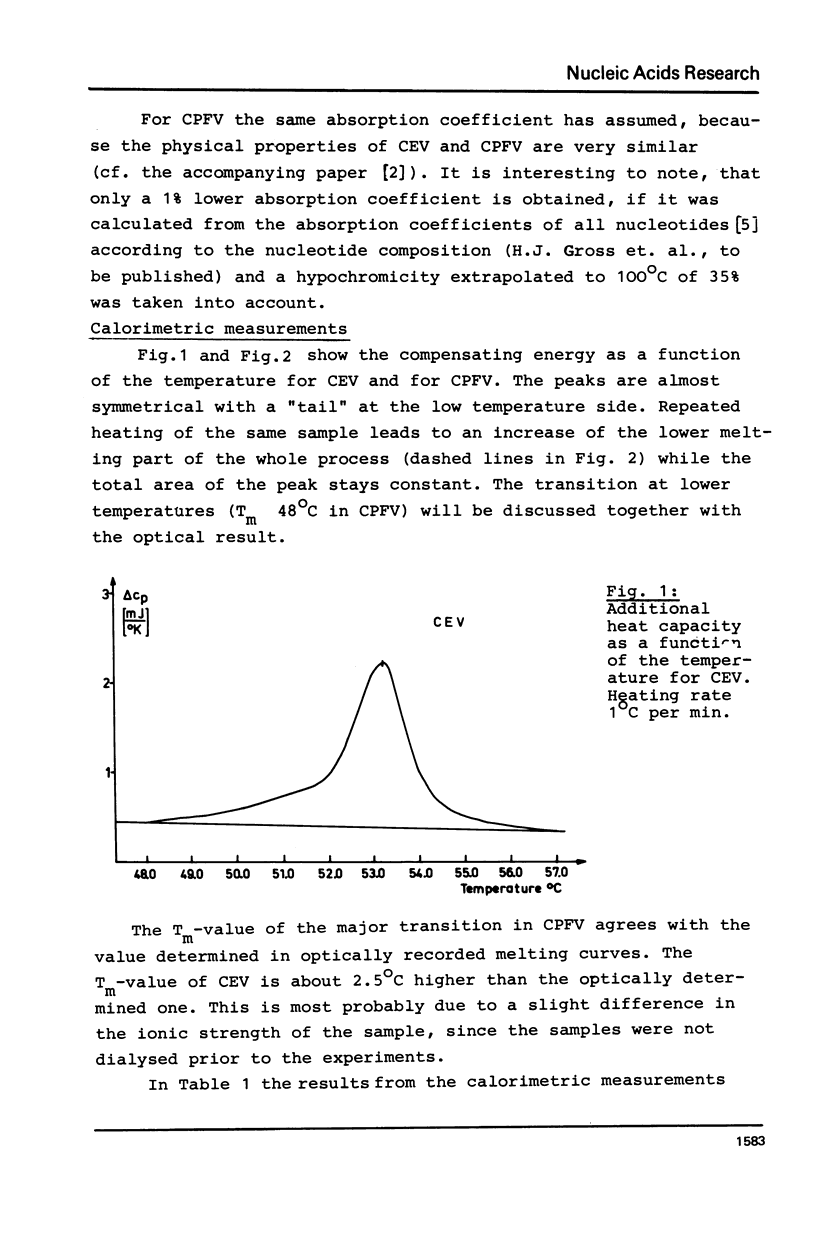
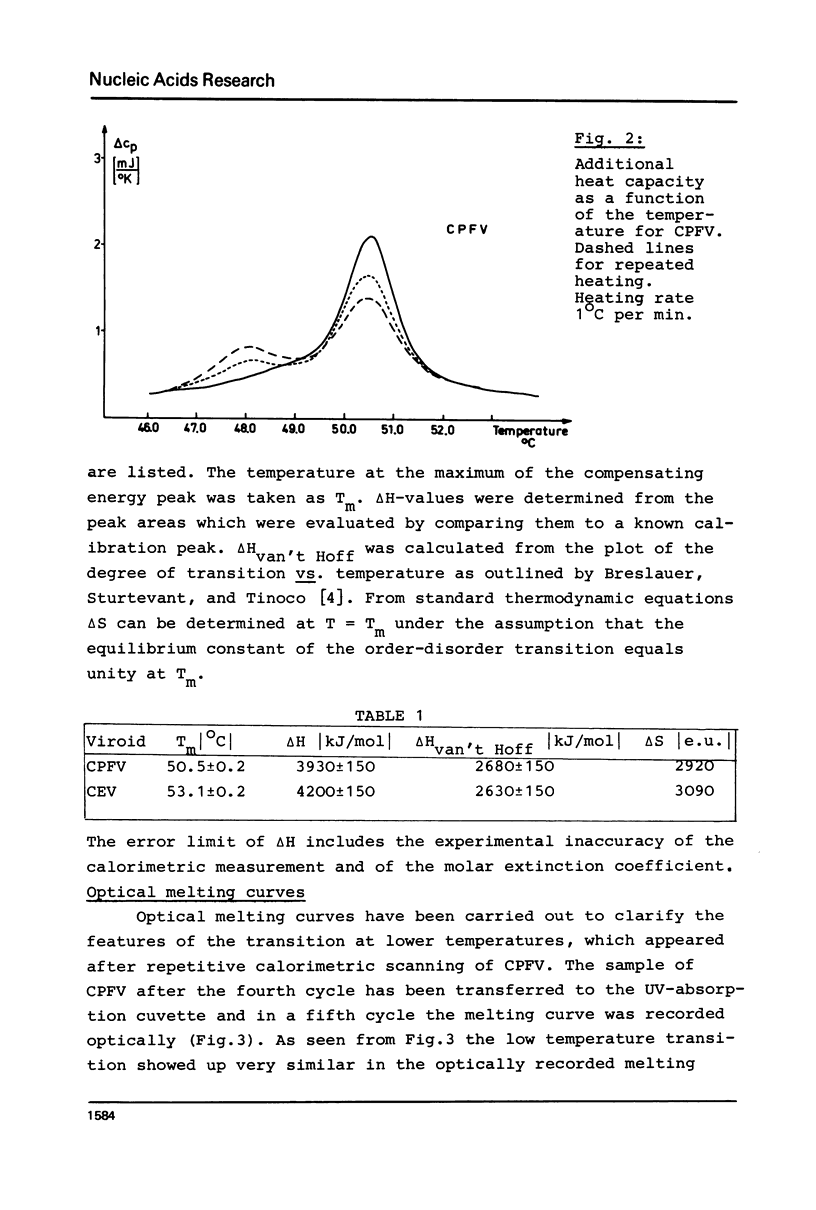
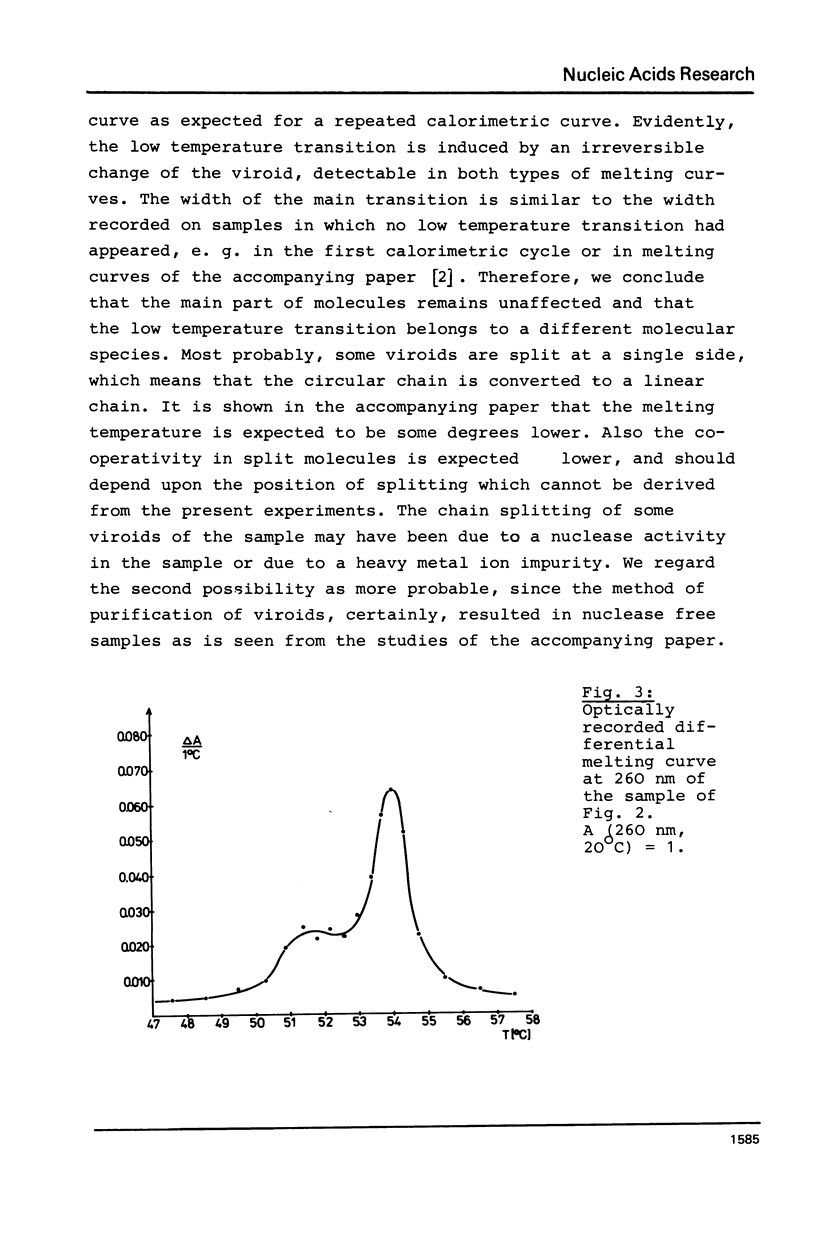
Abstract
Thermodynamic studies on highly purified viroid preparations were carried out with the help of a very sensitive adiabatic microcalorimeter. Parallel to the change of UV-absorption at 260 nm as a function of temperature, the additional heat capacity of the dilute viroid solution rises sharply within the melting interval, reaches a maximum at T = Tm and declines to a baseline again when the temperature is increased further. From the peak area the molar transition enthalpy can be calculated. The transition enthalpies of citrus exocortis viroid and cucumber pale fruit viroid are 4200 kJ/mol and 3930 kJ/mol, respectively. The calorimetric results are compared to the results obtained from melting studies using UV-absorption.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breslauer K. J., Sturtevant J. M., Tinoco I., Jr Calorimetric and spectroscopic investigation of the helix-to-coil transition of a ribo-oligonucleotide: rA7U7. J Mol Biol. 1975 Dec 25;99(4):549–565. doi: 10.1016/s0022-2836(75)80171-9. [DOI] [PubMed] [Google Scholar]
- Langowski J., Henco K., Riesner D., Sänger H. L. Common structural features of different viroids: serial arrangement of double helical sections and internal loops. Nucleic Acids Res. 1978 May;5(5):1589–1610. doi: 10.1093/nar/5.5.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger H. L., Klotz G., Riesner D., Gross H. J., Kleinschmidt A. K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]



