phan et al., 2011). These binding sites likely underlie the catalytic activity of the usher, by enabling optimal positioning of chaperone-subunit complexes to promote chaperone release and pilus assembly. Pili are assembled in a top-down order, with the adhesin incorporated first, followed by the rest of the pilus tip and finally the rod. The usher channel is only wide enough to allow secretion of a linear fiber of folded pilus subunits (Remaut et al., 2008; Phan et al., 2011). Therefore, the pilus rod is constrained to a linear fiber as it passes through the usher and only converts to its final helical form upon reaching the bacterial surface (Fig. 2).
All pilus subunits contain a single pilin domain, which is formed by an incomplete immunoglobulin (Ig)-like fold. Adhesin subunits contain an additional, N-terminal, receptor-binding or adhesin domain in addition to the pilin domain (Fig. 3). The pilin domain lacks the seventh, C-terminal β-strand present in canonical Ig folds, and thus pilus subunits are unable to complete their own structure (Choudhury et al., 1999; Sauer et al., 1999; Sauer et al., 2002; Zavialov et al., 2003). This missing strand results in a deep groove on the surface of the subunit, exposing its hydrophobic core. To complete their folds, pilins must rely on structural information provided by interaction with the chaperone in the periplasm or with neighboring subunits in the pilus fiber. In the periplasm, the chaperone donates a β-strand to complete the pilin domain Ig fold in a mechanism termed donor strand complementation (Figs. 2 and 3) (Choudhury et al., 1999; Sauer et al., 1999). In addition to the pilin domain, all pilus subunits (except the adhesin) contain a conserved N-terminal extension (Nte) (Fig. 2). During pilus assembly at the usher, the Nte from an incoming chaperone-subunit complex displaces the donated chaperone β-strand from the preceding subunit, completing the Ig fold of the preceding subunit by a mechanism termed donor strand exchange (Sauer et al., 2002; Zavialov et al., 2003). Thus, the pilus fiber is built from an array of Ig folds, with each subunit noncovalently bound to its neighboring subunit by the donor strand exchange reaction (Fig. 2). This arrangement provides great mechanical strength to the pilus fiber, which is reflected by the property that subunit-subunit interactions in the pilus are resistant to dissociation by SDS unless heated (Henderson et al., 2011). This mechanical strength is critical for the ability of the pili to maintain adhesion in the face of external forces such as the shear force encountered in the bladder from the flow of urine. The helical pilus rod also makes important contributions to the ability of pili to withstand shear forces and maintain adhesion. The helical pilus rod can convert or uncoil under stress to an extended linear fiber, in essence acting as a spring or shock absorber to extend the lifetime of pilus-receptor interactions (Bullitt & Makowski, 1995; Fallman et al., 2005; Miller et al., 2006).
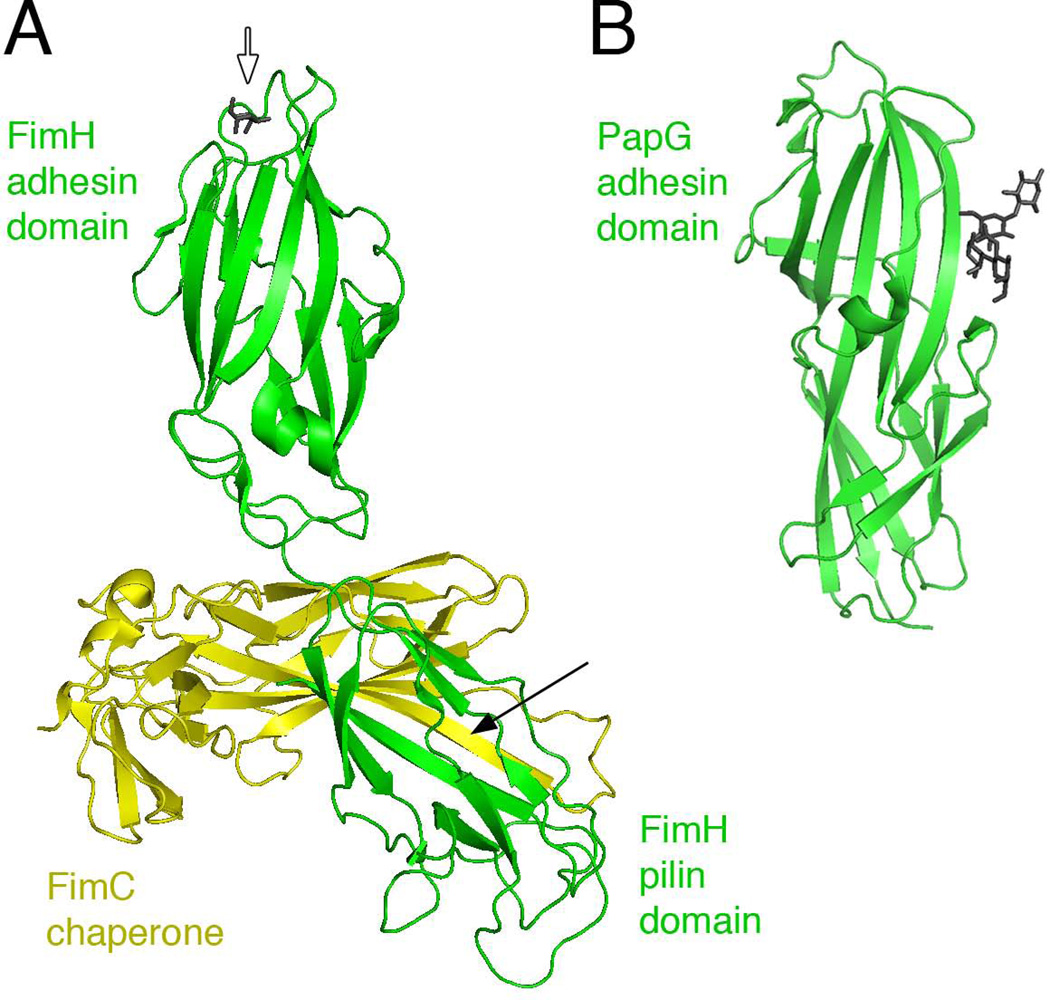
Fig. 3.
Structures of the FimH and PapG adhesins. (A) Crystal structure of the FimCH complex with bound mannose (PDB ID: 1KLF; (Hung et al., 2002)). The FimH adhesin and pilin domains are in green, with the bound mannose at the tip of the adhesin domain shown in dark gray stick representation (white arrow). The FimC chaperone is in yellow; the black arrow indicates the FimC β-strand engaged in donor strand complementation with the FimH pilin domain. (B) Crystal structure of the PapG adhesin domain with bound globoside (PDB ID: 1J8R; (Dodson et al., 2001)). The adhesin domain is in green and the tetrasaccharide bound at the side of the adhesin is in dark gray. Images were generated using PyMOL.
Adhesion mediated by CU pili
Many pili assembled by the CU pathway have a single, tip-located subunit (the adhesin) that contains the receptor-binding activity. Such pili have been termed monoadhesive pili and include the type 1 and P pili (Fig. 2) (Zav'yalov et al., 2010). However, not all pili assembled by the CU pathway contain a distinct tip structure or tip-located adhesin. For these pili, the structural subunit that builds the pilus fiber may also contain receptor-binding sites and thus the entire pilus fiber may function in adhesion. These polyadhesive pili are generally built from only 1–2 subunits and are exemplified by FGL pili such as the F1 capsule (Fig. 2) and the Afa/Dr family of adhesins (Zav'yalov et al., 2010). Structural and binding studies of the polyadhesive pili have revealed binding sites along the exposed surfaces of each pilus subunit (Anderson et al., 2004; Pettigrew et al., 2004; Korotkova et al., 2006).
Structures have been solved for a number of adhesins from monoadhesive pili (Choudhury et al., 1999; Dodson et al., 2001; Sung et al., 2001; Hung et al., 2002; Buts et al., 2003; Merckel et al., 2003; Li et al., 2007). The adhesins are two domain proteins, with a C-terminal pilin domain and an N-terminal adhesin domain (Fig. 3). The pilin domain comprises an incomplete Ig-like fold, as found for all CU pilins, and mediates incorporation of the adhesin into the pilus fiber. Adhesin domains have Ig-like folds similar to the pilin domains, but the adhesin domains are complete (not lacking the terminal β-strand) and structurally distinct (Fig. 3). Duplication and fusion events of a pilin domain likely led to specialization of the adhesin domains for adherence. Despite their common architecture, the adhesins vary greatly in sequence and employ distinct receptor binding mechanisms, reflecting their diverse functions within the host (Westerlund-Wikstrom & Korhonen, 2005). A comparison between the PapG and FimH adhesins is illustrative of these differences. The residues of PapG that interact with the glycolipid receptor lie in a shallow pocket on one side of the adhesin domain (Fig. 3B) (Dodson et al., 2001; Sung et al., 2001). P pili have a long, flexible tip fiber (Fig. 1B). The flexibility of the P pilus tip and side-on orientation of the PapG binding site likely function to facilitate docking of the adhesin onto the globoside moiety of the glycolipid receptor, which is oriented parallel to the membrane surface (Dodson et al., 2001). In contrast, the mannose-binding site of FimH is located in a deep pocket at the tip of the adhesin domain (Fig. 3A) (Choudhury et al., 1999; Hung et al., 2002). Type 1 pili have a shorter and less flexible tip fiber (Fig. 1C), placing the receptor binding site directly at the distal-most tip of the pilus.
Work on the FimH adhesin has revealed additional mechanisms by which pili have adapted to promote adhesion under specific environmental conditions. FimH binding to mannose is enhanced by shear stress, such as encountered during bacterial colonization in the urinary tract under conditions of urine flow (Thomas et al., 2002). The application of shear stress causes FimH to switch from a low-affinity to a high-affinity binding state. This greater affinity presumably allows UPEC to avoid being washed away by the flow of urine, and may also provide a mechanism for the bacteria to discriminate between surface-located and soluble receptors, as binding to the latter will not result in force generation on the pilus and FimH will stay in the low-affinity state. The shear-enhanced binding of FimH is mediated by a catch-bond mechanism that involves allosteric activation of the FimH adhesin domain (Yakovenko et al., 2008). Recent work has revealed the structural basis for the catch-bond behavior of FimH (Le Trong et al., 2010). When incorporated into the type 1 pilus tip fiber, the pilin domain of FimH interacts with the adhesin domain to cause structural alterations of the adhesin that weaken the mannose binding pocket. However, the application of force to the pilus fiber causes the FimH pilin and adhesin domains to separate, allowing the mannose binding pocket to clamp tightly around the mannose ligand. Moreover, the structures of both the type 1 pilus tip fiber and the helical pilus rod appear to be designed to optimize the shear-enhanced behavior of FimH, and the flexibility of the pilus tip likely provides maximum opportunity for FimH to find its target receptors (Forero et al., 2006; Aprikian et al., 2011).
Pilus function during pathogenesis: more than just adhesion to host cells
Pili assembled by the CU pathway are critical virulence factors of pathogenic bacteria, as they initiate contact with host cells and mediate colonization of host cell surfaces. However, CU pili may play more complex roles than acting simply as adhesins, as they may modulate host cell signaling pathways, promote or inhibit bacterial invasion inside host cells, or function in bacterial-bacterial interactions such as biofilm formation. The contributions of type 1 pili during infection of the bladder by UPEC, leading to cystitis, is illustrative of the multifunctional nature of CU pili during pathogenesis and is described below.
Upon entering the urinary tract, E. coli use type 1 pili to bind to mannosylated receptors, such as the uroplakins that coat the luminal surface of the bladder, allowing the bacteria to colonize the bladder and avoid being washed out by the flow of urine (Mulvey et al., 1998; Zhou et al., 2001). Type 1 pili not only mediate binding of UPEC to the bladder surface, but also trigger host cell signaling pathways that lead to actin rearrangement in the urothelial cells and bacterial invasion inside the cells by a zipper-like mechanism (Mulvey et al., 1998; Martinez et al., 2000). In addition to the uroplakin receptors, bacterial uptake is facilitated by binding of the FimH adhesin at the tip of type 1 pili to β1 and α3 integrins on the host cells, and FimH alone is sufficient to trigger uptake into bladder epithelial cells (Martinez et al., 2000; Eto et al., 2007). Following invasion, UPEC replicate rapidly within the host cells and form intracellular biofilm-like communities (Anderson et al., 2003). Bacteria within these intracellular communities are protected from host innate immune responses and shielded from antibiotics. Type 1 pili are known to contribute to the formation of extracellular biofilms (Pratt & Kolter, 1998). Surprisingly, type 1 pili are also expressed by the intracellular bacteria and are required for formation of the intracellular biofilm-like structures, separate from their function in host cell binding and invasion (Wright et al., 2007). In agreement with this, Chen and colleagues identified residues in FimH that did not affect host cell binding, but which were required for formation of intracellular bacterial communities (Chen et al., 2009). An E. coli strain expressing FimH mutated in these residues was defective for pathogenesis and rapidly cleared from the bladder, highlighting the requirement for functions of type 1 pili in addition to extracellular adhesion. Bladder urothelial cells respond to UPEC binding and invasion by exfoliating into the bladder lumen, a host-defense mechanism to wash out the colonizing bacteria (Mulvey et al., 1998). However, UPEC counter this by fluxing out of the host cells and undergoing additional rounds of attachment to and invasion of neighboring cells, presumably mediated by type 1 pili as in the initial round of infection (Mulvey et al., 2001; Justice et al., 2004). During this process, the E. coli may gain access to the underlying bladder epithelium, which may lead to the formation of quiescent bacterial reservoirs from which recurrent infections can be seeded to begin the infection process anew (Mulvey et al., 2001; Mysorekar & Hultgren, 2006). Therefore, type 1 pili have both extracellular and intracellular roles and function at multiple different points during UPEC pathogenesis in the urinary tract.
TYPE IV PILI: DYANMIC AND MULTIFUNCTIONAL SURFACE FIBERS
Introduction
Type IV pili (T4P) are multifunctional surface structures that function in adhesion to host cells and other surfaces, formation of bacterial aggregates or microcolonies, biofilm formation, cellular invasion, electron transfer, DNA and phage uptake, and twitching or gliding motility (for recent reviews, see (Burrows, 2005; Hansen & Forest, 2006; Craig & Li, 2008; Pelicic, 2008)). Many of these functions are important during pathogenesis and T4P are critical virulence factors. Some T4P are able to extend and retract dynamically, which provides the basis for their unique functions, including twitching motility – a form of bacterial movement on solid surfaces – and DNA uptake (Mattick, 2002; Burrows, 2005). T4P are widely distributed among Gram-negative bacteria, including Pseudomonas aeruginosa (Mattick et al., 1996), Neisseria gonorrhoeae and Neisseria meningitidis (Merz & So, 2000), E. coli (Giron et al., 1991), Vibrio cholerae (Taylor et al., 1987), Salmonella typhi (Zhang et al., 2000), Moraxella bovis (Marrs et al., 1985), Myxococcus xanthus (Sun et al., 2000), Francisella tularensis (Gil et al., 2004), Dichelobacter nodosus (Han et al., 2007), and the plasmid R64 thin pilus system (Yoshida et al., 1998). Type IV pili have ancient evolutionary origins, as evidenced by the presence of homologous systems in Gram-positive bacteria and in archea, where they also assemble archeal flagella (Rakotoarivonina et al., 2002; Peabody et al., 2003; Varga et al., 2006; Pelicic, 2008; Pohlschroder et al., 2011).
T4P are thin, flexible fibers, 6–9 nm in diameter and up to several micrometers in length. Despite their thin diameter, T4P have great mechanical strength and are capable of exerting and withstanding forces in excess of 100 pN (Maier et al., 2002). The pili may be present on the bacterial surface as individual fibers or interact laterally to form bundles. T4P can be categorized into type IVa and IVb subgroups based on characteristics of the major pilin protein and the organization and composition of the pilus genes (Pelicic, 2008). The pil T4P systems of P. aeruginosa and Neisseria spp. are prototype members of the type IVa subgroup, and the bundle-forming pili (BFP) of enteropathogenic E. coli (EPEC) and the toxin-coregulated pili (TCP) of V. cholerae are prototype members of the type IVb subgroup. These pili are all critical virulence factors and mediate colonization of host surfaces, either through direct adhesion to host cell receptors or by promoting bacterial-bacterial interactions that lead to the formation of bacterial microcolonies and biofilms. These well-studied pili will be used as examples throughout this section.
Structural details of T4P and pilin proteins
T4P fibers consist solely or predominantly of repeating subunits of the major pilin protein (Figs. 5 and 6B) (Parge et al., 1995; Craig et al., 2003; Ramboarina et al., 2005; Craig et al., 2006; Li et al., 2008; Campos et al., 2011). Type IVa and type IVb pili share a similar overall structural arrangement, but differences in the sizes of the subunits lead to different sized fibers, with the type IVa pili of P. aeruginosa and N. gonorrhoeae having diameters of 6 nm and the type IVb pili of V. cholerae and EPEC having diameters of 8–9 nm (Craig et al., 2004; Ramboarina et al., 2005; Craig et al., 2006). In addition, differences in the pilin subunits affects their packing in the pilus fiber, with models indicating that type IVa and type IVb pilins form right-handed or left handed one-start helices, respectively (Campos et al., 2011). The pilin subunits contain a conserved N-terminal prepilin leader sequence, which is cleaved by a dedicated prepilin peptidase to yield the mature pilin (Strom et al., 1993). The leader sequence for type IVa pilins is short (less than 10 residues) and positively charged. Cleavage of the leader sequence occurs following a conserved glycine and the first amino acid of the mature pilin is phenylalanine (Hobbs & Mattick, 1993; Strom & Lory, 1993). In addition, a conserved glutamate is present at position +5 of the mature pilin, followed by a highly conserved hydrophobic region of approximately 25 residues (Strom & Lory, 1993; Craig et al., 2003). The conserved glutamate is required for assembly of pilin subunits (Pasloske et al., 1989; Strom & Lory, 1991; Macdonald et al., 1993). The remainder of the pilin sequence exhibits variability, except for the presence of two C-terminal cysteines, which define a disulfide-bonded antigenic loop or D-region (Fig. 6A) (Strom & Lory, 1993). Type IVb pilins are larger than type IVa pilins, have a longer leader sequence, typically 15–30 residues, and the first mature amino acid is not phenylalanine.
Fig. 5.
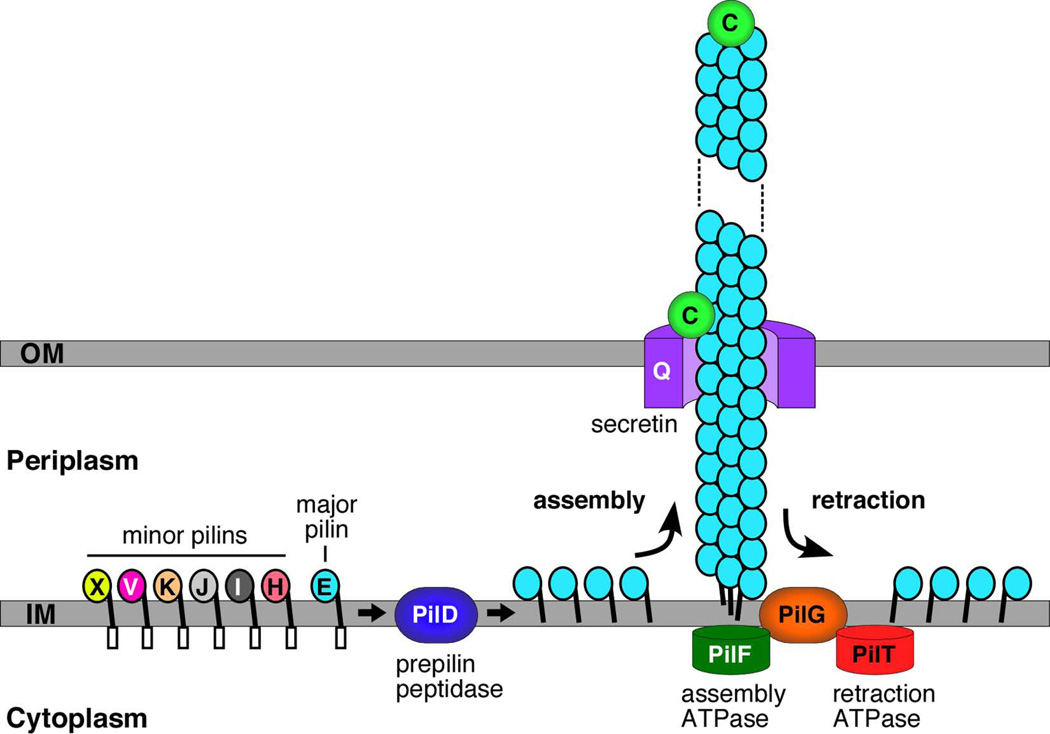
Model for biogenesis of T4P by N. meningitidis. The major (PilE) and minor (PilH, I, J, K, V and X) pilins are anchored in the inner membrane by their hydrophobic N-termini, with the conserved prepilin leader sequence exposed to the cytoplasm and globular head domain exposed to the periplasm. The leader sequence is cleaved and the mature pilins are N-methylated by the PilD prepilin peptidase. Processed pilin subunits are assembled into the pilus fiber from the periplasmic face of the inner membrane. PilF is the cytoplasmic ATPase that powers pilus assembly. The PilQ secretin provides the channel for secretion of the fiber across the outer membrane to the cell surface. Retraction of the pilus fiber is powered by the PilT cytoplasmic ATPase. During retraction, pilins are disassembled from the base of the pilus fiber and enter back into the inner membrane. The PilC protein functions as a tip-located adhesin, and also localizes to the outer membrane where it regulates pilus retraction.
Fig. 6.
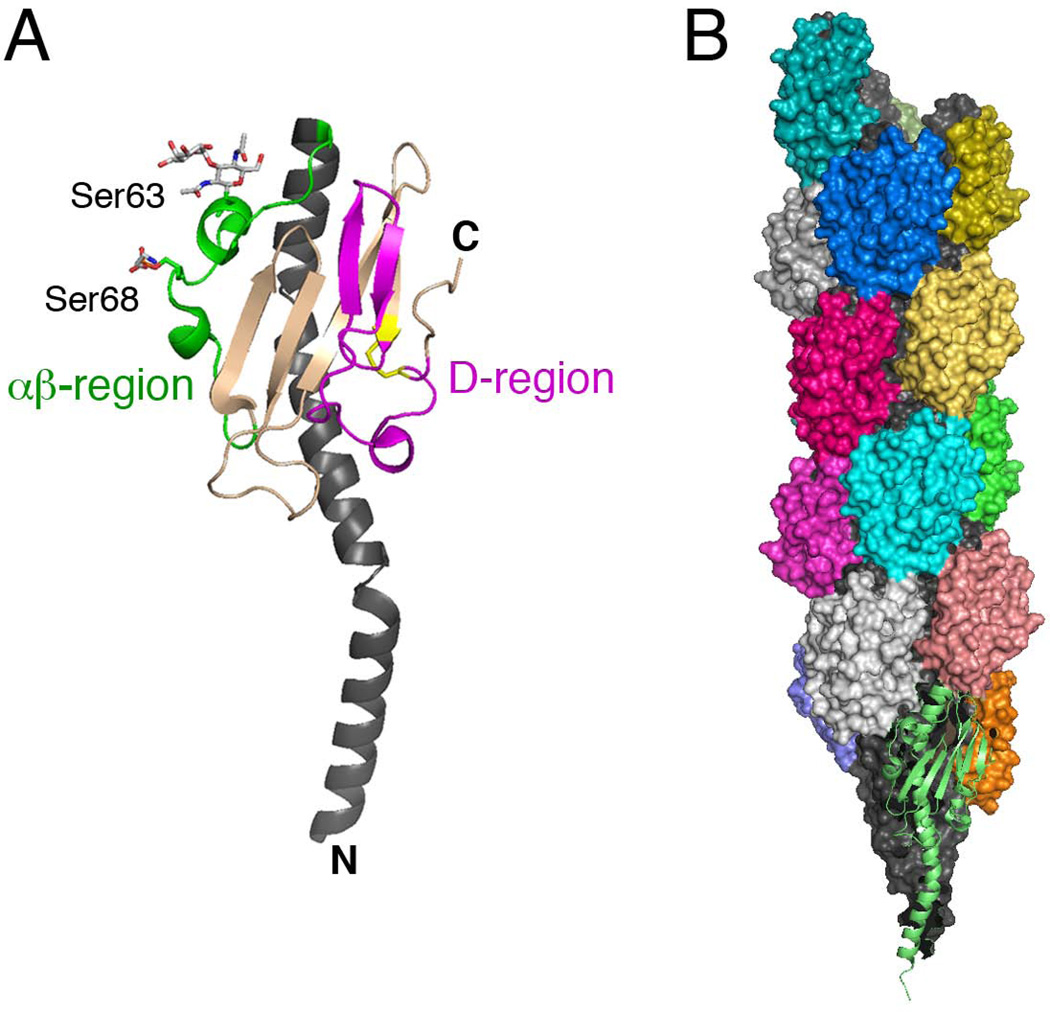
Structures of the N. gonorrhoeae pilin and assembled T4P fiber. (A) Crystal structure of PilE (PDB ID: 2HI2; (Craig et al., 2006)). The N-terminal α-helix is shown in dark gray, the αβ-region in green, and the D-region in magenta with the conserved disulfide bond in yellow. The phosphoethanolamine and disaccharide modifications to serine-68 and -63, respectively, in the αβ-region are shown in stick representation. (B) Atomic model of the T4P fiber based on docking of the PilE structure into a cryoEM density map (PDB ID: 2HIL; (Craig et al., 2006)). The pilin subunits are shown in surface representation, with their N-terminal α-helices in dark gray and surface-exposed C-terminal globular domains colored according to the individual pilin subunits. The bottom-most pilin is depicted in ribbon representation in light green. Images were generated using PyMOL.
Structures have been solved for several type IV pilins (Parge et al., 1995; Craig et al., 2003; Craig et al., 2006; Hansen & Forest, 2006; Craig & Li, 2008). The pilins adopt an elongated, ladle-like shape, with the N terminus forming an extended α-helix and the C terminus folding into a globular domain in which the α-helix packs against an antiparallel β-sheet (Fig. 6A). The conserved hydrophobic region of the N terminus forms the exposed ‘handle’ of the ladle. In assembly models for T4P, the hydrophobic N-terminal α-helices pack into the center of the pilus as a twisted three-helix bundle, forming a hydrophobic core that provides flexibility and tensile strength to the assembled fiber (Figs. 5 and 6B) (Parge et al., 1995; Craig et al., 2003; Craig et al., 2006; Craig & Li, 2008). The C-terminal globular domains are oriented towards the outside of the fiber, providing specific subunit-subunit contacts and exposed surfaces that confer pilus function (Fig. 6B). Two regions of the pilin in particular, the D-region and a region connecting the N-terminal α-helix to the C-terminal β-sheet, termed the αβ-region, form prominent surface-exposed edges in the assembled pilus fiber and make important contributions to pilus interactions (Fig. 6) (Craig et al., 2004). The surface of the pilus fiber is highly corrugated, with antigenically variable regions forming exposed ridges and conserved, functional residues lying shielded within deep grooves (Craig et al., 2006). The presence of the grooves likely enhances the flexibility of the pilus fiber and, together with the exposed ridges, provides specific binding sites along the length of the pilus.
For some type IV pilus systems, the pilus subunits are subject to both phase and antigenic variation, which provide mechanisms to prevent or escape the development of a protective host immune response and allows for modulation of functional activity. The surface-exposed D-region is an important site for host immune system recognition and a hotspot of pilin antigenic variation (Craig et al., 2004; Ramboarina et al., 2005). Antigenic variation has been extensively studied in Neisseria spp., where variation is driven by recombination between the active, expressed pilin gene and multiple silent partial copies present in the chromosome (Haas & Meyer, 1986; Howell-Adams & Seifert, 1999). Type IV pilins may also undergo different types of posttranslational modifications. These modifications are targeted to exposed regions of the pilus fiber and likely contribute to antigenic variation and immune evasion, and may also have important functional consequences as discussed below. Two serines in the exposed αβ-region of the Neisseria spp. PilE pilin are subject to varying O-linked modifications, with phosphocholine or phosphoethanolamine added to serine-68 and a disaccharide added to serine-63 (Fig. 6A) (Stimson et al., 1996; Warren & Jennings, 2003; Hegge et al., 2004). P. aeruginosa pilins are subject to glycosylation with an O-linked trisaccharide at their C terminus, which is surface exposed in the pilus fiber and in close proximity to the functionally and antigenically important D-loop region (Castric et al., 2001; Comer et al., 2002).
In addition to the major pilin subunit, T4P systems encode a number of prepilin-like proteins, termed minor pilins or pseudopilins, that contain the defining N-terminal prepilin leader sequence. These minor pilins are processed by the prepilin peptidase and may assemble into the pilus fiber as minor components that influence pilus assembly or function (Hobbs & Mattick, 1993; Mattick, 2002; Winther-Larsen et al., 2005; Cisneros et al., 2011). Pseudopilins are also present in type II secretion systems, where they form a pilus-like structure that is thought to aid secretion (Hobbs & Mattick, 1993; Mattick, 2002; Cisneros et al., 2011). In Neisseria spp., the PilH, I, J and K minor pilins are required for pilus biogenesis, as mutants lacking these proteins are non-piliated (Winther-Larsen et al., 2005; Carbonnelle et al., 2006). In contrast, the PilX, PilV and ComP minor pilins are not required for pilus assembly, but instead modify pilus function. The PilX minor pilin is required for T4P-mediated bacterial aggregation and the PilV minor pilin influences the level of phosphocholine/phosphoethanolamine modification of the PilE major pilin and triggers host cell signaling events leading to the formation of membrane protrusions (Hegge et al., 2004; Helaine et al., 2007; Mikaty et al., 2009). The ComP minor pilin acts to enhance DNA binding and uptake by T4P (Aas et al., 2002). Proteins in addition to the minor pilins may also associate with T4P fibers to modulate pilus function or act as adhesins. For example, the PilC1 and PilC2 proteins of Neisseria spp. function as tip-located adhesins and also localize to the outer membrane to regulate pilus retraction (Nassif et al., 1994; Rudel et al., 1995; Scheuerpflug et al., 1999; Morand et al., 2004).
Overview of the T4P assembly mechanism
The molecular details of T4P assembly are still not well understood. Biogenesis of T4P requires approximately 15 genes. For type IVA pili, these genes are found distributed on the chromosome in a scattered manner, but in defined clusters and conserved positions, such as for the pil genes of P. aeruginosa and Neisseria spp. (Mattick et al., 1996; Tonjum & Koomey, 1997). For type IVB pili, the pilus genes are found clustered together at a single chromosomal or plasmid-based location, such as for the tcp and bfp genes of V. cholera and EPEC, respectively (Sohel et al., 1996; Stone et al., 1996); Manning, 1997. Regulation of T4P biogenesis and function is complex and involves a number of signal transduction and chemosensory pathways, including quorum-sensing systems (Mattick, 2002). In P. aeruginosa, nearly 40 genes have been identified that affect T4P expression and function, with about half directly involved in pilus assembly and half contributing to regulation of pilus expression (Mattick, 2002). Biogenesis of T4P occurs by a mechanism closely related to the type II secretion pathway (see companion review in this issue and (Peabody et al., 2003)). This relationship is illustrated by the fact that T4P systems can function in the secretion of soluble proteins and type II secretion systems can be induced to assemble fibers resembling T4P (Sauvonnet et al., 2000; Durand et al., 2003; Kirn et al., 2003; Hager et al., 2006; Han et al., 2007). Moreover, in P. aeruginosa, which has both type II secretion and T4P systems, a single prepilin peptidase (PilD/XcpA) is shared between both systems and the major T4P subunit (PilA) participates in secretion by the type II system (Lu et al., 1997).
Fig. 5 presents a model for T4P biogenesis, as depicted for the N. meningitidis Pil system. Newly synthesized prepilins (the PilE major pilin plus additional minor pilins) are inserted into the cytoplasmic membrane by the Sec system, with their prepilin sequence exposed to the cytoplasm, their hydrophobic N-terminal α-helix spanning the membrane, and their globular C-terminal domain exposed to the periplasm (Zhang & Donnenberg, 1996; Arts et al., 2007; Francetic et al., 2007). The prepilin leader sequence is cleaved and the mature pilin N-methylated by the PilD prepilin peptidase (Strom & Lory, 1993; Zhang et al., 1994). Mature pilins are then extracted from the cytoplasmic membrane and polymerized into the pilus fiber by the T4P assembly machinery (Fig. 5). Assembly of pilins into the pilus fiber is thought to be mediated by interactions between the conserved hydrophobic N-terminal α-helices. This also provides a mechanism for incorporation of minor pilins, as these proteins contain the same conserved N-terminal sequence and structure (Craig et al., 2006). Pilus assembly is energized by the PilF cytoplasmic ATPase, which belongs to the secretion superfamily of hexameric ATPases that also power type II and type IV secretion systems (Planet et al., 2001; Peabody et al., 2003; Crowther et al., 2004; Satyshur et al., 2007). In addition, the T4P assembly machinery includes the integral inner membrane protein PilG, for which the precise function remains to be determined, and requires several minor pilins (PilH-K) (Tonjum et al., 1995; Crowther et al., 2004; Winther-Larsen et al., 2005; Carbonnelle et al., 2006). The assembled T4P fiber crosses from the periplasm to the cell surface through a large, multimeric gated channel in the outer membrane termed the secretin (PilQ; Fig. 5), which is also present in type II and III secretion systems (Bitter et al., 1998; Wolfgang et al., 2000; Collins et al., 2004; Reichow et al., 2010).
Type IV pilus retraction and twitching motility
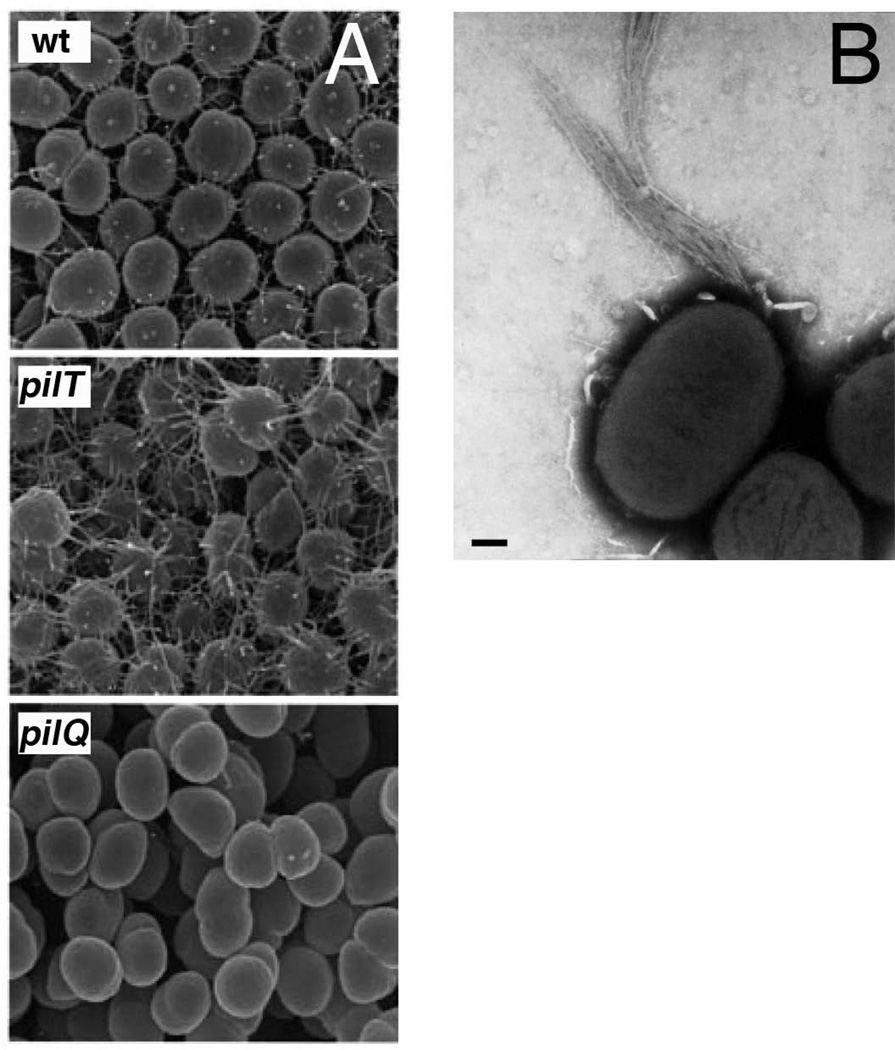
A distinctive feature of T4P is the ability of these fibers to dynamically extend and retract (Mattick, 2002; Nudleman & Kaiser, 2004; Burrows, 2005). In systems capable of pilus retraction, this is powered by a second cytoplasmic hexameric ATPase, the disassembly ATPase PilT (Fig. 5) (Satyshur et al., 2007; Misic et al., 2010). Mutant bacteria lacking PilT are typically hyperpiliated, because extension of the pilus fiber driven by the PilF assembly ATPase still occurs, but is no longer countered by pilus retraction (Fig. 4A). Available data suggest that PilT acts to disassemble pilin subunits from the base of the pilus fiber, with the liberated subunits entering back into the cytoplasmic membrane via their hydrophobic N termini (Fig. 5) (Morand et al., 2004). Thus, pilus extension and retraction appears to be a balance between pilin polymerization and depolymerization events occurring at the cytoplasmic membrane, caused by the action of opposing cytosolic ATPases (Burrows, 2005). Moreover, experimental evidence suggests that the primary function of a number of T4P machinery proteins is to stabilize the assembled pilus fiber or act as a counter to pilus retraction, rather than functioning directly in pilus assembly (Carbonnelle et al., 2006). The recent structure of the P. aeruginosa PilY1 protein provides interesting insights into possible mechanisms of regulation of pilus retraction (Orans et al., 2010). PilY1 is homologous to the Neisseria spp. PilC proteins, is associated with the pilus fiber, and functions similar to PilC in counteracting PilT-mediated retraction (Heiniger et al., 2010). The PilY1 structure revealed a modified β-propeller fold containing an EF-hand-like calcium binding site. A PilY1 mutation that prevented calcium binding, or removal of calcium using a chelator, caused loss of surface pili, whereas a mutation that mimicked a bound calcium ion caused hyperpiliation (Orans et al., 2010). Thus, PilY1 appears to use calcium sensing as a means to regulate pilus extension and retraction.
Fig. 4.
Electron micrographs of bacteria expressing T4P. (A) Scanning EM images showing T4P expressed by wild-type, and pilT and pilQ mutant N. gonorrhoeae. The pilT bacteria are hyperpiliated compared to wild-type, and the pilQ bacteria lack pili. (B) Micrograph of BFP expressed by EPEC showing a polar bundle of pili. Scale bar = 200 nm. Images in (A and B) reproduced with permission from (Wolfgang et al., 2000) and (Stone et al., 1996), respectively.
Pilus extension and retraction enables a form of movement on surfaces termed twitching motility, after the jerky motion produced (Mattick, 2002). Individual T4P fibers extend out from the bacteria, adhere to a surface, and then retract, pulling the bacteria toward the site of adhesion (Skerker & Berg, 2001). The retraction of T4P generates extremely high forces and PilT may be the strongest biological motor characterized to date (Biais et al., 2008). Measurement of N. gonorrhoeae T4P retraction forces using optical tweezers revealed that a single pilus fiber exerts forces of 50–100 pN, and an analysis of pilus fibers acting cooperatively in bundles revealed retraction forces in the nanonewton range (Maier et al., 2002; Biais et al., 2008). Twitching motility mediates bacterial-bacterial interactions and the formation of bacterial communities and biofilms (O'Toole & Kolter, 1998; Merz et al., 2000; Mattick, 2002). Type IV pilus-mediated twitching motility was recently shown to allow P. aeruginosa to orient perpendicular to a surface (‘walk upright’), facilitating surface exploration and influencing biofilm formation (Gibiansky et al., 2010). Pilus retraction is also required for functions such as DNA and phage uptake. Twitching motility performs essential functions during pathogenesis, as T4P assembled by pilT mutant bacteria are competent for adhesion but attenuated for host cell interactions and in host infection models (Bieber et al., 1998; Comolli et al., 1999; Merz et al., 1999; Pujol et al., 1999). Pilus retraction allows intimate adherence of bacteria to host cells and the formation of three-dimensional microcolonies on the host cell surface (Pujol et al., 1999; Jude & Taylor, 2011). In addition, the forces exerted by pilus retraction appear to be an important component in the elicitation of host cell responses during bacterial colonization (Merz et al., 1999; Howie et al., 2005; Dietrich et al., 2011).
Adhesion mediated by T4P: receptor binding and pilus bundling
T4P function as adhesins, and the ability to mediate adhesion to different surfaces, to other bacteria, and to DNA is critical for the various functions of T4P. Despite this central role, adhesion mechanisms of T4P are still not well understood. For most T4P, adhesion appears to be mediated by the major pilin subunit, and exposed surfaces such as the D- and αβ-regions are important sites for receptor binding (Lee et al., 1994; Xu et al., 2004; Hansen & Forest, 2006). P. aeruginosa T4P have been shown to bind to β-GalNAc(1–4)βGal moieties present in asialo-GM1 glycolipids expressed by epithelial cells, with binding mediated by the D-region of the C-terminal globular pilin domain (Lee et al., 1994; Hazes et al., 2000). However, the in vivo relevance of binding to asialo-GM1 remains to be established, as clinical P. aeruginosa isolates do not appear to use this receptor for adhesion to host cells (Schroeder et al., 2001). Moreover, Heiniger and coworkers recently provided evidence that the pilus-associated PilY1 protein may function as the P. aeruginosa T4P adhesin rather than the main pilin subunit (Heiniger et al., 2010). The use of a separate, tip-located protein as the pilus adhesin is best characterized for the homologous PilC1 and PilC2 proteins expressed by N. meningitidis and N. gonorrhoeae (Nassif et al., 1994; Rudel et al., 1995; Rudel et al., 1995). Neisseria spp. use their T4P for colonization of human mucosal surfaces. PilC localizes to the pilus tip and purified PilC has been shown to bind various human epithelial and endothelial cells (Scheuerpflug et al., 1999). The role of PilC is still controversial, however, as a direct requirement for PilC in host cell binding by Neisseria spp. T4P has not been demonstrated and there are conflicting reports on the host cell receptor for the Neisseria spp. T4P. CD46 has been identified as a pilus receptor and transgenic mice expressing human CD46 are susceptible to infection by piliated N. meningitidis (Johansson et al., 2003). In contrast, other studies have not been able to demonstrate a role for CD46 in cell culture binding experiments (Tobiason & Seifert, 2001; Kirchner et al., 2005). Understanding the function of PilC is further complicated by the fact that, as discussed above, it also localizes to the outer membrane and has been shown to promote pilus stability by counteracting PilT-mediated pilus retraction by an unknown mechanism (Morand et al., 2004).
While T4P mediate the initial attachment of bacteria to host cells, the primary function of some T4P may be less in the binding to specific host cell receptors and more in the formation of subsequent bacterial-bacterial and bacterial host-interactions, leading to intimate host cell contact and the generation of bacterial microcolonies. Analysis of high versus low adhesive strains of N. meningitidis revealed that high-level adhesion to host cells is mediated by bundling of T4P and the formation of bacterial aggregates, rather than increased adhesion to a host cell receptor. Thus, highly adhesive strains formed aggregates whereas less adhesive strains adhered as individual bacteria (Marceau et al., 1995). Similarly, the bundle-forming pili (BFP) of EPEC not only function in the initial attachment of bacteria to the intestinal epithelium, but also are required for bacterial autoaggregation and the formation of three-dimensional microcolonies, termed localized adherence (Giron et al., 1991; Donnenberg et al., 1992; Hyland et al., 2008). Furthermore, retraction of BFP allows intimate contact of EPEC with the host epithelium and facilitates the delivery of virulence factors into host cells via the EPEC type III secretion system (Zahavi et al., 2011).
T4P-driven bacterial aggregation is mediated by association of pilus fibers and pilus bundling. These pilus-pilus interactions appear to be formed by specific contacts between grooves and ridges along the length of the pilus fibers. The TCP of V. cholerae are required for bacterial colonization of the intestine, and pilus-mediated autoaggregation is a critical component of this process (Tacket et al., 1998; Kirn et al., 2000). Recent structural studies of V. cholerae TCP revealed that deep cavities are present along the surface of the fiber, produced by a loose packing of the surface-oriented C-terminal globular pilin domains (Li et al., 2008; Lim et al., 2010). Combined with mutagenesis studies that identified specific residues required for pilus-mediated aggregation, these structures suggest that pilus-pilus interactions are mediated by packing of charged residues in D-region bulges on one pilus fiber into cavities on another fiber (Kirn et al., 2000; Li et al., 2008; Lim et al., 2010). The cavities and grooves along the surface of the pilus fiber may also function in DNA binding and uptake by T4P, as modeling of the N. gonorrhoeae pilus fiber showed that some grooves are lined with positive charges that would facilitate interaction with negatively charged DNA (Craig et al., 2006).
Minor pilins may also influence or mediate pilus-pilus interactions. In N. meningitidis, the minor prepilin-like protein PilX is required for T4P-driven bacterial aggregation and microcolony formation (Helaine et al., 2005; Helaine et al., 2007). PilX is not required for pilus assembly, but incorporates along the length of the fiber. PilX adopts a typical pilin structure, with a hydrophobic N-terminal α-helix and globular C-terminal domain. Modeling and mutagenesis experiments identified the PilX D-region, which would be surface exposed in the pilus fiber, as required for N. meningitidis aggregation. Furthermore, bacteria expressing PilX could not form aggregates with bacteria lacking PilX, suggesting that PilX-PilX interactions between pilus fibers lead to bacterial aggregation (Helaine et al., 2005). Thus, the incorporation of minor pilins with distinct binding surfaces may confer specific functionality to T4P.
The many roles of T4P during pathogenesis: the example of N. meningitidis
Studies on the pathogenesis of N. meningitidis have revealed the multifunctional contributions of T4P during host-pathogen interactions. N. meningitidis is a causative agent of septicemia and cerebrospinal meningitis and an important human pathogen. N. meningitidis colonizes epithelial surfaces of the human nasopharynx. T4P function as adhesins to mediate the initial contact of N. meningitidis with epithelial cells, possibly via the PilC minor tip protein (Nassif et al., 1994). Following binding, the bacteria replicate on the epithelial cell surface and form tight, three-dimensional clusters or microcolonies. Microcolony formation requires pilus-pilus interactions mediated by the PilX minor pilin (Helaine et al., 2005; Helaine et al., 2007). As discussed below, binding via T4P also triggers host cell signaling pathways leading to cortical plaque formation and rearrangement of host cell actin (Merz et al., 1999; Merz & So, 2000). T4P retraction allows intimate contact of the bacteria with the host cell surface, facilitating stable colonization mediated by other adhesins such as the outer membrane opacity proteins (Virji et al., 1993; Pujol et al., 1999). Bacteria that colonize the nasopharynx may then invade across the epithelium to gain access to the blood stream, resulting in septicemia and ultimately meningitis if the bacteria are able to cross the blood-brain barrier. A recent study suggests that, in addition to mediating colonization, T4P play a central role in the dissemination step as well (Chamot-Rooke et al., 2011). N. meningitidis proliferating in association with host cells were found to upregulate the transferase that adds the phosphoglycerol posttranslational modification to the PilE major pilin. The phosphoglycerol modification introduces a negative charge into a positively charged patch of the αβ-region of the pilin, which is thought to be important for pilus-pilus interactions (Craig et al., 2006). The phosphoglycerol modification also sterically alters the surface of the pilus fiber. Thus, the increased levels of modified pilin will disrupt bacterial aggregation and promote detachment of bacteria from the microcolonies to facilitate dissemination (Chamot-Rooke et al., 2011). In agreement with this model, mutant N. meningitidis unable to modify their pilin showed decreased release from host cell-associated microcolonies and decreased transmigration across epithelial cell monolayers (Chamot-Rooke et al., 2011).
Once in the bloodstream, N. meningitidis must adhere to brain endothelial cells and cross the blood-brain barrier to cause meningitis. Adhesion to the endothelial cells is mediated by T4P and PilC plays a role in this process (Pron et al., 1997; Mairey et al., 2006). Similar to colonization of the nasopharynx, the bacteria multiply rapidly on the endothelial cell surface and form large microcolonies. N. meningitidis growing on the endothelial cells trigger the formation of filopodia-like protrusions. Generation of these host cell protrusions is dependent on T4P and on the PilV minor prepilin-like protein (Mikaty et al., 2009). PilV-dependent interactions with the endothelial cell surface result in cortical plaque formation, which drives localized actin polymerization and generation of the cellular protrusions. These protrusions protect the bacteria from shear forces caused by blood flow, possibly by providing more host surface area for bacterial contact (Mairey et al., 2006; Mikaty et al., 2009). A similar mechanism may also provide resistance to shear forces in the nasopharynx resulting from mucus flow and actions such as coughing and swallowing. In addition to formation of the cellular protrusions, host cell signals generated by N. meningitidis adhesion also appear to facilitate invasion of the bacteria across the blood-brain barrier. Adhesion via T4P triggers recruitment of host proteins involved in formation of intercellular junctions to sites of bacterial attachment (Coureuil et al., 2009). T4P, but not PilT-mediated pilus retraction, is required for this process. Recent work by Coureuil and colleagues demonstrated that the N. meningitidis-induced signaling occurs through the β2-adrenonergic receptor/β-arrestin pathway, which activates the Src tyrosine kinase to trigger actin polymerization and recruits host junctional proteins to sites of bacterial colonization (Coureuil et al., 2010). This depletes the junctional proteins from their normal positions at endothelial cell-cell interfaces and opens gaps to facilitate invasion of N. meningitidis across the blood-brain barrier (Coureuil et al., 2009). Thus, T4P provide a continuum of functions during host-pathogen interactions, from initial host cell contact, to colonization and remodeling of the host cell surface, to subsequent invasion across host barriers and dissemination to new sites of infection.
TYPE III SECRETION NEEDLE TIP COMPLEXES OF BACTERIAL PATHOGENS: KEY VIRULENCE STRUCTURES AT THE HOST-PATHOGEN INTERFACE
Introduction
Type III secretion systems (T3SS) are encoded by a large number of Gram-negative bacteria. Members of the T3SS superfamily are responsible for the export and assembly of two distinct types of surface organelles: flagella and needles (the latter have been called needle complexes or injectisomes) (Blocker et al., 2003; Macnab, 2004; Cornelis, 2006; Galan & Wolf-Watz, 2006) (Fig. 7). Flagella are used for bacterial motility, while needles are used for delivery of bacterial proteins into the membranes or cytosolic compartment of host eukaryotic cells. Assembly of T3SS needles and flagella is coordinately regulated by environmental signals. They are assembled in hierarchical fashion and made up of 20–30 proteins. T3SSs contain three main structural components: a set of core transmembrane proteins, a basal body, and surface exposed flagella or needle. The core transmembrane proteins are highly conserved between the two systems, while the basal body and flagella/needle components are more divergent at the level of primary sequence and three-dimensional structure, reflecting their distinct functions. For recent reviews of work on the structures of T3SS core transmembrane proteins, basal bodies, and needles see (Blocker et al., 2008; Marlovits & Stebbins, 2010; Worrall et al., 2011).
Fig. 7.
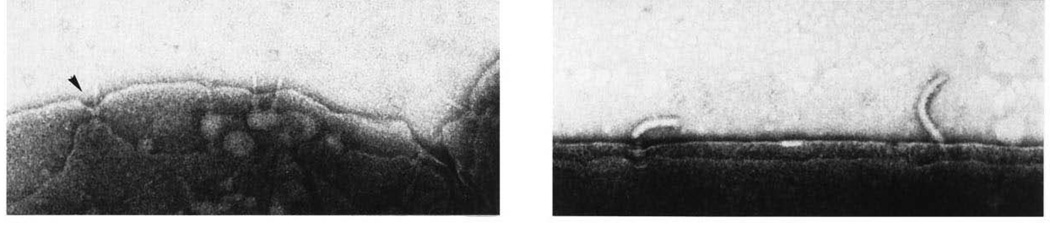
Electron micrographs of osmotically shocked S. enterica serovar Typhimurium bacteria. (Left) Nonflagellated ΔflhC S. Typhimurium exhibits needle complexes on the bacterial envelope. Note the depression at the insertion point of the needle complex (closed arrow). (Right) An S. Typhimurium fliK mutant exhibits flagellar polyhook basal bodies that span the inner and outer membranes. Scale bar = 100 nm. Reproduced with permission from (Kubori et al., 1998).
A description of the mechanism of type III secretion and an overview of the structures of the basal bodies and surface appendages of flagellar and needle T3SSs can be found in the accompanying review in this issue by Dalbey and Kuhn. Flagella and needles are helical structures with a central channel of ~25 angstroms. Flagellin monomers travel up the channel and assemble at the tip of the growing filament. It is assumed that needles assemble in the same manner. Substrates are believed to travel through this channel in needles in a partially unfolded state prior to delivery to host cells. Needle length is controlled by a ruler mechanism (Cornelis et al., 2006; Deane et al., 2010). A major distinction between flagella and needles can be seen by the structures of their tips. Flagella contain a cap protein that is required for subunit assembly at the growing tip (Macnab, 2004). In contrast, needles are capped by a “tip complex” (Mueller et al., 2005; Mueller et al., 2008). The tip complex is largely composed of a hydrophilic protein that multimerizes (Blocker et al., 2008; Mueller et al., 2008; Wang et al., 2008; Sato & Frank, 2011). In some bacteria (e.g. enteropathogenic E. coli, EPEC) the tip protein polymerizes into a long sheath that covers the needle, forming a pilus-like extension or filament (Sekiya et al., 2001; Garmendia et al., 2005; Enninga & Rosenshine, 2009). The tip complex is proposed to have several major functions, including regulation of secretion, sensing of host signals, transmitting activating signals to the needle, acting as a platform for recruitment and insertion of hydrophobic “translocator” proteins into host plasma membrane, and forming a conduit between the needle and the translocation channel (Blocker et al., 2008; Mueller et al., 2008; Wang et al., 2008; Sato & Frank, 2011). In addition, several of the tip proteins have been demonstrated to be the targets of immunoprotective antibodies (Mueller et al., 2008; Sato & Frank, 2011).
This section will focus on the structures of T3SS tip complexes and highlight recent studies in three areas: 1) the role of tip complexes as assembly platforms for translocators to form channels in host plasma membranes; 2) the function of tip complexes as conduits between the needle and channel; and 3) the role of tip complexes as protective antigens. This section will concentrate on the well-characterized tip proteins in Yersinia (LcrV), Pseudomonas (PcrV), Shigella (IpaD) and Salmonella (SipD) species. We will attempt to integrate structural data of the tip complexes with studies of surface localization, antibody neutralization and protective antibody epitope mapping (for a comprehensive recent review of the tip protein family, see (Sato & Frank, 2011)).
The end starts at the beginning: detection of organized LcrV protein on the bacterial surface
LcrV was identified as a protective antigen secreted by Y. pestis in 1956, and at that time it was called the V antigen (Burrows & Bacon, 1956). Forty-three years later the first evidence that LcrV is located in an organized manner on the surface of Y. pestis and Y. pseudotuberculosis was obtained by microscopy (Fields et al., 1999; Pettersson et al., 1999). Immunofluorescence microscopy showed that LcrV was present in “punctate zones” or “clusters” on the surface of Y. pseudotuberculosis and Y. pestis (Fields et al., 1999; Pettersson et al., 1999). A functional T3SS was shown to be required for this surface localization (Fields et al., 1999; Pettersson et al., 1999). The number of clusters per bacteria was estimated at 40–80 (Pettersson et al., 1999). Immunogold labeling in conjunction with EM gave a similar result, i.e. clusters of immunogold particles were found at discrete areas of the bacterial surface (Pettersson et al., 1999). Antibodies specific for LcrV reduced effector protein translocation into epithelial cells infected with Y. pseudotuberculosis, providing the first direct evidence that the surface localized protein had a critical function in effector translocation (see below) (Pettersson et al., 1999). In addition, LcrV released from the bacterial surface has been suggested to function as an immunomodulator (Brubaker, 2003).
Organized localization of tip proteins on bacterial surfaces has been detected in studies with Shigella (Espina et al., 2006; Sani et al., 2007), S. enterica (Lara-Tejero & Galan, 2009) and P. aeruginosa (Lee et al., 2010; Sato et al., 2011). The sheath forming tip protein of EPEC, EspA, was shown to form a fibrillar structure extending from the bacterial surface to the host cell plasma membrane (Knutton et al., 1998). Even earlier studies suggested that the Shigella tip protein IpaD was on the bacterial surface, but no evidence was obtained for an organized structure (Turbyfill et al., 1998).
Structure of LcrV and other tip proteins
The Y. pestis LcrV protein lacking its N-terminal 22 amino acids was purified and a crystal structure determined (Derewenda et al., 2004). In addition to lacking N-terminal residues, Lys40, Asp41 and Lys42 were converted to Ala, and Cys237 was changed to Ser to facilitate purification and crystallization of the protein. Although structural information for several N- (23–27), and C-(323–326) terminal residues and two internal loops (49–63 and 260–275) was not obtained, the visible electron maps allowed determination of the overall LcrV structure (PDB ID: 1R6F). LcrV has an overall dumbbell shape (see Fig. 8), with the “handle” comprising two helices (α-7 and α-12) that form a coiled-coil (Derewenda et al., 2004). The LcrV N terminus forms a globular domain at one end of the handle. A second globular domain that is formed by the region between α-7 and α-12 in LcrV is found at the other end of the handle. There is overall low sequence similarity among tip proteins, but the structural determination of IpaD (PDB ID: 2J0O; (Johnson et al., 2007)), BipD from Burkholderia (PD 2IZP; (Erskine et al., 2006; Johnson et al., 2007)) and EspA (PD ID code 1XOU; (Yip et al., 2005)) revealed an overall structurally similar dumbbell shape with long central coiled-coils. The globular domains among the family members vary in size, likely reflecting species-specific molecular functions. In IpaB and BipD, the N-terminal domain is proposed to function as an intramolecular chaperone for assembly of the subunits into the tip complex (Johnson et al., 2007).
Fig. 8.
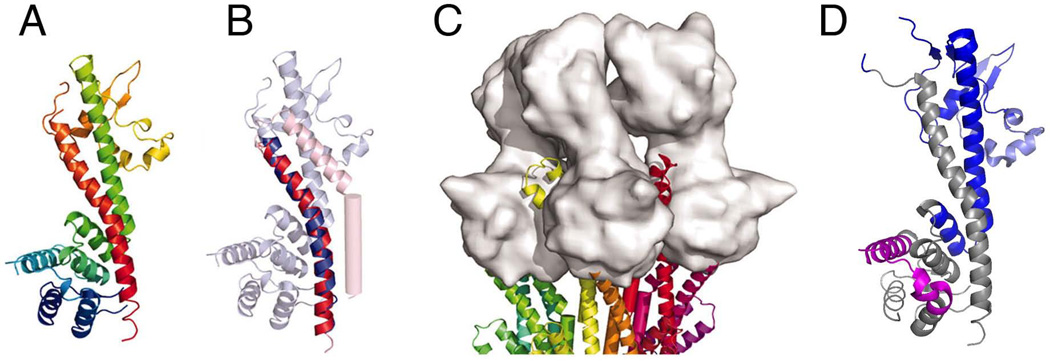
Structures of LcrV and the putative tip complex. (A) Ribbon diagram of LcrV (Derewenda et al., 2004) colored N (blue) to C (red) termini. (B) Overlay of the C-terminal helices of MxiH (red, residues 45–75) and LcrV (blue, residues 287–317), with all but the overlaid region made transparent to aid visualization. (C) Model of an LcrV tip complex (surface representation, gray) docked onto the tip of a T3SS needle. Reprinted with permission from (Deane et al., 2006). (D) Locations of protective epitopes in LcrV. Ribbon diagram of LcrV structure (PDB ID: 1R6F; (Derewenda et al., 2004)) shown in the same orientation as in panel A. Conformational epitope recognized by protective monoclonal antibody 7.3 (amino acids 135–275) (Hill et al., 1997; Hill et al., 2009) is shown in dark blue. Linear epitope recognized by protective monoclonal antibody BA5 (residues 196–225) (Eisele & Anderson, 2009; Quenee et al., 2010) is shown in light blue. Epitopes recognized by non-protective AH1 monoclonal antibody (Eisele & Anderson, 2009), corresponding to amino acids 76–105 of LcrV (D. Anderson, personal communication), are shown in magenta. Image was generated using PyMOL.
Structure of the needle and tip complex
T3SS basal bodies and needles were first isolated and characterized in Salmonella (Fig. 7) (Kubori et al., 1998). The needle is formed by polymerization of ~100–150 copies of ~9 kDa monomers into a helical structure. The 9.5 kDa protein YscF forms needles in Yersinia (Hoiczyk & Blobel, 2001). In Shigella there are 5.5 subunits of MxiH per turn, with a 4.6 Å axial rise per subunit (Cordes et al., 2003). The helical structure is slightly different in Salmonella as this needle contains 6.3 subunits of PrgI per turn (Galkin et al., 2010). X-ray crystal structures of several different needle subunits reveal a common fold for the central regions of these proteins: two α-helices connected by a short loop fold into a coiled-coil (Blocker et al., 2008). The N and C termini of the subunits are disordered in the structures, suggesting they are connected to the coiled-coil fold by flexible regions (Blocker et al., 2008). Mutational analysis of needle subunits has yielded evidence supporting a role for the needle in sensing host contact and regulating secretion (Kenjale et al., 2005; Davis et al., 2010; Martinez-Argudo & Blocker, 2010).
Fitting of the MxiH structure into the 16 Å resolution EM density map of the Shigella needle yielded a model for the assembled appendage (Deane et al., 2006; Blocker et al., 2008). The loop connecting the two helices is exposed on the surface of the needle, the shorter N-terminal helix lines the channel, and the longer C-terminal helix makes extensive subunit-subunit contacts. Small deletions at the C-terminus of IpaD prevent is association with needles (Espina et al., 2006), an observation which is consistent with a requirement for the C-terminal helix in needle-tip complex interactions.
Scanning transmission EM (STEM) analysis of needles sheared from the surface of Y. enterocolitica revealed a unique structure at the distal end (Fig. 9) (Mueller et al., 2005). Needles from an lcrV mutant of Yersinia lacked this tip complex, and antibodies specific for LcrV bound to the tip complex (Mueller et al., 2005). Immunogold labeling and TEM was used to show that IpaD localizes to the tip of Shigella needles (Espina et al., 2006; Sani et al., 2007). Modeling of PcrV and AcrV of Aeromonas on the structure of LcrV revealed that these three molecules have different sized globular domains. Analysis by STEM of complexes formed from hybrids of LcrV with PcrV or AcrV at the tips of Yersinia needles allowed determination that the N-terminal globular domain of LcrV is proximal to the needle, while the central globular domain is distal (Broz et al., 2007). The structure of the tip complex produced by averaging the electron densities from images obtained by STEM was used as a framework to estimate that five LcrV monomers are present (Broz et al., 2007). The pentameric structure arrived at has radial symmetry and a central channel (Broz et al., 2007). The hybrid LcrV proteins were also exploited to investigate the role of the globular N-terminal domain for function (Broz et al., 2007). Results showed that a hybrid LcrV protein containing the smaller PcrV N-terminal domain was defective for lysis of red blood cells and for insertion of the translocator YopB into red blood cell membranes (Broz et al., 2007). These results suggest that the needle proximal N-terminal domain of LcrV plays a key role in the insertion of the translocation channel into host membranes.
Fig. 9.
STEM images of negatively stained T3SS needles. (A) Characteristic tip complexes (arrow), comprising a head, a neck, and a base, of wild-type needles isolated from Y. enterocolitica grown in secretion-permissive conditions. (B) Needles formed by lcrV mutant bacteria. The needles of lcrV mutant bacteria are distinctly pointed at one end (asterisk). Scale bar = 20 nm. Reprinted with permission from (Mueller et al., 2005).
Structural similarity between the C-terminal helix of MxiH and the C-terminal helix of tip proteins such as LcrV suggested a model for the molecular interactions that allow assembly of the complex at the tip of the needle (Deane et al., 2006; Blocker et al., 2008). In the model, the C-terminal helix of an LcrV subunit would replace the C-terminal helix of a needle subunit, resulting in a homo-pentamer complex with helical symmetry and a channel of ~25 Å (Fig. 8). As discussed above, Broz et al. (Broz et al., 2007) arrived at an LcrV tip structure with radial symmetry. Thus, the two models for the structure of the LcrV tip complex differ with respect to radial (Broz et al., 2007) versus helical (Deane et al., 2006) symmetry, but arrive at the same overall role for the coiled-coil fold in assembly, the pentameric structure, orientation of globular domains and the presence of a channel. Interestingly, in both models the central globular domain would be oriented distal from the needle, which has implications for the functions of neutralizing antibodies that recognize epitopes in this domain (see below).
Determination of the crystal structure of IpaD allowed building of a model of the Shigella tip complex in which five monomers are tightly packed (Johnson et al., 2007). This structure lacks a central channel of sufficient size to allow passage of unfolded proteins, and therefore has been suggested to represent a “closed conformation” of the tip complex (Johnson et al., 2007; Blocker et al., 2008).
Evidence for association of translocators with the tip complex prior to host cell contact
It is thought that a major function of the tip complex is to act as an assembly platform for the hydrophobic translocators prior to their insertion into the host cell plasma membrane (Mueller et al., 2008; Mattei et al., 2011; Sato & Frank, 2011). Following the nomenclature of Mattei and colleagues (Mattei et al., 2011), the translocators fall into two classes, the so-called major (YopB, IpaB, SipB and PopB) and minor (YopD, IpaC, SipC and PopD) translocators of Yersinia, Shigella, Salmonella and Pseudomonas, respectively. Very little structural detail on either class of translocator is available due to their hydrophobicity and disordered character (Mattei et al., 2011). Evidence suggests that once inserted into the host plasma membrane SipB and IpaB adopt a hairpin structure, with the N and C termini exposed at the surface, and the two transmembrane domains spanning the lipid bilayer (McGhie et al., 2002; Hume et al., 2003).
YopB and YopD have not been detected at the tips of Yersinia needles, although YopD is reported to co-purify in small amounts with needle preparations (Mueller et al., 2005). Similarly, SipB and SipC were not detected on the surface of Salmonella prior to host cell contact (Lara-Tejero & Galan, 2009). In contrast, IpaB has been shown to localize to the tip of Shigella needles (Johnson et al., 2007; Olive et al., 2007; Veenendaal et al., 2007). IpaD is required for localization of IpaB to the needle tip (Olive et al., 2007; Veenendaal et al., 2007). Quantification of the relative amounts of IpaD and IpaB at needle tips suggest that one subunit of IpaB may associate with 4 subunits of IpaD (Johnson et al., 2007; Veenendaal et al., 2007). This observation lead to modeling of a modified complex in which four subunits of IpaD assemble at the tip via interactions with MxiH and adjacent IpaD proteins, continuing the helical structure of the needle. The fifth position of the complex is occupied by IpaB, interacting with MxiH below and laterally with staggered IpaD subunits (Johnson et al., 2007). The presence of two predicted α-helices that could form a coiled-coil structure in IpaB has lead to the predication that IpaB would have the same overall structure as IpaD, and that the C-terminal helix would be important for its association with the complex. Consistent with this idea is the observation that small deletions at the C terminus of IpaB prevent its association with needles (Roehrich et al., 2010; Shen et al., 2010).
Although IpaB has been detected at the Shigella needle tip in the absence of host cell contact, (Johnson et al., 2007; Veenendaal et al., 2007; Roehrich et al.; Shen et al.), there is evidence that sensing of host bile salts regulates localization of IpaB to the tip complex (Olive et al., 2007; Stensrud et al., 2008; Dickenson et al., 2010). Deoxycholate, a main component of bile salts, can bind to IpaD and induce a conformational change that is important for recruitment of IpaB (Dickenson et al., 2010). In addition, recruitment of IpaC to needle tips is stimulated by lipids such as cholesterol and sphingomyelin (Epler et al., 2009). As IpaB binds to cholesterol (Hayward et al., 2005; Lafont & van der Goot, 2005) it has been suggested that lipid binding to IpaB triggers a conformation change leading to recruitment of IpaC and insertion of the translocation channel into the host plasma membrane (Epler et al., 2009).
Evidence for direct contact between the tip complex and the translocon inserted in the host membrane
What evidence is there to support the concept that the tip complex maintains contact with the translocators inserted in the host plasma membrane, effectively forming a conduit for delivery of effectors? Several indirect pieces of evidence are worth discussing.
EspA filaments have been observed to form a direct link between EPEC and the host cell (Knutton et al., 1998). However, to date there is no direct evidence that EspA filaments are anchored to the host cell membrane via interactions with the translocators EspD or EspB.
Another interesting observation is that the tip protein SipD and the translocators SipB and SipC of Salmonella are required for a mode of bacterial-host cell contact that requires the T3SS (Lara-Tejero & Galan, 2009; Misselwitz et al., 2011). This mechanism of bacterial binding to epithelial cells was detected using strains lacking expression of effectors (so called “effectorless mutants”). The use of effectorless mutants is important to prevent the subsequent membrane ruffling and invasion by Salmonella that is normally triggered by effectors. Misselwitz et al. (Misselwitz et al., 2011) obtained evidence that the Sip-dependent binding mechanism, referred to as “docking” was irreversible, while binding mediated by type 1 pili was reversible. It was suggested that docking represents an intermediate step of translocation with a dual role: delivery of effectors, and maintenance of the bacteria at site of ruffling (Lara-Tejero & Galan, 2009; Misselwitz et al., 2011). Docking may physically represent binding of the tip complex to the translocon channel, although additional studies will be required to confirm this possibility.
Evidence that effectors located on the surface of bacteria can be translocated via a T3SS into host cells has recently been published (Akopyan et al., 2011). The authors argue that translocation of surface-localized effectors occurs by a different pathway from the standard “conduit” model (Akopyan et al., 2011). However, it has not been ruled out that the connection between the tip complex and the translocation channel is loose enough to allow access of extracellular effectors.
Tip proteins as targets of protective antibodies
Anti-tip protein antibodies have been shown to inhibit translocation function in T3SSs for LcrV (Pettersson et al., 1999), PcrV (Sawa et al., 1999), IpaD (Espina et al., 2006; Sani et al., 2007), and SipD (Desin et al., 2010). Antibody binding could directly interfere with tip protein function by blocking interaction with other subunits, with translocator proteins or host receptors. Opsonization is another mechanism by which antibodies that bind tip proteins could mediate host protection.
The mechanism of neutralizing polyclonal anti-LcrV antibodies was investigated using cytotoxicity in Yersinia-infected macrophages as a read-out of effector YopJ translocation (Weeks et al., 2002). Results showed that F(ab’)2 was as effective as whole antibody in inhibiting cytotoxicity (Weeks et al., 2002). However, F(ab’)2 was ineffective in promoting phagocytosis (Weeks et al., 2002). Cowen and colleagues measured YopH translocation into Yersinia-infected macrophages and showed that blocking Fc receptor function abrogated the neutralizing activity of anti-LcrV (Cowan et al., 2005). On the basis of these results it was suggested that Fc receptor-mediated phagocytosis is important for antibody neutralization of LcrV function (Cowan et al., 2005). A major protective epitope in LcrV is conformational and localizes to amino acids 135–275 (Hill et al., 1997; Vernazza et al., 2009). The region containing this epitope corresponds to α-helix 7 and the needle distal globular domain (Fig. 8D). A protective monoclonal antibody (7.3) that recognizes this conformational epitope (Hill et al., 1997; Hill et al., 2009) has been shown to promote phagocytosis and to reduce cytotoxicity in Yersinia-infected macrophages (Weeks et al., 2002; Noel et al., 2009). A second protective monoclonal antibody (BA5) that binds a linear epitope in LcrV (residues 196–225) promotes phagocytosis and reduces cytotoxicity in Yersinia-infected macrophages (Eisele & Anderson, 2009; Quenee et al., 2010). The epitope recognized by BA5 corresponds to part of α-helix 8, all of α-helix 9 and α-helix 10, and part of β-strand 4, all within the needle distal globular domain (Fig. 8D). Interestingly, a non-protective monoclonal antibody (AH1) was shown to inhibit cytotoxicity in Yersinia-infected macrophages but did not promote phagocytosis (Eisele & Anderson, 2009). The linear epitope recognized by AH1 corresponds to residues 76–105 of LcrV (D. Anderson, personal communication). This epitope maps to the needle proximal globular domain (Fig. 8D). Taken together, these results suggest that anti-LcrV antibodies must both neutralize effector translocation and promote phagocytosis to be fully protective (Eisele & Anderson, 2009). According the model of the LcrV tip complex (Fig. 8C) the epitopes recognized by 7.3 and BA5 should be exposed at the tips of the needles and oriented towards the center of the channel in an open complex (Fig. 8D). Such antibodies could simultaneously neutralize LcrV function and opsonize the bacteria. In contrast, the AH1 antibody, by binding to the laterally-exposed needle proximal globular domain (Fig. 8D), may inhibit tip complex formation and therefore would not opsonize the bacterial surface to promote phagocytosis.
A protective Mab specific for PcrV, Mab166, recognizes a conformational epitope between amino acids 144–257 (Sawa et al., 1999; Frank et al., 2002). This corresponds to the globular domain distal from the needle and is analogous to the domain recognized by protective anti-LcrV antibodies. Interestingly, F(ab’)2 of Mab166 has been shown to be protective against Pseudomonas in animal infection models (Frank et al., 2002; Sato & Frank, 2011), indicating that Fc receptor function may be differentially required for antibody-mediated protection against Pseudomonas vs. Yersinia.
Goure et al. showed that polyclonal anti-LcrV or anti-PcrV antibodies could prevent contact-mediated hemolysis of red blood cells infected with Yersinia or Pseudomonas, respectively (Goure et al., 2005). In addition, insertion of translocator proteins into red blood cell membranes was reduced in the presence of antibody, suggesting that insertion of the translocon pore was disrupted by neutralizing antibody (Goure et al., 2005). Polyclonal antibodies to IpaD inhibited contact-dependent hemolysis of red blood cells and invasion of Shigella into human epithelial cells (Espina et al., 2006; Sani et al., 2007). Epitope mapping studies suggested that the neutralizing antibodies recognized epitopes in the N-terminal 120 residues of IpaD (Espina et al., 2006). This region corresponds to an epitope in LcrV that is neutralizing in vitro but is not protective in vivo (Eisele & Anderson, 2009). Desin and colleagues showed that antibodies against the SPI-1 needle tip protein (SipD) inhibit Salmonella invasion (Desin et al., 2010). Epitope mapping studies have not been reported for SipD.
Translocator proteins as targets of protective or neutralizing antibodies
Evidence that translocator proteins can be targets of protective and or neutralizing antibodies is extremely limited. In fact, most studies suggest that translocator proteins are not effective protective antigens (Andrews et al., 1999; Sawa et al., 1999). An exception is that YopD was shown to be a protective antigen against Y. pestis lacking the F1 capsule (Andrews et al., 1999). Polyclonal antibodies specific for a complex of YopD and YopB were shown to be protective against F1− Y. pestis (Ivanov et al., 2008). Anti-YopD/B antibodies do not appear to inhibit Yop translocation into macrophages (J.B.B., unpublished data) and only weakly promote phagocytosis (Noel et al., 2009), and therefore the mechanism of protection is unknown. Taken together, these results suggest that the translocator proteins are generally not accessible to neutralizing or opsonizing antibodies in the context of wild-type pathogens. Of note, vaccination of mice with recombinant YscF, the subunit of the polymeric T3SS needle, has been shown to confer protection against Y. pestis infection (Matson et al., 2005; Swietnicki et al., 2005). Anti-YscF antibodies may mediate protection by opsonization.
Given the evidence for stable association of IpaB with the Shigella tip complex (Johnson et al., 2007; Olive et al., 2007; Veenendaal et al., 2007), it can be predicated that antibodies specific for IpaB should be neutralizing. In fact, a monoclonal antibody specific for IpaB was shown to partially neutralize S. flexneri T3SS function in an in vitro invasion assay (Mills et al., 1988). This antibody, 2F1, was reported to recognize an epitope in the N-terminal region of IpaB (Mills et al., 1988). Additional studies are needed to confirm and extend the observation that IpaB function can be neutralized by antibody binding.
THE TYPE IV SECRETION SYSTEM MACHINERY AND CONJUGATIVE PILUS
Introduction
The type IV secretion systems (T4SSs) are a phylogenetically and functionally diverse group of translocation systems (Baron & Coombes, 2007; Alvarez-Martinez & Christie, 2009; Llosa et al., 2009; Backert & Clyne, 2011; Fischer, 2011; Nagai & Kubori, 2011). These systems, present in nearly all bacterial and some archaeal species, translocate DNA and monomeric and multimeric protein substrates to target cells. T4SSs are composed of a few conserved machine subunits and variable numbers of other components from distinct ancestral lineages. These machine adaptations confer specialized functions exploited by bacterial pathogens and symbionts for successful colonization of particular host niches (Baron & Coombes, 2007; Alvarez-Martinez & Christie, 2009; Llosa et al., 2009; Backert & Clyne, 2011; Fischer, 2011; Nagai & Kubori, 2011).
T4SSs can be classified into functional groups as i) conjugation, ii) effector translocator, and iii) DNA uptake/release systems (Cascales & Christie, 2003). The conjugation machines translocate single-stranded DNA substrates within and across many bacterial species by a mechanism dependent on direct target cell contact (Alvarez-Martinez & Christie, 2009; Juhas et al., 2009). The effector translocators deliver protein substrates directly to eukaryotic target cells generally also by a contact-dependent mechanism; these systems are central to infection processes of a number of Gram-negative bacterial pathogens including Helicobacter pylori, Brucella spp., Bartonella spp., Rickettsial spp., and Legionella pneumophila (Baron & Coombes, 2007; Alvarez-Martinez & Christie, 2009; Llosa et al., 2009; Backert & Clyne, 2011; Fischer, 2011; Nagai & Kubori, 2011). The DNA uptake/release systems translocate DNA substrates across the cell envelope independently of target cell contact, as represented by the N. gonorrhoeae GGI system, which exports chromosomal DNA, and the H. pylori ComB system, which imports exogenous DNA (Karnholz et al., 2006; Ramsey et al., 2011).
Despite variability in subunit composition and function, the T4SSs likely adopt similar channel structures to convey substrates across the cell envelope. Consequently, recent structural findings for paradigmatic conjugation systems are probably thematic for T4SSs functioning in Gram-negative species. The Agrobacterium tumefaciens VirB/VirD4 T4SS is one of the best-characterized T4SSs and here will serve as a reference point for discussion of machine architecture and function. The A. tumefaciens system comprises 11 VirB proteins synthesized from the virB operon and the VirD4 subunit from the separate virD operon. These subunits coordinate the assembly of two structures, the envelope-spanning translocation channel and the extracellular conjugative pilus. Secretion substrates are recruited to the VirD4 substrate receptor, also termed the type IV coupling protein (T4CP), which sits at the entrance to the translocation channel. The channel spans the entire cell envelope and mediates substrate delivery to the cell surface in one step, reminiscent of the action of T3SSs (Christie, 2004; Alvarez-Martinez & Christie, 2009; Juhas et al., 2009).
The conjugative pilus is composed of the VirB2 pilin subunit forming the pilus fiber and VirB5, a pilus tip adhesion (Backert, 2000; Aly & Baron, 2007). All 11 VirB subunits are required for assembly of the conjugative pilus (Fullner et al., 1996). At this time, it is unknown whether the assembly of conjugative pili initiates from a platform of VirB subunits at the inner or outer membranes. If the former, pilus biogenesis might resemble the assembly pathway described for type IV pili (Fig. 5); these pili are unrelated to conjugative pili but instead cluster phylogenetically with type II secretion systems (Craig et al., 2006; Craig & Li, 2008; Ayers et al., 2010). If the latter, the assembly pathway could be more reminiscent of the CU pathway mediating assembly of P or type I fimbriae (Fig. 2).
Structural details of T4SS components
Structures of several T4SS subunits and subassemblies have been solved by X-ray crystallography, NMR, or cryoelectron microscopy (CryoEM) (Yeo et al., 2000; Gomis-Ruth et al., 2001; Terradot et al., 2005; Bailey et al., 2006; Hare et al., 2006; Bayliss et al., 2007; Chandran et al., 2009; Fronzes et al., 2009; Durand et al., 2011). A structure of the conjugative pilus encoded by the E. coli F plasmid T4SS also was solved by CryoEM and single-particle methods (Wang et al., 2009). These data allow for modeling of some features of the T4SS machines and general comparisons with other Gram-negative cell channels and surface fibers. Complementary biochemical findings from use of a ChIP-based, formaldehyde (FA) crosslinking assay (termed transfer DNA immunoprecipitation or TrIP) established that several of the VirB/VirD4 subunits (VirD4 VirB11, VirB6, VirB8, VirB2, VirB9) form close contacts with the translocating DNA substrate; these subunits are provisionally assigned as channel constituents (Atmakuri et al., 2004; Cascales & Christie, 2004; Jakubowski et al., 2004; Jakubowski et al., 2005). Here, we review recent advances in structure-function studies that formulate our current understanding of how T4SSs assemble and translocate substrates across the Gram-negative cell envelope. The reader is referred to several excellent reviews on other details of type IV secretion systems pertaining to biological diversity, regulation, and contributions to virulence (Baron & Coombes, 2007; Alvarez-Martinez & Christie, 2009; Fronzes et al., 2009; Llosa et al., 2009; Frost & Koraimann, 2010; Backert & Clyne, 2011; Nagai & Kubori, 2011)
Energetics of T4SS assembly/function: the inner-membrane-associated ATPases
The Gram-negative T4SSs typically possess two or three ATPases that each multimerize as dimers or ring-shaped hexamers. The ATPase complexes also physically and functionally interact with each other to form a higher-order, energy-generating factory to drive T4SS machine biogenesis and substrate translocation.
All conjugation systems and most effector translocators rely on VirD4 ATPases for substrate transfer. The VirD4 ATPases are members of the FtsK/SpoIIIE ATPase family (Gomis-Ruth et al., 2004). Most possess an N-terminal transmembrane domain (TMD) and a large C-terminal cytoplasmic domain (Alvarez-Martinez & Christie, 2009). The C-terminal domain of TrwB from the plasmid R388 conjugation system possesses two domains, a nucleotide-binding domain (NBD) similar to RecA and DNA ring helicases and an all α-domain (AAD) that exhibits structural similarity to site-specific recombinase, XerD of the λ integrase family. Six protomers of TrwB form a homohexameric ring that is 110 Å in diameter and 90 Å in height, with a 20 Å wide channel that constricts to 8 Å at the cytoplasmic pole (Fig. 10A) (Gomis-Ruth et al., 2001; Gomis-Ruth et al., 2002). TrwB ATP hydrolysis is stimulated by DNA binding and TrwB also undergoes conformational changes upon binding of DNA, consistent with a motor protein function during secretion (Tato et al., 2005).
Fig. 10.
T4SS structures and mechanisms of action of the T4SS ATPases in substrate transfer and machine biogenesis. (A) Upper: CryoEM (left) and X-ray (right) structures of the VirB7/VirB9/VirB10 core complex from pKM101. Lower: X-ray structure of the VirD4-like TrwB hexamer from plasmid R388; the N-terminal TMD was modeled onto the X-ray structure of the TrwB soluble domain. X-ray structure of B. suis VirB11 hexamer. Model of VirB4 dimer (left, schematic of predicted VirB4 topology; right, SAXS model of a L. pneumophila VirB4 monomer superimposed with the VirB4 C-terminus from A. tumefaciens atomic model in ribbon representation). (B) Model depicting substrate docking with the VirD4 T4CP, delivery to VirB11, passage through a channel composed of VirB6 and VirB8 with a contribution by VirB4, and a VirB2 conduit. (C) Model depicting the contributions of VirB4 and VirB11 to dislocation of VirB2 pilin from the inner membrane to build the conjugative pilus. Red arrows denote the substrate translocation route (B) and VirB2 pilin dislocation and polymerization (C). Structures are reprinted with permission from: TrwB (Gomis-Ruth et al., 2001); VirB11 (Yeo et al., 2000); VirB4 (Durand et al., 2011); VirB7/VirB10/VirB10 core complex CryoEM (Fronzes et al., 2009) and X-ray crystallography (Chandran et al., 2009); VIrB5 (Yeo et al., 2003).
The mechanism by which T4CPs engage with cognate secretion substrates has been elucidated only for the TraD T4CP of the E. coli F plasmid. TraD carries a C-terminal domain extending from its NBD. This C-terminal domain was shown to form a specific interaction with TraM, a component of the relaxosome responsible for nicking the F plasmid at its origin of transfer sequence (oriT) (Lu & Frost, 2005; Lu et al., 2008). The TraD-TraM interaction thus forms the basis for recognition of F plasmid substrate by the F T4SS (Lu et al., 2008). Although other T4CPs lack a C-terminal TraM-binding domain, they bind relaxases, other relaxosome components, or protein effectors. Studies suggest that the T4CP interacts with an unstructured C-terminal domain of protein substrates bearing clusters of positively-charged or hydrophobic amino acids (Atmakuri et al., 2003; Nagai et al., 2005; Vergunst et al., 2005). Some T4CPs also recognize internal translocation motifs shown to be required for translocation of protein substrates through some T4SSs (Schulein et al., 2005; Lang et al., 2010).
Results of the FA crosslinking studies suggest the A. tumefaciens VirD4 T4CP delivers secretion substrates to the VirB11 ATPase (Fig. 10B) (Cascales & Christie, 2004). VirB11 ATPases are members of a large family of structurally conserved ‘secretion ATPases’ also associated with the types II, III, and VI secretion systems (see companion review in this issue by Dalbey and Kuhn (Savvides et al., 2003; Savvides, 2007)). Like the other members of this superfamily, VirB11 subunits assemble as double-stacked homohexameric rings formed respectively by N- and C-terminal domains. The VirB11 hexamer is ~110 Å in diameter and ~70 Å in height with a central chamber of ~50 Å in diameter (Fig. 10A) (Yeo et al., 2000; Hare et al., 2006). VirB11 subunits undergo profound structural changes upon binding of ATP, as shown by a combination of X-ray crystallography and analytical ultracentrifugation (Savvides et al., 2003). In the absence of nucleotide, the N-terminal domains exhibit rigid-body conformations, and nucleotide binding 'locks' the hexamer into a symmetric and compact structure. It was proposed that VirB11 subunits use the mechanical leverage generated by nucleotide-dependent conformational changes to drive substrate export or T4SS machine assembly (Savvides et al., 2003). The VirB11 ATPases might use the energy of ATP hydrolysis to unfold protein substrates prior to translocation, reminiscent of unfoldase/chaperone functions described for similar hexameric ATPases associated with type III secretion systems (Akeda & Galan, 2005).
VirB4 ATPases also participate in substrate translocation across the inner membrane (Atmakuri et al., 2004; Cascales & Christie, 2004). These are large (>70 kDa) ATPases associated with all known T4SSs (Alvarez-Martinez & Christie, 2009). They display considerable variation with respect to the number of predicted TMDs, and those characterized fractionate as soluble, membrane-peripheral, or membrane-integral proteins. By small-angle X-ray scattering, low resolution structural models were presented for a membrane-extracted dimeric form of pKM101 TraB and a soluble monomeric form of LvhB4 from L. pneumophila (Fig. 10A) (Durand et al., 2011). These structures do not yet inform us how these ATPases function, but VirB4 homologs form contacts with multiple channel subunits, including the VirD4 and VirB11 ATPases, VirB3, and a complex composed of the VirB7, VirB9, and VirB10 proteins, which as described below serves as a structural scaffold for the VirB/VirD4 T4SS (Atmakuri et al., 2004; Yuan et al., 2005; Mossey et al., 2010). A. tumefaciens VirB4 is required for delivery of substrates to the inner membrane channel subunits VirB6 and VirB8, although underlying mechanistic details of this reaction step are currently unknown (Fig. 10B) (Atmakuri et al., 2004; Cascales & Christie, 2004).
Recently, evidence was presented that A. tumefaciens VirB4 modulates the structural state of membrane-integrated VirB2 pilin by a mechanism dependent on ATP binding/hydrolysis (Kerr & Christie, 2010). VirB4 catalyzes structural changes resulting in release of membrane-integrated VirB2 to the periplasm (Kerr & Christie, 2010), and VirB4 also is required for formation of VirB2 complexes with the minor pilin subunit VirB5 (Yuan et al., 2005). These findings support a proposal that VirB4 functions as a pilin dislocase, catalyzing extraction of pilin monomers from the inner membrane for incorporation into the secretion channel and the conjugative pilus (Fig. 10C). In the F plasmid T4SS, VirB4-like TraC is the only ATPase required for production of the F pilus (Lawley et al., 2003). F pili undergo cycles of polymerization and depolymerization (Clarke et al., 2008), suggesting a role for TraC in regulation of both processes.
The inner membrane translocase
Reminiscent of SecA ATPase (see companion review), the T4SS ATPases are envisioned to coordinate the docking of secretion substrates with the cognate T4SS channel. Although the inner membrane translocase is not structurally defined, it most likely is composed of a polytopic VirB6-like subunit and TMD’s contributed by bitopic VirB8- and VirB10-like subunits as well as other small membrane proteins, e.g. VirB3. Polytopic membrane proteins are common constituents of macromolecular translocation channels, e.g., SecY of the general secretory pathway (see companion review). Consistent with a direct role in substrate transfer, A. tumefaciens VirB6 forms FA-crosslinkable contacts with translocating DNA substrates, and crosslinking is dependent on the presence of the VirD4, VirB4, and VirB11 ATPases (Atmakuri et al., 2004; Cascales & Christie, 2004; Jakubowski et al., 2004). VirB6 also contributes to assembly and stabilization of other putative components of the inner membrane translocase, including VirB3 and a VirB7/VirB9/VirB10 core complex described below (Figs. 10B and C) (Hapfelmeier et al., 2000; Jakubowski et al., 2003).
The periplasmic- and outer membrane-spanning channel: A T4SS core complex
In A. tumefaciens, early studies showed that outer-membrane-associated VirB7 lipoprotein and VirB9 interact to form a stable dimer, which in turn interacts with and stabilizes bitopic VirB10 (Anderson et al., 1996; Fernandez et al., 1996; Spudich et al., 1996; Baron et al., 1997). This VirB7/VirB/VirB10 complex also exerts stabilizing effects on several other VirB channel subunits, suggesting that this complex forms an envelope-spanning structural scaffold early during machine biogenesis. Recently, a core complex composed of these subunits was visualized by CryoEM, and a portion was further solved by X-ray crystallography.
The pKM101 core complex, composed of 14 copies of each of the VirB7-like TraN, VirB9-like TraO, and VirB10-like TraF subunits, is a 1.05 MDa structure, 185 Å wide and 185 Å long, with the potential of spanning the entire Gram-negative bacterial cell envelope (Fig. 10A) (Fronzes et al., 2009). It is composed of two layers (I- and O-layers) that form a double-walled ring-like structure. The I-layer, composed of the N-terminal domains of TraO and TraF, is anchored in the inner membrane and is opened at the base by a 55 Å diameter hole. The O-layer, composed of TraN and the C-terminal domains of TraO and TraF, has a main body and a narrower cap on the outermost side of the complex that is inserted in the outer membrane. A narrow hole (10 Å) exists in the cap, resulting in a channel extending through the entire cylindrical structure. This channel could allow for substrate passage or exchange of small molecule signals between the cytoplasm and the extracellular milieu (Fronzes et al., 2009).
A crystal structure of the entire O-layer reveals that VirB10 forms the outer membrane channel (Fig. 10B) (Chandran et al., 2009). Specifically, 14 copies of a VirB10 domain comprising two α-helices separated by a loop, termed the antennae projection or AP, form the outer membrane channel. These findings are remarkable, first, because the insertion of α-helices into outer membranes of Gram-negative bacteria is highly unusual; most outer membrane channel proteins adopt a β-barrel structure. Second, VirB10 subunits are unique among described bacterial proteins in spanning the entire cell envelope; the N-terminal TM domain crosses the inner membrane, a Pro-rich region forms an extended structure across the periplasm, and the C-terminal α-helical AP forms an outer membrane pore (Chandran et al., 2009; Jakubowski et al., 2009).
This overall topology lends itself well to a structural contribution of VirB10 to T4SS channel assembly. The 14 N-terminal TMDs of VirB10 form a ~55 Å diameter ring that is predicted to encircle the inner membrane translocase composed of the 3 ATPases (VirD4, VirB4, and VirB11), VirB3, VirB6, and the N-terminal domain of VirB8 (Christie, 2009; Fronzes et al., 2009). Extending across the periplasm, the central chamber of the core complex is approximately 100 Å in diameter, sufficiently large to house VirB subunits including the VirB8 C-terminal domain, the pilin subunits VirB2 and VirB5, and the N-terminal domain of VirB9 (this domain was not part of the O-layer X-ray structure). The VirB2 and VirB9 channel components form close contacts with translocating substrate as shown by FA crosslinking studies (Cascales & Christie, 2004).
The outer membrane pore includes but is probably not limited to the VirB10 AP. In the pKM101 crystal structure, the 14 copies of the TraF AP form a pore with a hole of 32 Å (Chandran et al., 2009). It is possible that translocating substrates exit the outer membrane directly through the AP pore. However, large deletions of the VirB10 AP do not abolish substrate transfer, and VirB10 does not form crosslinkable contacts with DNA substrate (Cascales & Christie, 2004; Jakubowski et al., 2009). These observations suggest the AP pore might encircle another protein complex configured as the translocation channel. In the FA-crosslinking studies, VirB2 pilin was crosslinked to the DNA substrate, raising the possibility that a pilus or pseudopilus structure extending across the outer membrane forms a conduit for translocating substrate (Cascales & Christie, 2004).
Structural variations of the core complex
The core complex and associated inner membrane translocase composed of VirB6 through VirB10 subunits are probably universal structural features of T4SSs of Gram-negative bacteria. However, in many systems, these core subunits possess novel domains that confer structural variability and probably modulate surface interactions with target cells. For example, some VirB6 subunits possess large central or C-terminal extensions predicted to reside in the periplasm or extracellularly (Fig. 11A). Such proteins often exceed 60 kDa, more than twice the molecular size of A. tumefaciens VirB6 (Alvarez-Martinez & Christie, 2009). Studies showed that these C-terminal extensions are important for entry exclusion, a property that prevents redundant transfer of conjugative elements to donor cells (Audette et al., 2007; Marrero & Waldor, 2007). The entry exclusion phenotype is attributed to the projection of the C-terminal extension across the donor and recipient cell outer membranes and formation of specific contacts with an entry exclusion protein (Eex) protein in the inner membrane of the recipient cell (Fig. 11A) (Audette et al., 2007; Marrero & Waldor, 2007). These so-called ‘extended-VirB6’ subunits therefore function not only as channel subunits in the donor cell; they also specify an extracellular restriction system preventing transmission of mobile DNA elements among donor cell populations.
Fig. 11.
Examples of T4SS subunit acquisitions of structural folds for modulation of target cell interactions. (A) Some VirB6-like subunits possess C-terminal extensions implicated in surface exposure, e.g. Rickettsia spp. VirB6 (not shown), or extension through the T4SS channel and the recipient cell to the inner membrane where contact is established with the entry exclusion protein Eex, e.g. V. cholera SXT TraG and F plasmid TraG, which prevents redundant transfer to donor cells. (B) Several T4SSs possess variant forms of the VirB7/VirB9/VirB10 core complex subunits. X. citri VirB7XAC2622, a VirB7-like lipoprotein with an N-terminal (red) domain, implicated in binding VirB9XAC2620, and a C-terminal N0 domain (globular domain in yellow), which could mediate binding to target cells or substrate translocation through the T4SS. H. pylori VirB7-like CagT and VirB10-like CagY possess repeat domains implicated in forming a surface-variable sheath structure. (C) H. pylori VirB5-like CagL has an RGD motif implicated in binding of the β-integrin receptor on the host cell. Bartonella and Rickettsia spp. code for multiple copies of VirB2-like pilins, TrwL and VirB2, respectively, implicated in forming surface-variable pilins for immunomodulation. The X. citri VirB7 X-ray structure is reprinted with permission from (Souza et al., 2011). The modeled structure of VirB5-like CagL is reprinted with permission from (Backert et al., 2008). The schematics in Figs. B and C are reprinted with permission from (Alvarez-Martinez & Christie, 2009).
Recently, two variant forms of the VirB7 lipoprotein were structurally characterized and found to contain N0 domains. An NMR solution structure of a VirB7-like lipoprotein in a Xanthomonas citri T4SS showed that the N terminus forms contacts with a VirB9 homolog similar to contacts identified for the pKM101 VirB7 and VirB9 homologs. However, a large C-terminal globular domain (residues 52–133) adopts a structure similar to N0 domains of proteins from other systems involved in outer membrane transport (Fig. 11B) (Souza et al., 2011). In the X. citri lipoprotein, the N0 domains mediate formation of VirB7-VirB7 homooligomers, with a result that a tetradodecamer modeled from the pKM101 core complex has an extra layer of structure surrounding it (Souza et al., 2011). DotD, a lipoprotein associated with the L. pneumophila Dot/Icm T4BSS (Nakano et al., 2010), also has an N0 domain structure in its C terminus (Fig. 11B). In considering a role for the DotD N0 domain, the authors noted that similar structural folds recently were identified in N-terminal domains of secretins from the types II (GpsD) (Fig. 11B) and III (EscC) secretion systems, and the T4P (PilQ) assembly system, as well in subunits of cell-puncturing organelles including bacteriophage subunits (gf27, gp44) and a T6SS (VgrG) (Reichow et al.; Korotkov et al., 2009; Spreter et al., 2009). CryoEM and biochemical studies supplied evidence that the N0 domain of the V. cholera secretin GspD forms a ring within the periplasm that engages with the cholera toxin substrate. Structural similarities between DotD and GspD led to the suggestion that DotD might similarly form a ring in the periplasmic portion of the L. pneumophila Dot/Icm core complex to regulate substrate trafficking through this T4SS (Fig. 11B) (Nakano et al., 2010).
A number of other T4SS surface components possess novel structural features. For example, in the H. pylori Cag T4SS, CagT and CagY are classified as VirB7- and VirB10-like, respectively, but both subunits are considerably larger than their VirB counterparts (Alvarez-Martinez & Christie, 2009) (Fig. 11B). Both localize extracellularly as a component of a large sheathed filament produced by the Cag T4SS, and both possess repeat regions that differ in size and composition among Cag systems of different H. pylori isolates. Similarly, with the H. pylori Cag, Bartonella Trw, and Rickettsial VirB/VirD4 T4SSs, multiple pilin genes exist often in tandem array, suggesting that variable pilus structures are elaborated through differential expression or intergenic recombination (Alvarez-Martinez & Christie, 2009) (Fig. 11C). In these systems, the production of surface variable proteins or structures might serve to modulate pathogen-host cell interactions or contribute to immune evasion.
Pilin subunits and conjugative pili
Pilin subunits of T4SSs exhibit low overall levels of sequence identity. They are typically small (~5–10 kDa) proteins with an unusually long signal sequence and two predicted α-helical domains (Lawley et al., 2003). Upon cleavage of the signal sequence, pilins are posttranslationally modified. The N terminus of TraA from the F-type T4SSs is N-acetylated, whereas two P-type pilins, TrbB from plasmid RP4 and VirB2 from A. tumefaciens, undergo novel cyclization reactions resulting in covalent linkage of their N and C termini (Eisenbrandt et al., 1999; Kalkum et al., 2002; Lawley et al., 2003). The processed pilin subunits integrate into the cytoplasmic membrane via their hydrophobic α-helices; as mentioned above, the integrated pilins form a pool for incorporation into the translocation channel and pilus in response to an unknown signal.
VirB5 subunits also contribute to substrate transfer and pilus biogenesis. Early complementation studies supplied evidence that TraC, a VirB5 homolog from the pKM101 system could be delivered by TraC-producing strains to neighboring traC mutants to restore transfer competence of the mutant strains (Winans & Walker, 1985). This finding led to a proposal that VirB5 subunits localize extracellularly, which has now been confirmed by EM studies showing that VirB5 localizes at the tip of the A. tumefaciens T pilus (Aly et al., 2008). The structure of pKM101 TraC, solved by X-ray crystallography, comprises a 3-helix bundle flanked by a smaller globular part (Yeo et al., 2003). TraC and another VirB5 homolog, CagL, of the H. pylori Cag PAI, are structurally similar, and both are thought to mediate adherence to target cells. A solvent-exposed Arg-Gly-Asp (RGD) motif near the C terminus of α-helix 1 is implicating in binding of target cell receptors (Figs. 10C and 11C).
The pili of Gram-negative bacterial conjugation systems are the best characterized of the surface structures elaborated by T4SSs (Lawley et al., 2003; Clarke et al., 2008; Wang et al., 2009). The conjugative pili are composed of a VirB2-like pilin subunit and a VirB5-like minor pilin subunit attached at the pilus tip and/or base. The pili can be long (2 – 20 µm) and flexible with a diameter of 8 nm with a central lumen of 2 nm, as represented by the F-type pili, or short (< 1 µm) and rigid with a diameter of 8–12 nm, as represented by P-type pili. F-type pili enable E. coli donor cells to transfer DNA in liquid media, whereas P-type pili support efficient transfer only on solid surfaces. F-type pili are easily detected on the surfaces of F plasmid-carrying cells and undergo cycles of extension and retraction to promote formation of conjugative junctions. P-type pili are rarely detected on donor cell surfaces and are instead sloughed from the cell surface where they mediate aggregation of donor and recipient cells through hydrophobic interactions.
F pili are tubular structures of ~8.5 nm in diameter with two different subunit packing arrangements (Wang et al., 2009). One is a stack of pilin rings of C4 symmetry and the second is a one-start helical symmetry with an axial rise of ~3.5 Å per subunit and a pitch of ~12.2 Å. These two packing arrangements seem to coexist within the pilus structure. Interestingly, the central lumen of pilus is estimated at ~30 Å, large enough to accommodate ssDNA and unfolded protein(s), although whether the conjugative pilus serves as a conduit for T4SS substrates remains a subject of debate.
F pilus extension and retraction has been visualized on living cells by laser-scanning confocal microscopy (Clarke et al., 2008). E. coli donor cells appear to extend and retract their pili to sample the immediate surroundings. Extension involves the addition of pilin subunits to the cell-proximal base of the pilus. If the extended pilus establishes contact with a recipient cell, retraction generates a force sufficient to bring the cells together. As noted above, VirB4-like TraC is the only ATPase involved in F pilus dynamics, but at this time there is little mechanistic understanding of how energy drives extension or retraction.
It is also presently unknown whether the conjugative pilus represents the outgrowth of a pilin fiber formed within the core complex, or initiates polymerization on an outer membrane VirB platform. For most T4SSs, extracellular pili do not directly convey substrates as has been shown for the needle or pilus structures associated with the T3SSs. Rather, pili mediate formation of tight contacts or mating junctions between donor and target cell membranes. EM images of E. coli mating junctions show zones of tight membrane-membrane contacts extending sometimes along half the lengths of the mating cells (Dreiseikelmann, 1994; Samuels et al., 2000). No pilus fibers connect these cells, supporting the notion that the primary function for such extracellular filaments is to promote formation of tight membrane junctions.
Activating signals for type IV secretion
T4SSs are dynamic organelles that translocate substrates only upon establishment of productive target cell contacts. Recent studies suggest the T4SSs transduce signals bidirectionally to activate the translocation process. The activating signals within the cell include binding of substrate by the T4CP and stimulation of ATP hydrolysis among the ATPases. Conversely, activating signals at the cell exterior include contact with target cells or binding of bacteriophage to pilus receptors.
Activation of substrate translocation from the interior was shown to occur through an energy-mediated change in the conformation of VirB10. Upon sensing of ATP energy use by the VirD4 and VirB11 ATPases at the inner membrane, A. tumefaciens VirB10 undergoes a conformational change as shown by a difference in protease susceptibility in the unactivated and activated states (Cascales & Christie, 2004). Energy activation of VirB10 in turn is required for passage of DNA substrates through the distal portion of the translocation channel. A role for VirB10 in energy-dependent gating of the outer membrane pore is suggested by isolation of a mutation near the AP that ‘locks’ VirB10 in the energized conformation and allows for release of secretion substrates to the cell surface independently of target cell contact (Banta et al., 2011). It is also of interest to note that the ATPase activity of TrwB, the T4CP from the plasmid R388 conjugation system, is stimulated by binding of DNA and relaxosome components (Tato et al., 2007). In a generalized model, therefore, the DNA substrate - T4CP docking reaction might serve as a signal to activate the T4SS through the coupled stimulation of T4CP ATPase activity, VirB10 energization, and OM channel opening.
A recent study reported the interesting finding that the uptake of bacteriophage R17, whose receptor is the conjugative pilus encoded by the IncF plasmid R1, requires docking of the R1 relaxosome with the TraD T4CP (Lang et al., 2011). Nicking activity of the R1 relaxase is also required, establishing that the relaxosome must be catalytically active. In this system, the TraD T4CP does not display detectable ATPase activity even when bound by relaxosome. Binding of bacteriophage R17 to the conjugative pilus stimulates TraD activity and, correspondingly, phage uptake. Activation of the R1 T4SS therefore requires both relaxosome docking at the T4CP and an external signal. Phage binding represents one activating external signal, and the authors suggest that another such signal is transduced during conjugation in response to formation of productive contacts with recipient cells (Lang et al., 2011).
These findings advance early findings that F plasmid transfer requires a ‘mating signal’ propagated by recipient cells to donor cells (Lu & Frost, 2005). The nature of this mating signal remains unknown, but the recent work suggests it is transduced across the T4SS to the T4CP. Existence of such a mating signal also supports the notion that T4SSs are gated and activated to translocate substrates only upon formation of productive mating junctions. In other recent work on the H. pylori Cag T4SS, VirB5-like CagL was shown to bind and activate β1-integrin receptors on mammalian epithelial cells through its RGD motif (Kwok et al., 2007; Backert et al., 2008; Tegtmeyer et al., 2010; Conradi et al., 2011). Integrin activation in turn activates the Cag T4SS, possibly by inducing channel opening to enable delivery of the CagA effector to the mammalian cell. Other T4SS subunits including CagI and CagY, as well as CagA itself, bind integrin receptors, suggesting that a complex network of protein – protein interactions at the H. pylori-mammalian cell interface is required for Cag T4SS activation and CagA translocation (Tegtmeyer et al., 2011).
CONCLUDING REMARKS
The diversity of surface organelles assembled by Gram-negative bacteria is remarkable and reflects the exquisite tuning of these appendages by evolutionary forces to perform specific tasks under specific environmental conditions. Pili and other surface structures assembled by bacterial secretion systems enable the bacteria to act at a distance, extending the functional reach of the bacteria while maintaining the protective integrity of the bacterial envelope. The surface organelles are engineered to withstand extracellular forces such as shear stress and enable the bacteria to maintain contact with their targets under adverse conditions. The ability to initiate contact at a distance provides a means for pathogenic bacteria to avoid detection or uptake by host cells. In addition, surface extensions of bacterial secretion systems, such as provided by the T3SS needle complex and T4SS pilus, allow the direct delivery of effector molecules to host cells, in contrast to the non-specific release of virulence factors into the extracellular environment.
The different morphologies and functions of bacterial surface structures do not necessarily imply that the structures are unrelated or assembled by different secretion systems. This is perhaps best exemplified by the CU pathway, which assembles a variety of pili, ranging from rigid, rod-like fibers to amorphous, capsular structures. Despite their different architectures, all pili assembled by the CU pathway use the same structural building blocks, assembly mechanism and secretion machinery. Thus, attempts to group surface appendages by appearance alone will lead to inappropriate classifications. In contrast, secretion systems and assembly machineries are highly conserved and provide a more reliable means for classifying pili and other surface organelles.
The plasticity and adaptability of the surface structures provides a means for the bacteria to evade detection by the host immune system, through mechanisms including phase variable expression, antigenic variation, and post-translational modifications that alter the physical and functional attributes of the organelles. Unfortunately, these features also complicate the use of bacterial surface organelles as targets for vaccines and therapeutics. Nevertheless, an improved understanding of the structure and function of bacterial secretion systems and their surface-exposed appendages will facilitate the exploitation of these systems for antimicrobial development.
One promising approach is to target conserved, receptor-binding domains of pili for vaccination. Vaccination with the E. coli type 1 pilus adhesin FimH was found to be effective in protecting monkeys from colonization and infection of the urinary tract by UPEC (Langermann et al., 2000). Interestingly, Tchesnokova and colleagues recently showed that the host antibody response may actually enhance binding of FimH to its ligands by stabilizing the adhesin’s high-affinity binding state (Tchesnokova et al., 2011). These findings suggest that an improved understanding of pilus adhesion mechanisms may allow tailoring of the antigen used for vaccination to provoke a more effective immune response. Receptor-binding sequences of T4P have also been targeted for vaccination. Kao and coworkers demonstrated that a peptide-based, consensus sequence vaccine targeting the receptor-binding domain of P. aeruginosa T4P could overcome antigenic variation and provide cross-protection against different P. aeruginosa strains (Kao et al., 2007). Another approach for overcoming antigenic variation of T4P is to target conserved, functionally important minor pilin subunits, which are not subject to the high level antigenic variation observed with the major pilins. The potential of such an approach was recently demonstrated for the PilX, PilV and ComP minor pilins of the N. meningitidis T4P (Cehovin et al., 2011). The targeting of conserved functional elements may also prove effective for vaccination against surface-exposed components of bacterial secretion machineries, such as described above for the Y. pestis LcrV and related proteins of the T3SS tip complexes.
An alternative approach to vaccination is to disrupt bacterial adhesion to host cells through the use of small molecule competitive inhibitors of pilus-receptor interactions. Examples include the use of galabiose- and mannose-based inhibitors to interfere with adhesion mediated by P and type 1 pili, respectively, with the goal of preventing UPEC colonization of the urinary tract (Salminen et al., 2007; Wellens et al., 2008). An additional approach is to develop small molecule inhibitors that disrupt the secretion and assembly machinery itself, thereby preventing assembly of the surface organelles. Almqvist and colleagues have advanced the development of a class of small molecules termed pilicides that prevent pilus assembly by the CU pathway (Pinkner et al., 2006). Pilicides target the periplasmic chaperone and appear to disrupt pilus assembly by interfering with binding of chaperone-subunit complexes to the outer membrane usher assembly platform. High-throughput screens have been used to identify small molecules that inhibit protein secretion by T3SSs from a number of Gram-negative pathogens (Felise et al., 2008; Keyser et al., 2008). One such inhibitor also showed efficacy against type II secretion and T4P systems (Felise et al., 2008). The inhibitor affected assembly of the T3SS needle complex and appears to act on the outer membrane secretin protein, which is shared by the type III, type II, and T4P secretion pathways. Finally, interference with regulatory proteins is another approach to inhibit the assembly and function of bacterial surface structures. The small molecule virstatin prevents dimerization of the V. cholerae ToxT transcriptional activator and blocks expression of both cholera toxin and the toxin-coregulated T4P (Shakhnovich et al., 2007). These examples highlight the potential for a new class of antibiotics that target virulence factor secretion systems and the assembly of virulence-associated surface structures rather than essential biological processes (Cegelski et al., 2008). Such antibiotics should place less pressure on the bacteria than traditional antibiotics, and should be less prone to the development of resistance mechanisms. Such antibiotics should also allow the selective targeting of pathogenic bacteria while sparing the normal bacterial flora.
ACKNOWLDEGEMENTS
J.B.B extends thanks to Deborah Anderson for access to unpublished data. Research in the Thanassi laboratory is supported by NIH grants GM62987 and AI055621. Research in the Bliska laboratory on the subject of the review is supported by funding from the Northeast Biodefense Center U54-AI057158-Lipkin. Work in the Christie laboratory is supported by NIH grants GM48746 and BARD IS-4245-09.
REFERENCES
- Aas FE, Lovold C, Koomey M. An inhibitor of DNA binding and uptake events dictates the proficiency of genetic transformation in Neisseria gonorrhoeae: mechanism of action and links to Type IV pilus expression. Mol Microbiol. 2002;46:1441–1450. doi: 10.1046/j.1365-2958.2002.03265.x. [DOI] [PubMed] [Google Scholar]
- Abraham SN, Sun D, Dale JB, Beachey EH. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobactericeae. Nature. 1988;336:682–684. doi: 10.1038/336682a0. [DOI] [PubMed] [Google Scholar]
- Akeda Y, Galan JE. Chaperone release and unfolding of substrates in type III secretion. Nature. 2005;437:911–915. doi: 10.1038/nature03992. [DOI] [PubMed] [Google Scholar]
- Akopyan K, Edgren T, Wang-Edgren H, Rosqvist R, Fahlgren A, Wolf-Watz H, Fallman M. Translocation of surface-localized effectors in type III secretion. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1013888108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Martinez CE, Christie PJ. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev. 2009;73:775–808. doi: 10.1128/MMBR.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly KA, Baron C. The VirB5 protein localizes to the T-pilus tips in Agrobacterium tumefaciens. Microbiology. 2007;153:3766–3775. doi: 10.1099/mic.0.2007/010462-0. [DOI] [PubMed] [Google Scholar]
- Aly KA, Krall L, Lottspeich F, Baron C. The type IV secretion system component VirB5 binds to the trans-zeatin biosynthetic enzyme Tzs and enables its translocation to the cell surface of Agrobacterium tumefaciens. Journal of Bacteriology. 2008;190:1595–1604. doi: 10.1128/JB.01718-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantha RP, McVeigh AL, Lee LH, et al. Evolutionary and functional relationships of colonization factor antigen i and other class 5 adhesive fimbriae of enterotoxigenic Escherichia coli. Infect Immun. 2004;72:7190–7201. doi: 10.1128/IAI.72.12.7190-7201.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- Anderson KL, Billington J, Pettigrew D, et al. An atomic resolution model for assembly, architecture, and function of the Dr adhesins. Mol Cell. 2004;15:647–657. doi: 10.1016/j.molcel.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Anderson LB, Hertzel AV, Das A. Agrobacterium tumefaciens VirB7 and VirB9 form a disulfide-linked protein complex. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8889–8894. doi: 10.1073/pnas.93.17.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews GP, Strachan ST, Benner GE, et al. Protective efficacy of recombinant Yersinia outer proteins against bubonic plague caused by encapsulated and nonencapsulated Yersinia pestis. Infect Immun. 1999;67:1533–1537. doi: 10.1128/iai.67.3.1533-1537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprikian P, Interlandi G, Kidd BA, et al. The bacterial fimbrial tip acts as a mechanical force sensor. PLoS Biology. 2011;9:e1000617. doi: 10.1371/journal.pbio.1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts J, van Boxtel R, Filloux A, Tommassen J, Koster M. Export of the pseudopilin XcpT of the Pseudomonas aeruginosa type II secretion system via the signal recognition particle-Sec pathway. J Bacteriol. 2007;189:2069–2076. doi: 10.1128/JB.01236-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmakuri K, Ding Z, Christie PJ. VirE2, a type IV secretion substrate, interacts with the VirD4 transfer protein at cell poles of Agrobacterium tumefaciens. Mol. Microbiol. 2003;49:1699–1713. doi: 10.1046/j.1365-2958.2003.03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmakuri K, Cascales E, Christie PJ. Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol Microbiol. 2004;54:1199–1211. doi: 10.1111/j.1365-2958.2004.04345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audette GF, Manchak J, Beatty P, Klimke WA, Frost LS. Entry exclusion in F-like plasmids requires intact TraG in the donor that recognizes its cognate TraS in the recipient. Microbiology. 2007;153:442–451. doi: 10.1099/mic.0.2006/001917-0. [DOI] [PubMed] [Google Scholar]
- Ayers M, Howell PL, Burrows LL. Architecture of the type II secretion and type IV pilus machineries. Future Microbiology. 2010;5:1203–1218. doi: 10.2217/fmb.10.76. [DOI] [PubMed] [Google Scholar]
- Backert S. Strand switching during rolling circle replication of plasmid-like DNA circles in the mitochondria of the higher plant Chenopodium album (L.) Plasmid. 2000;43:166–170. doi: 10.1006/plas.1999.1437. [DOI] [PubMed] [Google Scholar]
- Backert S, Clyne M. Pathogenesis of Helicobacter pylori Infection. Helicobacter. 2011;16(Suppl 1):19–25. doi: 10.1111/j.1523-5378.2011.00876.x. [DOI] [PubMed] [Google Scholar]
- Backert S, Fronzes R, Waksman G. VirB2 and VirB5 proteins: specialized adhesins in bacterial type-IV secretion systems? Trends Microbiol. 2008;16:409–413. doi: 10.1016/j.tim.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Baga M, Norgren M, Normark S. Biogenesis of E. coli Pap pili: PapH, a minor pilin subunit involved in cell anchoring and length modulation. Cell. 1987;49:241–251. doi: 10.1016/0092-8674(87)90565-4. [DOI] [PubMed] [Google Scholar]
- Bailey S, Ward D, Middleton R, Grossmann JG, Zambryski PC. Agrobacterium tumefaciens VirB8 structure reveals potential protein-protein interaction sites. Proc Natl Acad Sci U S A. 2006;103:2582–2587. doi: 10.1073/pnas.0511216103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta LM, Kerr JE, Cascales E, et al. An Agrobacterium VirB10 mutation conferring a type IV secretion system gating defect. J Bacteriol. 2011;193:2566–2574. doi: 10.1128/JB.00038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C, Coombes B. Targeting bacterial secretion systems: benefits of disarmament in the microcosm. Infect Disord Drug Targets. 2007;7:19–27. doi: 10.2174/187152607780090685. [DOI] [PubMed] [Google Scholar]
- Baron C, Thorstenson YR, Zambryski PC. The lipoprotein VirB7 interacts with VirB9 in the membranes of Agrobacterium tumefaciens. J Bacteriol. 1997;179:1211–1218. doi: 10.1128/jb.179.4.1211-1218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss R, Harris R, Coutte L, et al. NMR structure of a complex between the VirB9/VirB7 interaction domains of the pKM101 type IV secretion system. Proc Natl Acad Sci U S A. 2007;104:1673–1678. doi: 10.1073/pnas.0609535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biais N, Ladoux B, Higashi D, So M, Sheetz M. Cooperative Retraction of Bundled Type IV Pili Enables Nanonewton Force Generation. PLoS Biology. 2008;6:e87. doi: 10.1371/journal.pbio.0060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber D, Ramer SW, Wu C-Y, Murray WJ, Tobe T, Fernandez R, Schoolnik GK. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Eschichia coli. Science. 1998;208:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- Bitter W, Koster M, Latijnhouwers M, de Cock H, Tommassen J. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol. 1998;27:209–219. doi: 10.1046/j.1365-2958.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- Blocker A, Komoriya K, Aizawa S. Type III secretion systems and bacterial flagella: insights into their function from structural similarities. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3027–3030. doi: 10.1073/pnas.0535335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocker AJ, Deane JE, Veenendaal AK, Roversi P, Hodgkinson JL, Johnson S, Lea SM. What's the point of the type III secretion system needle? Proc Natl Acad Sci U S A. 2008;105:6507–6513. doi: 10.1073/pnas.0708344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomfield IC. The regulation of pap and type 1 fimbriation in Escherichia coli. Adv Microb Physiol. 2001;45:1–49. doi: 10.1016/s0065-2911(01)45001-6. [DOI] [PubMed] [Google Scholar]
- Bock K, Breimer ME, Brignole A, et al. Specificity of binding of a strain of uropathogenic Escherichia coli to Gala(1–4)Gal-containing glycosphingolipids. J Biol Chem. 1985;260:8545–8551. [PubMed] [Google Scholar]
- Brinton CC. Non-flagellar appendages of bacteria. Nature. 1959;183:782–786. doi: 10.1038/183782a0. [DOI] [PubMed] [Google Scholar]
- Broz P, Mueller CA, Muller SA, Philippsen A, Sorg I, Engel A, Cornelis GR. Function and molecular architecture of the Yersinia injectisome tip complex. Mol Microbiol. 2007;65:1311–1320. doi: 10.1111/j.1365-2958.2007.05871.x. [DOI] [PubMed] [Google Scholar]
- Brubaker RR. Interleukin-10 and inhibition of innate immunity to Yersiniae: roles of Yops and LcrV (V antigen) Infect. Immun. 2003;71:3673–3681. doi: 10.1128/IAI.71.7.3673-3681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt E, Makowski L. Structural polymorphism of bacterial adhesion pili. Nature. 1995;373:164–167. doi: 10.1038/373164a0. [DOI] [PubMed] [Google Scholar]
- Burrows LL. Weapons of mass retraction. Mol Microbiol. 2005;57:878–888. doi: 10.1111/j.1365-2958.2005.04703.x. [DOI] [PubMed] [Google Scholar]
- Burrows TW, Bacon GA. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br. J. Exp. Pathol. 1956;37:481–493. [PMC free article] [PubMed] [Google Scholar]
- Buts L, Bouckaert J, De Genst E, et al. The fimbrial adhesin F17-G of enterotoxigenic Escherichia coli has an immunoglobulin-like lectin domain that binds N-acetylglucosamine. Mol Microbiol. 2003;49:705–715. doi: 10.1046/j.1365-2958.2003.03600.x. [DOI] [PubMed] [Google Scholar]
- Campos M, Francetic O, Nilges M. Modeling pilus structures from sparse data. J Struct Biol. 2011;173:436–444. doi: 10.1016/j.jsb.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Carbonnelle E, Helaine S, Nassif X, Pelicic V. A systematic genetic analysis in Neisseria meningitidis defines the Pil proteins required for assembly, functionality, stabilization and export of type IV pili. Mol Microbiol. 2006;61:1510–1522. doi: 10.1111/j.1365-2958.2006.05341.x. [DOI] [PubMed] [Google Scholar]
- Cascales E, Christie PJ. The versatile bacterial type IV secretion systems. Nat. Rev. Microbiol. 2003;1:137–150. doi: 10.1038/nrmicro753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales E, Christie PJ. Agrobacterium VirB10, an ATP energy sensor required for type IV secretion. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17228–17233. doi: 10.1073/pnas.0405843101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales E, Christie PJ. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science. 2004;304:1170–1173. doi: 10.1126/science.1095211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castric P, Cassels FJ, Carlson RW. Structural characterization of the Pseudomonas aeruginosa 1244 pilin glycan. J Biol Chem. 2001;276:26479–26485. doi: 10.1074/jbc.M102685200. [DOI] [PubMed] [Google Scholar]
- Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat Rev Micro. 2008;6:17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cehovin A, Kroll JS, Pelicic V. Testing the vaccine potential of PilV, PilX and ComP, minor subunits of Neisseria meningitidis type IV pili. Vaccine. 2011;29:6858–6865. doi: 10.1016/j.vaccine.2011.07.060. [DOI] [PubMed] [Google Scholar]
- Chamot-Rooke J, Mikaty G, Malosse C, et al. Posttranslational modification of pili upon cell contact triggers N. meningitidis dissemination. Science. 2011;331:778–782. doi: 10.1126/science.1200729. [DOI] [PubMed] [Google Scholar]
- Chandran V, Fronzes R, Duquerroy S, Cronin N, Navaza J, Waksman G. Structure of the outer membrane complex of a type IV secretion system. Nature. 2009;462:1011–1015. doi: 10.1038/nature08588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Hung CS, Pinkner JS, et al. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc Natl Acad Sci USA. 2009;106:22439–22444. doi: 10.1073/pnas.0902179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TH, Elberg SS. Scanning electron microscopic study of virulent Yersinia pestis and Yersinia pseudotuberculosis type 1. Infect Immun. 1977;15:972–977. doi: 10.1128/iai.15.3.972-977.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury D, Thompson A, Stojanoff V, Langermann S, Pinkner J, Hultgren SJ, Knight SD. X-ray structure of the FimC-FimH chaperone-adhesin complex from uropathogenic Escherichia coli. Science. 1999;285:1061–1066. doi: 10.1126/science.285.5430.1061. [DOI] [PubMed] [Google Scholar]
- Christie PJ. Bacterial type IV secretion: The Agrobacterium VirB/D4 and related conjugation systems. Biochem Biophys Acta. 2004;1694:219–234. doi: 10.1016/j.bbamcr.2004.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie PJ. Structural biology: Translocation chamber's secrets. Nature. 2009;462:992–994. doi: 10.1038/462992b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros DA, Bond PJ, Pugsley AP, Campos M, Francetic O. Minor pseudopilin self-assembly primes type II secretion pseudopilus elongation. EMBO J. 2011;31:1041–1053. doi: 10.1038/emboj.2011.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M, Maddera L, Harris RL, Silverman PM. F-pili dynamics by live-cell imaging. Proc Natl Acad Sci U S A. 2008;105:17978–17981. doi: 10.1073/pnas.0806786105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RF, Frye SA, Kitmitto A, Ford RC, Tonjum T, Derrick JP. Structure of the Neisseria meningitidis outer membrane PilQ secretin complex at 12 A resolution. J Biol Chem. 2004;279:39750–39756. doi: 10.1074/jbc.M405971200. [DOI] [PubMed] [Google Scholar]
- Comer JE, Marshall MA, Blanch VJ, Deal CD, Castric P. Identification of the Pseudomonas aeruginosa 1244 pilin glycosylation site. Infect Immun. 2002;70:2837–2845. doi: 10.1128/IAI.70.6.2837-2845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comolli JC, Hauser AR, Waite L, Whitchurch CB, Mattick JS, Engel JN. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect Immun. 1999;67:3625–3630. doi: 10.1128/iai.67.7.3625-3630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradi J, Huber S, Gaus K, et al. Cyclic RGD peptides interfere with binding of the Helicobacter pylori protein CagL to integrins alpha(V)beta (3) and alpha (5)beta (1) Amino Acids. 2011 doi: 10.1007/s00726-011-1066-0. [DOI] [PubMed] [Google Scholar]
- Cordes FS, Komoriya K, Larquet E, Yang S, Egelman EH, Blocker A, Lea SM. Helical structure of the needle of the type III secretion system of Shigella flexneri. J Biol Chem. 2003;278:17103–17107. doi: 10.1074/jbc.M300091200. [DOI] [PubMed] [Google Scholar]
- Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- Cornelis GR, Agrain C, Sorg I. Length control of extended protein structures in bacteria and bacteriophages. Curr Opin Microbiol. 2006;9:201–206. doi: 10.1016/j.mib.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Coureuil M, Lecuyer H, Scott MG, et al. Meningococcus Hijacks a beta2-adrenoceptor/beta-Arrestin pathway to cross brain microvasculature endothelium. Cell. 2010;143:1149–1160. doi: 10.1016/j.cell.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Coureuil M, Mikaty G, Miller F, et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–87. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C, Philipovskiy AV, Wulff-Strobel CR, Ye Z, Straley SC. Anti-LcrV antibody inhibits delivery of Yops by Yersinia pestis KIM5 by directly promoting phagocytosis. Infect Immun. 2005;73:6127–6137. doi: 10.1128/IAI.73.9.6127-6137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig L, Li J. Type IV pili: paradoxes in form and function. Curr Opin Struct Biol. 2008;18:267–277. doi: 10.1016/j.sbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig L, Li J. Type IV pili: paradoxes in form and function. Curr Opin Struct Biol. 2008;18:267–277. doi: 10.1016/j.sbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig L, Pique ME, Tainer JA. Type IV pilus structure and bacterial pathogenicity. Nat Rev Microbiol. 2004;2:363–378. doi: 10.1038/nrmicro885. [DOI] [PubMed] [Google Scholar]
- Craig L, Volkmann N, Arvai AS, Pique ME, Yeager M, Egelman EH, Tainer JA. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol Cell. 2006;23:651–662. doi: 10.1016/j.molcel.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Craig L, Taylor RK, Pique ME, et al. Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol Cell. 2003;11:1139–1150. doi: 10.1016/s1097-2765(03)00170-9. [DOI] [PubMed] [Google Scholar]
- Crowther LJ, Anantha RP, Donnenberg MS. The inner membrane subassembly of the enteropathogenic Escherichia coli bundle-forming pilus machine. Mol Microbiol. 2004;52:67–79. doi: 10.1111/j.1365-2958.2003.03963.x. [DOI] [PubMed] [Google Scholar]
- Davis AJ, Diaz DA, Mecsas J. A dominant-negative needle mutant blocks type III secretion of early but not late substrates in Yersinia. Mol Microbiol. 2010;76:236–259. doi: 10.1111/j.1365-2958.2010.07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JE, Abrusci P, Johnson S, Lea SM. Timing is everything: the regulation of type III secretion. Cell Mol Life Sci. 2010;67:1065–1075. doi: 10.1007/s00018-009-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JE, Roversi P, Cordes FS, et al. Molecular model of a type III secretion system needle: Implications for host-cell sensing. Proc Natl Acad Sci U S A. 2006;103:12529–12533. doi: 10.1073/pnas.0602689103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derewenda U, Mateja A, Devedjiev Y, Routzahn KM, Evdokimov AG, Derewenda ZS, Waugh DS. The structure of Yersinia pestis V-antigen, an essential virulence factor and mediator of immunity against plague. Structure (Camb) 2004;12:301–306. doi: 10.1016/j.str.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Desin TS, Mickael CS, Lam PK, Potter AA, Koster W. Protection of epithelial cells from Salmonella enterica serovar Enteritidis invasion by antibodies against the SPI-1 type III secretion system. Can J Microbiol. 2010;56:522–526. doi: 10.1139/w10-034. [DOI] [PubMed] [Google Scholar]
- Dickenson NE, Zhang L, Epler CR, Adam PR, Picking WL, Picking WD. Conformational Changes in IpaD from Shigella flexneri upon Binding Bile Salts Provide Insight into the Second Step of Type III Secretion. Biochemistry. 2010 doi: 10.1021/bi101365f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich M, Bartfeld S, Munke R, Lange C, Ogilvie LA, Friedrich A, Meyer TF. Activation of NF-kappaB by Neisseria gonorrhoeae is associated with microcolony formation and type IV pilus retraction. Cell Microbiol. 2011;13:1168–1182. doi: 10.1111/j.1462-5822.2011.01607.x. [DOI] [PubMed] [Google Scholar]
- Dodson KW, Pinkner JS, Rose T, Magnusson G, Hultgren SJ, Waksman GJ. Structural basis of the Interaction of the pyelonephritic E. coli adhesin to Its human kidney receptor. Cell. 2001;105:733–743. doi: 10.1016/s0092-8674(01)00388-9. [DOI] [PubMed] [Google Scholar]
- Donnenberg M, Giron J, Nataro J, Kaper J. A plasmid encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Molecular Microbiology. 1992;6:3427. doi: 10.1111/j.1365-2958.1992.tb02210.x. [DOI] [PubMed] [Google Scholar]
- Dreiseikelmann B. Translocation of DNA across bacterial membranes. Microbiol. Rev. 1994;58:293–316. doi: 10.1128/mr.58.3.293-316.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen AJ, Nouwen N. Protein translocation across the bacterial cytoplasmic membrane. Annu Rev Biochem. 2008;77:643–667. doi: 10.1146/annurev.biochem.77.061606.160747. [DOI] [PubMed] [Google Scholar]
- Du Y, Rosqvist R, Forsberg A. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect Immun. 2002;70:1453–1460. doi: 10.1128/IAI.70.3.1453-1460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid JP, Anderson ES, Campbell I. Fimbriae and adhesive properties in Salmonellae. J. Pathol. Bacteriol. 1966;92:107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Duguid JP, Smith IW, Dempster G, Edmunds PN. Non-flagellar filamentous appendages ("fimbriae") and hemagglutinating activity in bacterium coli. J. Pathol. Bacteriol. 1955;70:335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Durand E, Waksman G, Receveur-Brechot V. Structural insights into the membrane-extracted dimeric form of the ATPase TraB from the Escherichia coli pKM101 conjugation system. BMC structural biology. 2011;11:4. doi: 10.1186/1472-6807-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand E, Bernadac A, Ball G, Lazdunski A, Sturgis JN, Filloux A. Type II protein secretion in Pseudomonas aeruginosa: the pseudopilus is a multifibrillar and adhesive structure. J Bacteriol. 2003;185:2749–2758. doi: 10.1128/JB.185.9.2749-2758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele NA, Anderson DM. Dual-function antibodies to Yersinia pestis LcrV required for pulmonary clearance of plague. Clin Vaccine Immunol. 2009;16:1720–1727. doi: 10.1128/CVI.00333-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbrandt R, Kalkum M, Lai EM, Lurz R, Kado CI, Lanka E. Conjugative pili of IncP plasmids, and the Ti plasmid T pilus are composed of cyclic subunits. J. Biol. Chem. 1999;274:22548–22555. doi: 10.1074/jbc.274.32.22548. [DOI] [PubMed] [Google Scholar]
- Enninga J, Rosenshine I. Imaging the assembly, structure and activity of type III secretion systems. Cell Microbiol. 2009;11:1462–1470. doi: 10.1111/j.1462-5822.2009.01360.x. [DOI] [PubMed] [Google Scholar]
- Epler CR, Dickenson NE, Olive AJ, Picking WL, Picking WD. Liposomes recruit IpaC to the Shigella flexneri type III secretion apparatus needle as a final step in secretion induction. Infect Immun. 2009;77:2754–2761. doi: 10.1128/IAI.00190-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine PT, Knight MJ, Ruaux A, et al. High resolution structure of BipD: an invasion protein associated with the type III secretion system of Burkholderia pseudomallei. J Mol Biol. 2006;363:125–136. doi: 10.1016/j.jmb.2006.07.069. [DOI] [PubMed] [Google Scholar]
- Espina M, Olive AJ, Kenjale R, et al. IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect Immun. 2006;74:4391–4400. doi: 10.1128/IAI.00440-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto DS, Jones TA, Sundsbak JL, Mulvey MA. Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog. 2007;3:e100. doi: 10.1371/journal.ppat.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallman E, Schedin S, Jass J, Uhlin BE, Axner O. The unfolding of the P pili quaternary structure by stretching is reversible, not plastic. EMBO reports. 2005;6:52–56. doi: 10.1038/sj.embor.7400310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felek S, Jeong JJ, Runco LM, Murray S, Thanassi D, Krukonis ES. Contributions of chaperone/usher systems to cell binding, biofilm formation and Yersinia pestis virulence. Microbiology. 2010 doi: 10.1099/mic.0.044826-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felise HB, Nguyen HV, Pfuetzner RA, et al. An inhibitor of gram-negative bacterial virulence protein secretion. Cell Host Microbe. 2008;4:325–336. doi: 10.1016/j.chom.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez D, Spudich GM, Zhou XR, Christie PJ. The Agrobacterium tumefaciens VirB7 lipoprotein is required for stabilization of VirB proteins during assembly of the T-complex transport apparatus. J. Bacteriol. 1996;178:3168–3176. doi: 10.1128/jb.178.11.3168-3176.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields KA, Nilles ML, Cowan C, Straley SC. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect. Immun. 1999;67:5395–5408. doi: 10.1128/iai.67.10.5395-5408.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W. Assembly and molecular mode of action of the Helicobacter pylori Cag type IV secretion apparatus. FEBS J. 2011;278:1203–1212. doi: 10.1111/j.1742-4658.2011.08036.x. [DOI] [PubMed] [Google Scholar]
- Forero M, Yakovenko O, Sokurenko EV, Thomas WE, Vogel V. Uncoiling mechanics of Escherichia coli type I fimbriae are optimized for catch bonds. PLoS Biology. 2006;4:e298. doi: 10.1371/journal.pbio.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francetic O, Buddelmeijer N, Lewenza S, Kumamoto CA, Pugsley AP. Signal recognition particle-dependent inner membrane targeting of the PulG Pseudopilin component of a type II secretion system. J Bacteriol. 2007;189:1783–1793. doi: 10.1128/JB.01230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DW, Vallis A, Wiener-Kronish JP, et al. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J Infect Dis. 2002;186:64–73. doi: 10.1086/341069. [DOI] [PubMed] [Google Scholar]
- Fronzes R, Christie PJ, Waksman G. The structural biology of type IV secretion systems. Nature reviews. Microbiology. 2009;7:703–714. doi: 10.1038/nrmicro2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronzes R, Schafer E, Wang L, Saibil HR, Orlova EV, Waksman G. Structure of a type IV secretion system core complex. Science. 2009;323:266–268. doi: 10.1126/science.1166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost LS, Koraimann G. Regulation of bacterial conjugation: balancing opportunity with adversity. Future Microbiology. 2010;5:1057–1071. doi: 10.2217/fmb.10.70. [DOI] [PubMed] [Google Scholar]
- Fullner KJ, Lara JC, Nester EW. Pilus assembly by Agrobacterium T-DNA transfer genes. Science. 1996;273:1107–1109. doi: 10.1126/science.273.5278.1107. [DOI] [PubMed] [Google Scholar]
- Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- Galkin VE, Schmied WH, Schraidt O, Marlovits TC, Egelman EH. The structure of the Salmonella typhimurium type III secretion system needle shows divergence from the flagellar system. J Mol Biol. 2010;396:1392–1397. doi: 10.1016/j.jmb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galyov EE, Smirnov O, Karlishev AV, et al. Nucleotide sequence of the Yersinia pestis gene encoding F1 antigen and the primary structure of the protein. Putative T and B cell epitopes. FEBS Lett. 1990;277:230–232. doi: 10.1016/0014-5793(90)80852-a. [DOI] [PubMed] [Google Scholar]
- Garmendia J, Frankel G, Crepin VF. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect Immun. 2005;73:2573–2585. doi: 10.1128/IAI.73.5.2573-2585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibiansky ML, Conrad JC, Jin F, et al. Bacteria use type IV pili to walk upright and detach from surfaces. Science. 2010;330:197. doi: 10.1126/science.1194238. [DOI] [PubMed] [Google Scholar]
- Gil H, Benach JL, Thanassi DG. Presence of pili on the surface of Francisella tularensis. Infect Immun. 2004;72:3042–3047. doi: 10.1128/IAI.72.5.3042-3047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron JA, Ho ASY, Schoolnik GK. An inducible bundle-forming pilus of Enteropathogenic Escherichia coli. Science. 1991;254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- Gomis-Ruth FX, de la Cruz F, Coll M. Structure and role of coupling proteins in conjugal DNA transfer. Res. Microbiol. 2002;153:199–204. doi: 10.1016/s0923-2508(02)01313-x. [DOI] [PubMed] [Google Scholar]
- Gomis-Ruth FX, Sola M, de la Cruz F, Coll M. Coupling factors in macromolecular type-IV secretion machineries. Curr Pharm Des. 2004;10:1551–1565. doi: 10.2174/1381612043384817. [DOI] [PubMed] [Google Scholar]
- Gomis-Ruth FX, Moncalian G, Perez-Luque R, Gonzalez A, Cabezon E, de la Cruz F, Coll M. The bacterial conjugation protein TrwB resembles ring helicases and F1-ATPase. Nature. 2001;409:637–641. doi: 10.1038/35054586. [DOI] [PubMed] [Google Scholar]
- Goure J, Broz P, Attree O, Cornelis GR, Attree I. Protective anti-V antibodies inhibit Pseudomonas and Yersinia translocon assembly within host membranes. J Infect Dis. 2005;192:218–225. doi: 10.1086/430932. [DOI] [PubMed] [Google Scholar]
- Haas R, Meyer T. The repertoire of silent pilus genes in Neisseria gonorrhoeae: Evidence for gene conversion. Cell. 1986;44:107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- Hager AJ, Bolton DL, Pelletier MR, et al. Type IV pili-mediated secretion modulates Francisella virulence. Mol Microbiol. 2006;62:227–237. doi: 10.1111/j.1365-2958.2006.05365.x. [DOI] [PubMed] [Google Scholar]
- Hahn E, Wild P, Hermanns U, et al. Exploring the 3D molecular architecture of Escherichia coli type 1 pili. J Mol Biol. 2002;323:845–857. doi: 10.1016/s0022-2836(02)01005-7. [DOI] [PubMed] [Google Scholar]
- Han X, Kennan RM, Parker D, Davies JK, Rood JI. Type IV fimbrial biogenesis is required for protease secretion and natural transformation in Dichelobacter nodosus. J Bacteriol. 2007;189:5022–5033. doi: 10.1128/JB.00138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JK, Forest KT. Type IV pilin structures: insights on shared architecture, fiber assembly, receptor binding and type II secretion. J Mol Microbiol Biotechnol. 2006;11:192–207. doi: 10.1159/000094054. [DOI] [PubMed] [Google Scholar]
- Hapfelmeier S, Domke N, Zambryski PC, Baron C. VirB6 is required for stabilization of VirB5 and VirB3 and formation of VirB7 homodimers in Agrobacterium tumefaciens. J. Bacteriol. 2000;182:4505–4511. doi: 10.1128/jb.182.16.4505-4511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare S, Bayliss R, Baron C, Waksman G. A large domain swap in the VirB11 ATPase of Brucella suis leaves the hexameric assembly intact. J Mol Biol. 2006;360:56–66. doi: 10.1016/j.jmb.2006.04.060. [DOI] [PubMed] [Google Scholar]
- Hayward RD, Cain RJ, McGhie EJ, Phillips N, Garner MJ, Koronakis V. Cholesterol binding by the bacterial type III translocon is essential for virulence effector delivery into mammalian cells. Mol Microbiol. 2005;56:590–603. doi: 10.1111/j.1365-2958.2005.04568.x. [DOI] [PubMed] [Google Scholar]
- Hazes B, Sastry PA, Hayakawa K, Read RJ, Irvin RT. Crystal structure of Pseudomonas aeruginosa PAK pilin suggests a main-chain-dominated mode of receptor binding. J Mol Biol. 2000;299:1005–1017. doi: 10.1006/jmbi.2000.3801. [DOI] [PubMed] [Google Scholar]
- Hegge FT, Hitchen PG, Aas FE, et al. Unique modifications with phosphocholine and phosphoethanolamine define alternate antigenic forms of Neisseria gonorrhoeae type IV pili. Proc Natl Acad Sci USA. 2004;101:10798–10803. doi: 10.1073/pnas.0402397101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiniger RW, Winther-Larsen HC, Pickles RJ, Koomey M, Wolfgang MC. Infection of human mucosal tissue by Pseudomonas aeruginosa requires sequential and mutually dependent virulence factors and a novel pilus-associated adhesin. Cell Microbiol. 2010;12:1158–1173. doi: 10.1111/j.1462-5822.2010.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helaine S, Dyer DH, Nassif X, Pelicic V, Forest KT. 3D structure/function analysis of PilX reveals how minor pilins can modulate the virulence properties of type IV pili. Proc Natl Acad Sci U S A. 2007;104:15888–15893. doi: 10.1073/pnas.0707581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helaine S, Carbonnelle E, Prouvensier L, Beretti JL, Nassif X, Pelicic V. PilX, a pilus-associated protein essential for bacterial aggregation, is a key to pilus-facilitated attachment of Neisseria meningitidis to human cells. Mol Microbiol. 2005;55:65–77. doi: 10.1111/j.1365-2958.2004.04372.x. [DOI] [PubMed] [Google Scholar]
- Henderson NS, Ng TW, Talukder I, Thanassi DG. Function of the usher N-terminus in catalysing pilus assembly. Mol Microbiol. 2011;79:954–967. doi: 10.1111/j.1365-2958.2010.07505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Leary SE, Griffin KF, Williamson ED, Titball RW. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect Immun. 1997;65:4476–4482. doi: 10.1128/iai.65.11.4476-4482.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Leary S, Smither S, et al. N255 is a key residue for recognition by a monoclonal antibody which protects against Yersinia pestis infection. Vaccine. 2009;27:7073–7079. doi: 10.1016/j.vaccine.2009.09.061. [DOI] [PubMed] [Google Scholar]
- Hobbs M, Mattick JS. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol. Microbiol. 1993;10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- Hoiczyk E, Blobel G. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc Natl Acad Sci U S A. 2001;98:4669–4674. doi: 10.1073/pnas.071065798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell-Adams B, Seifert HS. Insertion mutations in pilE differentially alter gonococcal pilin antigenic variation. J Bacteriol. 1999;181:6133–6141. doi: 10.1128/jb.181.19.6133-6141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie HL, Glogauer M, So M. The N. gonorrhoeae type IV pilus stimulates mechanosensitive pathways and cytoprotection through a pilT-dependent mechanism. PLoS Biology. 2005;3:e100. doi: 10.1371/journal.pbio.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume PJ, McGhie EJ, Hayward RD, Koronakis V. The purified Shigella IpaB and Salmonella SipB translocators share biochemical properties and membrane topology. Mol. Microbiol. 2003;49:425–439. doi: 10.1046/j.1365-2958.2003.03559.x. [DOI] [PubMed] [Google Scholar]
- Hung CS, Bouckaert J, Hung D, et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol Microbiol. 2002;44:903–915. doi: 10.1046/j.1365-2958.2002.02915.x. [DOI] [PubMed] [Google Scholar]
- Hung DL, Knight SD, Woods RM, Pinkner JS, Hultgren SJ. Molecular basis of two subfamilies of immunoglobulin-like chaperones. EMBO J. 1996;15:3792–3805. [PMC free article] [PubMed] [Google Scholar]
- Hyland RM, Sun J, Griener TP, Mulvey GL, Klassen JS, Donnenberg MS, Armstrong GD. The bundlin pilin protein of enteropathogenic Escherichia coli is an N-acetyllactosamine-specific lectin. Cell Microbiol. 2008;10:177–187. doi: 10.1111/j.1462-5822.2007.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov MI, Noel BL, Rampersaud R, Mena P, Benach JL, Bliska JB. Vaccination of mice with a Yop translocon complex elicits antibodies that are protective against infection with F1- Yersinia pestis. Infect Immun. 2008 doi: 10.1128/IAI.00189-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob-Dubuisson F, Heuser J, Dodson K, Normark S, Hultgren SJ. Initiation of assembly and association of the structural elements of a bacterial pilus depend on two specialized tip proteins. EMBO J. 1993;12:837–847. doi: 10.1002/j.1460-2075.1993.tb05724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski SJ, Krishnamoorthy V, Christie PJ. Agrobacterium tumefaciens VirB6 protein participates in formation of VirB7 and VirB9 complexes required for type IV secretion. J. Bacteriol. 2003;185:2867–2878. doi: 10.1128/JB.185.9.2867-2878.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski SJ, Krishnamoorthy V, Cascales E, Christie PJ. Agrobacterium tumefaciens VirB6 domains direct the ordered export of a DNA substrate through a type IV secretion system. J Mol Biol. 2004;341:961–977. doi: 10.1016/j.jmb.2004.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski SJ, Cascales E, Krishnamoorthy V, Christie PJ. Agrobacterium tumefaciens VirB9, an outer-membrane-associated component of a type IV secretion system, regulates substrate selection and T-pilus biogenesis. J Bacteriol. 2005;187:3486–3495. doi: 10.1128/JB.187.10.3486-3495.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski SJ, Kerr JE, Garza I, Krishnamoorthy V, Bayliss R, Waksman G, Christie PJ. Agrobacterium VirB10 domain requirements for type IV secretion and T pilus biogenesis. Mol Microbiol. 2009;71:779–794. doi: 10.1111/j.1365-2958.2008.06565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Rytkonen A, Bergman P, et al. CD46 in meningococcal disease. Science. 2003;301:373–375. doi: 10.1126/science.1086476. [DOI] [PubMed] [Google Scholar]
- Johnson S, Roversi P, Espina M, et al. Self-chaperoning of the type III secretion system needle tip proteins IpaD and BipD. J Biol Chem. 2007;282:4035–4044. doi: 10.1074/jbc.M607945200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CH, Pinkner JS, Roth R, Heuser J, Nicholoes AV, Abraham SN, Hultgren SJ. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc. Natl. Acad. Sci. USA. 1995;92:2081–2085. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jude BA, Taylor RK. The physical basis of type 4 pilus-mediated microcolony formation by Vibrio cholerae O1. Journal of structural biology. 2011 doi: 10.1016/j.jsb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhas M, van der Meer JR, Gaillard M, Harding RM, Hood DW, Crook DW. Genomic islands: tools of bacterial horizontal gene transfer and evolution. FEMS microbiology reviews. 2009;33:376–393. doi: 10.1111/j.1574-6976.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkum M, Eisenbrandt R, Lurz R, Lanka E. Tying rings for sex. Trends Microbiol. 2002;10:382–387. doi: 10.1016/s0966-842x(02)02399-5. [DOI] [PubMed] [Google Scholar]
- Kao DJ, Churchill ME, Irvin RT, Hodges RS. Animal protection and structural studies of a consensus sequence vaccine targeting the receptor binding domain of the type IV pilus of Pseudomonas aeruginosa. J. Mol. Biol. 2007;374:426–442. doi: 10.1016/j.jmb.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnholz A, Hoefler C, Odenbreit S, Fischer W, Hofreuter D, Haas R. Functional and topological characterization of novel components of the comB DNA transformation competence system in Helicobacter pylori. J Bacteriol. 2006;188:882–893. doi: 10.1128/JB.188.3.882-893.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenjale R, Wilson J, Zenk SF, Saurya S, Picking WL, Picking WD, Blocker A. The needle component of the type III secreton of Shigella regulates the activity of the secretion apparatus. J Biol Chem. 2005;280:42929–42937. doi: 10.1074/jbc.M508377200. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Christie PJ. Evidence for VirB4-mediated dislocation of membrane-integrated VirB2 pilin during biogenesis of the Agrobacterium VirB/VirD4 type IV secretion system. J Bacteriol. 2010;192:4923–4934. doi: 10.1128/JB.00557-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser P, Elofsson M, Rosell S, Wolf-Watz H. Virulence blockers as alternatives to antibiotics: type III secretion inhibitors against Gram-negative bacteria. J Intern Med. 2008;264:17–29. doi: 10.1111/j.1365-2796.2008.01941.x. [DOI] [PubMed] [Google Scholar]
- Kirchner M, Heuer D, Meyer TF. CD46-independent binding of neisserial type IV pili and the major pilus adhesin, PilC, to human epithelial cells. Infect Immun. 2005;73:3072–3082. doi: 10.1128/IAI.73.5.3072-3082.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn TJ, Bose N, Taylor RK. Secretion of a soluble colonization factor by the TCP type 4 pilus biogenesis pathway in Vibrio cholerae. Mol Microbiol. 2003;49:81–92. doi: 10.1046/j.1365-2958.2003.03546.x. [DOI] [PubMed] [Google Scholar]
- Kirn TJ, Lafferty MJ, Sandoe CM, Taylor RK. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol Microbiol. 2000;35:896–910. doi: 10.1046/j.1365-2958.2000.01764.x. [DOI] [PubMed] [Google Scholar]
- Knutton S, Rosenshine I, Pallen MJ, et al. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korea CG, Badouraly R, Prevost MC, Ghigo JM, Beloin C. Escherichia coli K-12 possesses multiple cryptic but functional chaperone-usher fimbriae with distinct surface specificities. Environmental microbiology. 2010;12:1957–1977. doi: 10.1111/j.1462-2920.2010.02202.x. [DOI] [PubMed] [Google Scholar]
- Korotkov KV, Pardon E, Steyaert J, Hol WG. Crystal structure of the N-terminal domain of the secretin GspD from ETEC determined with the assistance of a nanobody. Structure. 2009;17:255–265. doi: 10.1016/j.str.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova N, Le Trong I, Samudrala R, et al. Crystal structure and mutational analysis of the DaaE adhesin of Escherichia coli. J Biol Chem. 2006;281:22367–22377. doi: 10.1074/jbc.M604646200. [DOI] [PubMed] [Google Scholar]
- Kubori T, Matsushima Y, Nakamura D, et al. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- Kuehn MJ, Heuser J, Normark S, Hultgren SJ. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature. 1992;356:252–255. doi: 10.1038/356252a0. [DOI] [PubMed] [Google Scholar]
- Kwok T, Zabler D, Urman S, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862–866. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- Lafont F, van der Goot FG. Oiling the key hole. Mol Microbiol. 2005;56:575–577. doi: 10.1111/j.1365-2958.2005.04570.x. [DOI] [PubMed] [Google Scholar]
- Lang S, Gruber CJ, Redzej A, Raffl S, Zellnig G, Zangger K, Zechner E. An activation domain of plasmid R1 TraI protein delineates stages of gene transfer initiation. Mol. Microbiol. 2011 doi: 10.1111/j.1365-2958.2011.07872.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Gruber K, Mihajlovic S, et al. Molecular recognition determinants for type IV secretion of diverse families of conjugative relaxases. Molecular Microbiology. 2010;78:1539–1555. doi: 10.1111/j.1365-2958.2010.07423.x. [DOI] [PubMed] [Google Scholar]
- Langermann S, Möllby R, Burlein JE, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherhichia coli. J. Infect. Dis. 2000;181:774–778. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- Lara-Tejero M, Galan JE. Salmonella enterica serovar typhimurium pathogenicity island 1-encoded type III secretion system translocases mediate intimate attachment to nonphagocytic cells. Infect Immun. 2009;77:2635–2642. doi: 10.1128/IAI.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley TD, Klimke WA, Gubbins MJ, Frost LS. F factor conjugation is a true type IV secretion system. FEMS Microbiol. Lett. 2003;224:1–15. doi: 10.1016/S0378-1097(03)00430-0. [DOI] [PubMed] [Google Scholar]
- Le Trong I, Aprikian P, Kidd BA, et al. Structural basis for mechanical force regulation of the adhesin FimH via finger trap-like beta sheet twisting. Cell. 2010;141:645–655. doi: 10.1016/j.cell.2010.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Sheth HB, Wong WY, et al. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol Microbiol. 1994;11:705–713. doi: 10.1111/j.1365-2958.1994.tb00348.x. [DOI] [PubMed] [Google Scholar]
- Lee PC, Stopford CM, Svenson AG, Rietsch A. Control of effector export by the Pseudomonas aeruginosa type III secretion proteins PcrG and PcrV. Mol Microbiol. 2010;75:924–941. doi: 10.1111/j.1365-2958.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lim MS, Li S, Brock M, Pique ME, Woods VL, Jr, Craig L. Vibrio cholerae toxin-coregulated pilus structure analyzed by hydrogen/deuterium exchange mass spectrometry. Structure. 2008;16:137–148. doi: 10.1016/j.str.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ng TW, Dodson KW, et al. The differential affinity of the usher for chaperone-subunit complexes is required for assembly of complete pili. Mol Microbiol. 2010;76:159–172. doi: 10.1111/j.1365-2958.2010.07089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Poole S, Rasulova F, McVeigh AL, Savarino SJ, Xia D. A Receptor-binding Site as Revealed by the Crystal Structure of CfaE, the Colonization Factor Antigen I Fimbrial Adhesin of Enterotoxigenic Escherichia coli. J Biol Chem. 2007;282:23970–23980. doi: 10.1074/jbc.M700921200. [DOI] [PubMed] [Google Scholar]
- Lim MS, Ng D, Zong S, Arvai AS, Taylor RK, Tainer JA, Craig L. Vibrio Cholerae El Tor Tcpa Crystal Structure and Mechanism for Pilus-Mediated Microcolony Formation. Mol Microbiol. 2010;77:755–770. doi: 10.1111/j.1365-2958.2010.07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Chen H, Galvan EM, Lasaro MA, Schifferli DM. Effects of Psa and F1 on the adhesive and invasive interactions of Yersinia pestis with human respiratory tract epithelial cells. Infect Immun. 2006;74:5636–5644. doi: 10.1128/IAI.00612-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llosa M, Roy C, Dehio C. Bacterial type IV secretion systems in human disease. Mol Microbiol. 2009 doi: 10.1111/j.1365-2958.2009.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D, Braaten B, van der Woude M. Fimbriae. In: Neidhardt FC, editor. Escherichia Coli and Salmonella; Cellular and Molecular Biology. Washington DC: ASM Press; 1996. pp. 146–157. [Google Scholar]
- Lu HM, Motley ST, Lory S. Interactions of the components of the general secretion pathway: role of Pseudomonas aeruginosa type IV pilin subunits in complex formation and extracellular protein secretion. Mol Microbiol. 1997;25:247–259. doi: 10.1046/j.1365-2958.1997.4561818.x. [DOI] [PubMed] [Google Scholar]
- Lu J, Frost LS. Mutations in the C-terminal region of TraM provide evidence for in vivo TraM-TraD interactions during F-plasmid conjugation. J Bacteriol. 2005;187:4767–4773. doi: 10.1128/JB.187.14.4767-4773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wong JJ, Edwards RA, Manchak J, Frost LS, Glover JN. Structural basis of specific TraD-TraM recognition during F plasmid-mediated bacterial conjugation. Mol Microbiol. 2008;70:89–99. doi: 10.1111/j.1365-2958.2008.06391.x. [DOI] [PubMed] [Google Scholar]
- Macdonald DL, Pasloske BL, Paranchych W. Mutations in the fifth-position glutamate in Pseudomonas aeruginosa pilin affect the transmethylation of the N-terminal phenylalanine. Can J Microbiol. 1993;39:500–505. doi: 10.1139/m93-071. [DOI] [PubMed] [Google Scholar]
- Macnab RM. Type III flagellar protein export and flagellar assembly. Biochim Biophys Acta. 2004;1694:207–217. doi: 10.1016/j.bbamcr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Maier B, Potter L, So M, Long CD, Seifert HS, Sheetz MP. Single pilus motor forces exceed 100 pN. Proc Natl Acad Sci U S A. 2002;99:16012–16017. doi: 10.1073/pnas.242523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairey E, Genovesio A, Donnadieu E, et al. Cerebral microcirculation shear stress levels determine Neisseria meningitidis attachment sites along the blood-brain barrier. J Exp Med. 2006;203:1939–1950. doi: 10.1084/jem.20060482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning PA. The tcp gene cluster of Vibrio cholerae. Gene. 1997;192:63–70. doi: 10.1016/s0378-1119(97)00036-x. [DOI] [PubMed] [Google Scholar]
- Marceau M, Beretti JL, Nassif X. High adhesiveness of encapsulated Neisseria meningitidis to epithelial cells is associated with the formation of bundles of pili. Mol Microbiol. 1995;17:855–863. doi: 10.1111/j.1365-2958.1995.mmi_17050855.x. [DOI] [PubMed] [Google Scholar]
- Marlovits TC, Stebbins CE. Type III secretion systems shape up as they ship out. Curr Opin Microbiol. 2010;13:47–52. doi: 10.1016/j.mib.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero J, Waldor MK. Determinants of entry exclusion within Eex and TraG are cytoplasmic. J Bacteriol. 2007;189:6469–6473. doi: 10.1128/JB.00522-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs CF, Schoolnik G, Koomey JM, Hardy J, Rothbard J, Falkow S. Cloning and sequencing of aMoraxella bovis pilin gene. J. Bact. 1985;163:132–139. doi: 10.1128/jb.163.1.132-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000;19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Argudo I, Blocker AJ. The Shigella T3SS needle transmits a signal for MxiC release, which controls secretion of effectors. Mol Microbiol. 2010;78:1365–1378. doi: 10.1111/j.1365-2958.2010.07413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson JS, Durick KA, Bradley DS, Nilles ML. Immunization of mice with YscF provides protection from Yersinia pestis infections. BMC Microbiol. 2005;5:38. doi: 10.1186/1471-2180-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei PJ, Faudry E, Job V, Izore T, Attree I, Dessen A. Membrane targeting and pore formation by the type III secretion system translocon. FEBS J. 2011;278:414–426. doi: 10.1111/j.1742-4658.2010.07974.x. [DOI] [PubMed] [Google Scholar]
- Mattick JS. Type IV pili and twitching motility. Annu Rev Microbiol. 2002;56:289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- Mattick JS, Whitchurch CB, Alm RA. The molecular genetics of type-4 fimbriae in Pseudomonas aeruginosa--a review. Gene. 1996;179:147–155. doi: 10.1016/s0378-1119(96)00441-6. [DOI] [PubMed] [Google Scholar]
- McGhie EJ, Hume PJ, Hayward RD, Torres J, Koronakis V. Topology of the Salmonella invasion protein SipB in a model bilayer. Mol. Microbiol. 2002;44:1309–1321. doi: 10.1046/j.1365-2958.2002.02958.x. [DOI] [PubMed] [Google Scholar]
- Merckel MC, Tanskanen J, Edelman S, Westerlund-Wikstrom B, Korhonen TK, Goldman A. The structural basis of receptor-binding by Escherichia coli associated with diarrhea and septicemia. J Mol Biol. 2003;331:897–905. doi: 10.1016/s0022-2836(03)00841-6. [DOI] [PubMed] [Google Scholar]
- Merz AJ, So M. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu Rev Cell Dev Biol. 2000;16:423–457. doi: 10.1146/annurev.cellbio.16.1.423. [DOI] [PubMed] [Google Scholar]
- Merz AJ, Enns CA, So M. Type IV pili of pathogenic Neisseriae elicit cortical plaque formation in epithelial cells. Mol Microbiol. 1999;32:1316–1332. doi: 10.1046/j.1365-2958.1999.01459.x. [DOI] [PubMed] [Google Scholar]
- Merz AJ, So M, Sheetz MP. Pilus retraction powers bacterial twitching motility. Nature. 2000;407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- Mikaty G, Soyer M, Mairey E, et al. Extracellular bacterial pathogen induces host cell surface reorganization to resist shear stress. PLoS Pathog. 2009;5:e1000314. doi: 10.1371/journal.ppat.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Garcia T, Hultgren S, Oberhauser AF. The mechanical properties of E. coli type 1 pili measured by atomic force microscopy techniques. Biophys J. 2006;91:3848–3856. doi: 10.1529/biophysj.106.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, Williamson ED, Lakey JH, Pearce MJ, Jones SM, Titball RW. Macromolecular organisation of recombinant Yersinia pestis F1 antigen and the effect of structure on immunogenicity. FEMS Immunol Med Microbiol. 1998;21:213–221. doi: 10.1111/j.1574-695X.1998.tb01168.x. [DOI] [PubMed] [Google Scholar]
- Mills JA, Buysse JM, Oaks EV. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect. Immun. 1988;56:2933–2941. doi: 10.1128/iai.56.11.2933-2941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misic AM, Satyshur KA, Forest KT. P. aeruginosa PilT structures with and without nucleotide reveal a dynamic type IV pilus retraction motor. Journal of Molecular Biology. 2010;400:1011–1021. doi: 10.1016/j.jmb.2010.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misselwitz B, Kreibich SK, Rout S, Stecher B, Periaswamy B, Hardt WD. Salmonella enterica serovar Typhimurium binds to HeLa cells via Fim-mediated reversible adhesion and irreversible type three secretion system 1-mediated docking. Infect Immun. 2011;79:330–341. doi: 10.1128/IAI.00581-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand PC, Bille E, Morelle S, et al. Type IV pilus retraction in pathogenic Neisseria is regulated by the PilC proteins. EMBO J. 2004;23:2009–2017. doi: 10.1038/sj.emboj.7600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossey P, Hudacek A, Das A. Agrobacterium tumefaciens type IV secretion protein VirB3 is an inner membrane protein and requires VirB4, VirB7, and VirB8 for stabilization. Journal of Bacteriology. 2010;192:2830–2838. doi: 10.1128/JB.01331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu XQ, Bullitt E. Structure and assembly of P-pili: a protruding hinge region used for assembly of a bacterial adhesion filament. Proc Natl Acad Sci U S A. 2006;103:9861–9866. doi: 10.1073/pnas.0509620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller CA, Broz P, Cornelis GR. The type III secretion system tip complex and translocon. Mol Microbiol. 2008;68:1085–1095. doi: 10.1111/j.1365-2958.2008.06237.x. [DOI] [PubMed] [Google Scholar]
- Mueller CA, Broz P, Muller SA, et al. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science. 2005;310:674–676. doi: 10.1126/science.1118476. [DOI] [PubMed] [Google Scholar]
- Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci USA. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Kubori T. Type IVB Secretion Systems of Legionella and Other Gram-Negative Bacteria. Front Microbiol. 2011;2:136. doi: 10.3389/fmicb.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Cambronne ED, Kagan JC, Amor JC, Kahn RA, Roy CR. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc Natl Acad Sci U S A. 2005;102:826–831. doi: 10.1073/pnas.0406239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano N, Kubori T, Kinoshita M, Imada K, Nagai H. Crystal structure of Legionella DotD: insights into the relationship between type IVB and type II/III secretion systems. PLoS pathogens. 2010;6:e1001129. doi: 10.1371/journal.ppat.1001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif X, Beretti JL, Lowy J, et al. Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proc Natl Acad Sci USA. 1994;91:3769–3773. doi: 10.1073/pnas.91.9.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, Ishikawa T, Rechsteiner H, Glockshuber R. Reconstitution of Pilus Assembly Reveals a Bacterial Outer Membrane Catalyst. Science. 2008;320:376–379. doi: 10.1126/science.1154994. [DOI] [PubMed] [Google Scholar]
- Noel BL, Lilo S, Capurso D, Hill J, Bliska JB. Yersinia pestis can bypass protective antibodies to LcrV and activation with gamma interferon to survive and induce apoptosis in murine macrophages. Clin Vaccine Immunol. 2009;16:1457–1466. doi: 10.1128/CVI.00172-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccio SP, Bäumler AJ. Evolution of the chaperone/usher assembly pathway: fimbrial classification goes Greek. Microbiol Mol Biol Rev. 2007;71:551–575. doi: 10.1128/MMBR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudleman E, Kaiser D. Pulling together with type IV pili. J Mol Microbiol Biotechnol. 2004;7:52–62. doi: 10.1159/000077869. [DOI] [PubMed] [Google Scholar]
- O'Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- Olive AJ, Kenjale R, Espina M, Moore DS, Picking WL, Picking WD. Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect Immun. 2007;75:2626–2629. doi: 10.1128/IAI.01599-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orans J, Johnson MD, Coggan KA, Sperlazza JR, Heiniger RW, Wolfgang MC, Redinbo MR. Crystal structure analysis reveals Pseudomonas PilY1 as an essential calcium-dependent regulator of bacterial surface motility. Proc Natl Acad Sci U S A. 2010;107:1065–1070. doi: 10.1073/pnas.0911616107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I, Orskov F. Serologic classification of fimbriae. Curr. Top. Microbiol. Immunol. 1990;151:71–90. [PubMed] [Google Scholar]
- Ottow JCG. Ecology, physiology and genetics of fimbriae and pili. Annual Review of Microbiology. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Parge HE, Forest KT, Hickey MJ, Christensen DA, Getzoff ED, Tainer JA. Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature. 1995;378:32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- Pasloske BL, Scraba DG, Paranchych W. Assembly of mutant pilins in Pseudomonas aeruginosa: formation of pili composed of heterologous subunits. J Bacteriol. 1989;171:2142–2147. doi: 10.1128/jb.171.4.2142-2147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody CR, Chung YJ, Yen MR, Vidal-Ingigliardi D, Pugsley AP, Saier MH., Jr Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology. 2003;149:3051–3072. doi: 10.1099/mic.0.26364-0. [DOI] [PubMed] [Google Scholar]
- Pelicic V. Type IV pili: e pluribus unum? Mol Microbiol. 2008;68:827–837. doi: 10.1111/j.1365-2958.2008.06197.x. [DOI] [PubMed] [Google Scholar]
- Pettersson J, Holmstrom A, Hill J, et al. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol Microbiol. 1999;32:961–976. doi: 10.1046/j.1365-2958.1999.01408.x. [DOI] [PubMed] [Google Scholar]
- Pettigrew D, Anderson KL, Billington J, et al. High resolution studies of the Afa/Dr adhesin DraE and its interaction with chloramphenicol. J Biol Chem. 2004;279:46851–46857. doi: 10.1074/jbc.M409284200. [DOI] [PubMed] [Google Scholar]
- Phan G, Remaut H, Wang T, et al. Crystal structure of the FimD usher bound to its cognate FimC-FimH substrate. Nature. 2011;474:49–53. doi: 10.1038/nature10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkner JS, Remaut H, Buelens F, et al. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc Natl Acad Sci U S A. 2006;103:17897–17902. doi: 10.1073/pnas.0606795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planet PJ, Kachlany SC, DeSalle R, Figurski DH. Phylogeny of genes for secretion NTPases: identification of the widespread tadA subfamily and development of a diagnostic key for gene classification. Proc Natl Acad Sci U S A. 2001;98:2503–2508. doi: 10.1073/pnas.051436598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlschroder M, Ghosh A, Tripepi M, Albers SV. Archaeal type IV pilus-like structures-evolutionarily conserved prokaryotic surface organelles. Curr Opin Microbiol. 2011;14:357–363. doi: 10.1016/j.mib.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Poole ST, McVeigh AL, Anantha RP, et al. Donor strand complementation governs intersubunit interaction of fimbriae of the alternate chaperone pathway. Mol Microbiol. 2007;63:1372–1384. doi: 10.1111/j.1365-2958.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- Pron B, Taha MK, Rambaud C, et al. Interaction of Neisseria maningitidis with the components of the blood-brain barrier correlates with an increased expression of PilC. The Journal of infectious diseases. 1997;176:1285–1292. doi: 10.1086/514124. [DOI] [PubMed] [Google Scholar]
- Pujol C, Eugene E, Marceau M, Nassif X. The meningococcal PilT protein is required for induction of intimate attachment to epithelial cells following pilus-mediated adhesion. Proc Natl Acad Sci USA. 1999;96:4017–4022. doi: 10.1073/pnas.96.7.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenee LE, Berube BJ, Segal J, Elli D, Ciletti NA, Anderson D, Schneewind O. Amino acid residues 196–225 of LcrV represent a plague protective epitope. Vaccine. 2010;28:1870–1876. doi: 10.1016/j.vaccine.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakotoarivonina H, Jubelin G, Hebraud M, Gaillard-Martinie B, Forano E, Mosoni P. Adhesion to cellulose of the Gram-positive bacterium Ruminococcus albus involves type IV pili. Microbiology. 2002;148:1871–1880. doi: 10.1099/00221287-148-6-1871. [DOI] [PubMed] [Google Scholar]
- Ramboarina S, Fernandes PJ, Daniell S, et al. Structure of the bundle-forming pilus from enteropathogenic Escherichia coli. J Biol Chem. 2005;280:40252–40260. doi: 10.1074/jbc.M508099200. [DOI] [PubMed] [Google Scholar]
- Ramsey ME, Woodhams KL, Dillard JP. The Gonococcal Genetic Island and Type IV Secretion in the Pathogenic Neisseria. Frontiers in microbiology. 2011;2:61. doi: 10.3389/fmicb.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichow SL, Korotkov KV, Hol WG, Gonen T. Structure of the cholera toxin secretion channel in its closed state. Nature structural & molecular biology. 17:1226–1232. doi: 10.1038/nsmb.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichow SL, Korotkov KV, Hol WG, Gonen T. Structure of the cholera toxin secretion channel in its closed state. Nature structural & molecular biology. 2010;17:1226–1232. doi: 10.1038/nsmb.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut H, Tang C, Henderson NS, et al. Fiber Formation across the Bacterial Outer Membrane by the Chaperone/Usher Pathway. Cell. 2008;133:640–652. doi: 10.1016/j.cell.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Marklund B-I, Ilver D, et al. The Galα(1–4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl. Acad. Sci. USA. 1994;91:11889–11893. doi: 10.1073/pnas.91.25.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrich AD, Martinez-Argudo I, Johnson S, Blocker AJ, Veenendaal AK. The extreme C terminus of Shigella flexneri IpaB is required for regulation of type III secretion, needle tip composition, and binding. Infect Immun. 2010;78:1682–1691. doi: 10.1128/IAI.00645-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel T, Scheuerpflug I, Meyer TF. Neisseria PilC protein identified as type 4 pilus tip-located adhesin. Nature. 1995;373:357–359. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- Rudel T, Boxberger HJ, Meyer TF. Pilus biogenesis and epithelial cell adherence of Neisseria gonorrhoeaepilC double knock-out mutants. Mol Microbiol. 1995;17:1057–1071. doi: 10.1111/j.1365-2958.1995.mmi_17061057.x. [DOI] [PubMed] [Google Scholar]
- Runco LM, Myrczek S, Bliska JB, Thanassi DG. Biogenesis of the fraction 1 capsule and analysis of the ultrastructure of Yersinia pestis. J Bacteriol. 2008;190:3381–3385. doi: 10.1128/JB.01840-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakellaris H, Scott JR. New tools in an old trade: CS1 pilus morphogenesis. Mol Microbiol. 1998;30:681–687. doi: 10.1046/j.1365-2958.1998.01088.x. [DOI] [PubMed] [Google Scholar]
- Salminen A, Loimaranta V, Joosten JA, Khan AS, Hacker J, Pieters RJ, Finne J. Inhibition of P-fimbriated Escherichia coli adhesion by multivalent galabiose derivatives studied by a live-bacteria application of surface plasmon resonance. J Antimicrob Chemother. 2007;60:495–501. doi: 10.1093/jac/dkm251. [DOI] [PubMed] [Google Scholar]
- Samuels AL, Lanka E, Davies JE. Conjugative junctions in RP4-mediated mating of Escherichia coli. J. Bacteriol. 2000;182:2709–2715. doi: 10.1128/jb.182.10.2709-2715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sani M, Botteaux A, Parsot C, Sansonetti P, Boekema EJ, Allaoui A. IpaD is localized at the tip of the Shigella flexneri type III secretion apparatus. Biochim Biophys Acta. 2007;1770:307–311. doi: 10.1016/j.bbagen.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Sato H, Frank DW. Multi-Functional Characteristics of the Pseudomonas aeruginosa Type III Needle-Tip Protein, PcrV; Comparison to Orthologs in other Gram-negative Bacteria. Front Microbiol. 2011;2:142. doi: 10.3389/fmicb.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Hunt ML, Weiner JJ, Hansen AT, Frank DW. Modified needle-tip PcrV proteins reveal distinct phenotypes relevant to the control of type III secretion and intoxication by Pseudomonas aeruginosa. PLoS One. 2011;6:e18356. doi: 10.1371/journal.pone.0018356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyshur KA, Worzalla GA, Meyer LS, Heiniger EK, Aukema KG, Misic AM, Forest KT. Crystal structures of the pilus retraction motor PilT suggest large domain movements and subunit cooperation drive motility. Structure. 2007;15:363–376. doi: 10.1016/j.str.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer FG, Pinkner JS, Waksman G, Hultgren SJ. Chaperone priming of pilus subunits facilitates a topological transition that drives fiber formation. Cell. 2002;111:543–551. doi: 10.1016/s0092-8674(02)01050-4. [DOI] [PubMed] [Google Scholar]
- Sauer FG, Fütterer K, Pinkner JS, Dodson KW, Hultgren SJ, Waksman G. Structural basis of chaperone function and pilus biogenesis. Science. 1999;285:1058–1061. doi: 10.1126/science.285.5430.1058. [DOI] [PubMed] [Google Scholar]
- Sauvonnet N, Vignon G, Pugsley AP, Gounon P. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J. 2000;19:2221–2228. doi: 10.1093/emboj/19.10.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvides SN. Secretion superfamily ATPases swing big. Structure. 2007;15:255–257. doi: 10.1016/j.str.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Savvides SN, Yeo HJ, Beck MR, et al. VirB11 ATPases are dynamic hexameric assemblies: new insights into bacterial type IV secretion. EMBO J. 2003;22:1969–1980. doi: 10.1093/emboj/cdg223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa T, Yahr TL, Ohara M, Kurahashi K, Gropper MA, Wiener-Kronish JP, Frank DW. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med. 1999;5:392–398. doi: 10.1038/7391. [DOI] [PubMed] [Google Scholar]
- Scheuerpflug I, Rudel T, Ryll R, Pandit J, Meyer TF. Roles of PilC and PilE proteins in pilus-mediated adherence of Neisseria gonorrhoeae and Neisseria meningitidis to human erythrocytes and endothelial and epithelial cells. Infect Immun. 1999;67:834–843. doi: 10.1128/iai.67.2.834-843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder TH, Zaidi T, Pier GB. Lack of adherence of clinical isolates of Pseudomonas aeruginosa to asialo-GM(1) on epithelial cells. Infect Immun. 2001;69:719–729. doi: 10.1128/IAI.69.2.719-729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulein R, Guye P, Rhomberg TA, et al. A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:856–861. doi: 10.1073/pnas.0406796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbane F, Jarrett C, Gardner D, Long D, Hinnebusch BJ. The Yersinia pestis caf1M1A1 fimbrial capsule operon promotes transmission by flea bite in a mouse model of bubonic plague. Infect Immun. 2009;77:1222–1229. doi: 10.1128/IAI.00950-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya K, Ohishi M, Ogino T, Tamano K, Sasakawa C, Abe A. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc Natl Acad Sci U S A. 2001;98:11638–11643. doi: 10.1073/pnas.191378598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhnovich EA, Hung DT, Pierson E, Lee K, Mekalanos JJ. Virstatin inhibits dimerization of the transcriptional activator ToxT. Proc Natl Acad Sci U S A. 2007;104:2372–2377. doi: 10.1073/pnas.0611643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DK, Saurya S, Wagner C, Nishioka H, Blocker AJ. Domains of the Shigella flexneri type III secretion system IpaB protein involved in secretion regulation. Infect Immun. 2010;78:4999–5010. doi: 10.1128/IAI.00470-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerker JM, Berg HC. Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci USA. 2001;98:6901–6904. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohel I, Puente JL, Ramer SW, Bieber D, Wu CY, Schoolnik GK. Enteropathogenic Escherichia coli: identification of a gene cluster coding for bundle-forming pilus morphogenesis. J Bacteriol. 1996;178:2613–2628. doi: 10.1128/jb.178.9.2613-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza DP, Andrade MO, Alvarez-Martinez CE, Arantes GM, Farah CS, Salinas RK. A component of the Xanthomonadaceae type IV secretion system combines a VirB7 motif with a N0 domain found in outer membrane transport proteins. PLoS Pathog. 2011;7:e1002031. doi: 10.1371/journal.ppat.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreter T, Yip CK, Sanowar S, et al. A conserved structural motif mediates formation of the periplasmic rings in the type III secretion system. Nature structural & molecular biology. 2009;16:468–476. doi: 10.1038/nsmb.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich GM, Fernandez D, Zhou XR, Christie PJ. Intermolecular disulfide bonds stabilize VirB7 homodimers and VirB7/VirB9 heterodimers during biogenesis of the Agrobacterium tumefaciens T-complex transport apparatus. Proc. Natl. Acad. Sci. U.S.A. 1996;93:7512–7517. doi: 10.1073/pnas.93.15.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensrud KF, Adam PR, La Mar CD, et al. Deoxycholate interacts with IpaD of Shigella flexneri in inducing the recruitment of IpaB to the type III secretion apparatus needle tip. J Biol Chem. 2008;283:18646–18654. doi: 10.1074/jbc.M802799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimson E, Virji M, Barker S, et al. Discovery of a novel protein modification: alpha-glycerophosphate is a substituent of meningococcal pilin. Biochem J. 1996;316:29–33. doi: 10.1042/bj3160029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KD, Zhang H-Z, Carlson LK, Donnenberg MS. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for the biogenesis of a type IV pilus. Mol. Microbiol. 1996;20:325–337. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- Strom MS, Lory S. Amino acid substitutions in pilin of Pseudomonas aeruginosa. Effect on leader peptide cleavage, amino-terminal methylation, and pilus assembly. J Biol Chem. 1991;266:1656–1664. [PubMed] [Google Scholar]
- Strom MS, Lory S. Structure-function and biogenesis of type IV pili. Annu. Rev. Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- Strom MS, Nunn DN, Lory S. A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proceeding of the National Academy of Sciences. 1993;90:2404. doi: 10.1073/pnas.90.6.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Zusman DR, Shi W. Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr Biol. 2000;10:1143–1146. doi: 10.1016/s0960-9822(00)00705-3. [DOI] [PubMed] [Google Scholar]
- Sung MA, Fleming K, Chen HA, Matthews S. The solution structure of PapGII from uropathogenic Escherichia coli and its recognition of glycolipid receptors. EMBO reports. 2001;2:621–627. doi: 10.1093/embo-reports/kve133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietnicki W, Powell BS, Goodin J. Yersinia pestis Yop secretion protein F: purification, characterization, and protective efficacy against bubonic plague. Protein Expr Purif. 2005;42:166–172. doi: 10.1016/j.pep.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Tacket CO, Taylor RK, Losonsky G, Lim Y, Nataro JP, Kaper JB, Levine MM. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect Immun. 1998;66:692–695. doi: 10.1128/iai.66.2.692-695.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tato I, Zunzunegui S, de la Cruz F, Cabezon E. TrwB, the coupling protein involved in DNA transport during bacterial conjugation, is a DNA dependent ATPase. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8156–8161. doi: 10.1073/pnas.0503402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tato I, Matilla I, Arechaga I, Zunzunegui S, de la Cruz F, Cabezon E. The ATPase activity of the DNA transporter TrwB is modulated by protein TrwA: implications for a common assembly mechanism of DNA translocating motors. J Biol Chem. 2007;282:25569–25576. doi: 10.1074/jbc.M703464200. [DOI] [PubMed] [Google Scholar]
- Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchesnokova V, Aprikian P, Kisiela D, Gowey S, Korotkova N, Thomas W, Sokurenko E. Type 1 fimbrial adhesin FimH elicits an immune response that enhances cell adhesion of Escherichia coli. Infect Immun. 2011;79:3895–3904. doi: 10.1128/IAI.05169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer N, Wessler S, Backert S. Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis. FEBS J. 2011;278:1190–1202. doi: 10.1111/j.1742-4658.2011.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer N, Hartig R, Delahay RM, et al. A small fibronectin-mimicking protein from bacteria induces cell spreading and focal adhesion formation. J Biol Chem. 2010;285:23515–23526. doi: 10.1074/jbc.M109.096214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terradot L, Bayliss R, Oomen C, Leonard, Baron C, Waksman G. Structures of two core subunits of the bacterial type IV secretion system, VirB8 from Brucella suis and ComB10 from Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:4956–4961. doi: 10.1073/pnas.0408927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanassi DG, Nuccio S-P, Shu Kin So S, Bäumler AJ. Fimbriae: Classification and Biochemistry. In: Bôck A, Curtiss R III, Kaper JB, Neidhardt FC, Nyström T, Rudd KE, Squires CL, editors. EcoSal–Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 2007. Vol. [Online] http://www.ecosal.org. [DOI] [PubMed] [Google Scholar]
- Thomas WE, Trintchina E, Forero M, Vogel V, Sokurenko EV. Bacterial adhesion to target cells enhanced by shear force. Cell. 2002;109:913–923. doi: 10.1016/s0092-8674(02)00796-1. [DOI] [PubMed] [Google Scholar]
- Titball RW, Williamson ED. Vaccination against bubonic and pneumonic plague. Vaccine. 2001;19:4175–4184. doi: 10.1016/s0264-410x(01)00163-3. [DOI] [PubMed] [Google Scholar]
- Tobiason DM, Seifert HS. Inverse relationship between pilus-mediated gonococcal adherence and surface expression of the pilus receptor, CD46. Microbiology. 2001;147:2333–2340. doi: 10.1099/00221287-147-8-2333. [DOI] [PubMed] [Google Scholar]
- Tonjum T, Koomey M. The pilus colonization factor of pathogenic neisserial species: organelle biogenesis and structure/function relationships--a review. Gene. 1997;192:155–163. doi: 10.1016/s0378-1119(97)00018-8. [DOI] [PubMed] [Google Scholar]
- Tonjum T, Freitag NE, Namork E, Koomey M. Identification and characterization of pilG, a highly conserved pilus-assembly gene in pathogenic Neisseria. Mol Microbiol. 1995;16:451–464. doi: 10.1111/j.1365-2958.1995.tb02410.x. [DOI] [PubMed] [Google Scholar]
- Turbyfill KR, Mertz JA, Mallett CP, Oaks EV. Identification of epitope and surface-exposed domains of Shigella flexneri invasion plasmid antigen D (IpaD) Infect Immun. 1998;66:1999–2006. doi: 10.1128/iai.66.5.1999-2006.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden AW, Bäumler AJ, Tsolis RM, Heffron F. Multiple fimbrial adhesins are required for full virulence of Salmonella typhimurium in mice. Infect Immun. 1998;66:2803–2808. doi: 10.1128/iai.66.6.2803-2808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Woude M, Braaten B, Low D. Epigenetic phase variation of the pap operon in Escherichia coli. Trends Microbiol. 1996;4:5–9. doi: 10.1016/0966-842x(96)81498-3. [DOI] [PubMed] [Google Scholar]
- Varga JJ, Nguyen V, O'Brien DK, Rodgers K, Walker RA, Melville SB. Type IV pili-dependent gliding motility in the Gram-positive pathogen Clostridium perfringens and other Clostridia. Mol Microbiol. 2006;62:680–694. doi: 10.1111/j.1365-2958.2006.05414.x. [DOI] [PubMed] [Google Scholar]
- Veenendaal AK, Hodgkinson JL, Schwarzer L, Stabat D, Zenk SF, Blocker AJ. The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol Microbiol. 2007;63:1719–1730. doi: 10.1111/j.1365-2958.2007.05620.x. [DOI] [PubMed] [Google Scholar]
- Verger D, Miller E, Remaut H, Waksman G, Hultgren S. Molecular mechanism of P pilus termination in uropathogenic Escherichia coli. EMBO reports. 2006;7:1228–1232. doi: 10.1038/sj.embor.7400833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergunst AC, van Lier MC, den Dulk-Ras A, Grosse Stuve TA, Ouwehand A, Hooykaas PJ. Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. Proc Natl Acad Sci U S A. 2005;102:832–837. doi: 10.1073/pnas.0406241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernazza C, Lingard B, Flick-Smith HC, Baillie LW, Hill J, Atkins HS. Small protective fragments of the Yersinia pestis V antigen. Vaccine. 2009;27:2775–2780. doi: 10.1016/j.vaccine.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Virji M, Makepeace K, Ferguson DJ, Achtman M, Moxon ER. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol Microbiol. 1993;10:499–510. doi: 10.1111/j.1365-2958.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]
- Waksman G, Hultgren SJ. Structural biology of the chaperone-usher pathway of pilus biogenesis. Nat Rev Microbiol. 2009;7:765–774. doi: 10.1038/nrmicro2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang L, Picking WL, Picking WD, De Guzman RN. Structural dissection of the extracellular moieties of the type III secretion apparatus. Mol Biosyst. 2008;4:1176–1180. doi: 10.1039/b808271p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YA, Yu X, Silverman PM, Harris RL, Egelman EH. The structure of F-pili. J Mol Biol. 2009;385:22–29. doi: 10.1016/j.jmb.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MJ, Jennings MP. Identification and characterization of pptA: a gene involved in the phase-variable expression of phosphorylcholine on pili of Neisseria meningitidis. Infect Immun. 2003;71:6892–6898. doi: 10.1128/IAI.71.12.6892-6898.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks S, Hill J, Friedlander A, Welkos S. Anti-V antigen antibody protects macrophages from Yersinia pestis -induced cell death and promotes phagocytosis. Microb Pathog. 2002;32:227–237. doi: 10.1006/mpat.2002.0498. [DOI] [PubMed] [Google Scholar]
- Weening EH, Cathelyn JS, Kaufman G, Lawrenz MB, Price P, Goldman WE, Miller VL. The dependence of the Yersinia pestis capsule on pathogenesis is influenced by the mouse background. Infect Immun. 2011;79:644–652. doi: 10.1128/IAI.00981-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellens A, Garofalo C, Nguyen H, et al. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS One. 2008;3:e2040. doi: 10.1371/journal.pone.0002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund-Wikstrom B, Korhonen TK. Molecular structure of adhesin domains in Escherichia coli fimbriae. Int J Med Microbiol. 2005;295:479–486. doi: 10.1016/j.ijmm.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Winans SC, Walker GC. Conjugal transfer system of the IncN plasmid pKM101. J. Bacteriol. 1985;161:402–410. doi: 10.1128/jb.161.1.402-410.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther-Larsen HC, Wolfgang M, Dunham S, et al. A conserved set of pilin-like molecules controls type IV pilus dynamics and organelle-associated functions in Neisseria gonorrhoeae. Mol Microbiol. 2005;56:903–917. doi: 10.1111/j.1365-2958.2005.04591.x. [DOI] [PubMed] [Google Scholar]
- Wolfgang M, van Putten JP, Hayes SF, Dorward D, Koomey M. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 2000;19:6408–6418. doi: 10.1093/emboj/19.23.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall LJ, Lameignere E, Strynadka NC. Structural overview of the bacterial injectisome. Curr Opin Microbiol. 2011;14:3–8. doi: 10.1016/j.mib.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol. 2007;9:2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- Xia Y, Gally D, Forsman-Semb K, Uhlin BE. Regulatory cross-talk between adhesin operons in Escherichia coli: inhibition of type 1 fimbriae expression by the PapB operon. EMBO J. 2000;19:1450–1457. doi: 10.1093/emboj/19.7.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XF, Tan YW, Lam L, Hackett J, Zhang M, Mok YK. NMR structure of a type IVb pilin from Salmonella typhi and its assembly into pilus. J Biol Chem. 2004;279:31599–31605. doi: 10.1074/jbc.M404727200. [DOI] [PubMed] [Google Scholar]
- Yakovenko O, Sharma S, Forero M, et al. FimH forms catch bonds that are enhanced by mechanical force due to allosteric regulation. J Biol Chem. 2008;283:11596–11605. doi: 10.1074/jbc.M707815200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen MR, Peabody CR, Partovi SM, Zhai Y, Tseng YH, Saier MH. Protein-translocating outer membrane porins of Gram-negative bacteria. Biochim Biophys Acta. 2002;1562:6–31. doi: 10.1016/s0005-2736(02)00359-0. [DOI] [PubMed] [Google Scholar]
- Yeo H-J, Yuan Q, Beck MR, Baron C, Waksman G. Structural and functional characterization of the VirB5 protein from the type IV secretion system encoded by the conjugative plasmid pKM101. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15947–15952. doi: 10.1073/pnas.2535211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo HJ, Savvides SN, Herr AB, Lanka E, Waksman G. Crystal structure of the hexameric traffic ATPase of the Helicobacter pylori type IV secretion system. Mol Cell. 2000;6:1461–1472. doi: 10.1016/s1097-2765(00)00142-8. [DOI] [PubMed] [Google Scholar]
- Yip CK, Finlay BB, Strynadka NC. Structural characterization of a type III secretion system filament protein in complex with its chaperone. Nat Struct Mol Biol. 2005;12:75–81. doi: 10.1038/nsmb879. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Furuya N, Ishikura M, Isobe T, Haino-Fukushima K, Ogawa T, Komano T. Purification and characterization of thin pili of IncI1 plasmids ColIb-P9 and R64: formation of PilV-specific cell aggregates by type IV pili. J Bacteriol. 1998;180:2842–2848. doi: 10.1128/jb.180.11.2842-2848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Carle A, Gao C, et al. Identification of the VirB4-VirB8-VirB5-VirB2 pilus assembly sequence of type IV secretion systems. J Biol Chem. 2005;280:26349–26359. doi: 10.1074/jbc.M502347200. [DOI] [PubMed] [Google Scholar]
- Zahavi EE, Lieberman JA, Donnenberg MS, et al. Bundle forming pilus retraction enhances enteropathogenic Escherichia coli infectivity. Molec Biol Cell. 2011;22:2436–2447. doi: 10.1091/mbc.E11-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zav'yalov V, Zavialov A, Zav'yalova G, Korpela T. Adhesive organelles of Gram-negative pathogens assembled with the classical chaperone/usher machinery: structure and function from a clinical standpoint. FEMS Microbiol Rev. 2010;34:317–378. doi: 10.1111/j.1574-6976.2009.00201.x. [DOI] [PubMed] [Google Scholar]
- Zavialov A, Zav'yalova G, Korpela T, Zav'yalov V. FGL chaperone-assembled fimbrial polyadhesins: anti-immune armament of Gram-negative bacterial pathogens. FEMS Microbiol Rev. 2007;31:478–514. doi: 10.1111/j.1574-6976.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- Zavialov AV, Berglund J, Pudney AF, Fooks LJ, Ibrahim TM, MacIntyre S, Knight SD. Structure and biogenesis of the capsular F1 antigen from Yersinia pestis: preserved folding energy drives fiber formation. Cell. 2003;113:587–596. doi: 10.1016/s0092-8674(03)00351-9. [DOI] [PubMed] [Google Scholar]
- Zhang HZ, Donnenberg MS. DsbA is required for stability of the type IV pilin of enteropathogenic escherichia coli. Mol Microbiol. 1996;21:787–797. doi: 10.1046/j.1365-2958.1996.431403.x. [DOI] [PubMed] [Google Scholar]
- Zhang HZ, Lory S, Donnenberg MS. A plasmid-encoded prepilin peptidase gene from enteropathogenic Escherichia coli. J Bacteriol. 1994;176:6885–6891. doi: 10.1128/jb.176.22.6885-6891.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, Tsui IS, Yip CM, et al. Salmonella enterica serovar typhi uses type IVB pili to enter human intestinal epithelial cells. Infect Immun. 2000;68:3067–3073. doi: 10.1128/iai.68.6.3067-3073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Mo WJ, Sebbel P, et al. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. Journal of cell science. 2001;114:4095–4103. doi: 10.1242/jcs.114.22.4095. [DOI] [PubMed] [Google Scholar]