Introduction
Angiogenesis, or the formation of new blood vessels, is essential for solid tumor growth and metastasis and it is regulated by proangiogenic soluble mediators including the vascular endothelial growth factor (VEGF).1 Antiangiogenic drugs that block the VEGF signaling pathway prolong progression free survival in several cancers2-4 and are now in broad clinical use to treat advanced stage solid tumors. Hypertension is among the most common toxicities of this therapeutic class, with recent meta-analyses reporting the incidence of hypertension induced by a single antiangiogenic agent to be ~20%,5-7 and single studies have reported incidence as high as 87%.8 Thus, the appropriate diagnosis and management of antiangiogenic therapy-induced hypertension is now a common clinical problem. In this report, we present a patient who developed antiangiogenic therapy-induced hypertension and review the mechanisms, management and emerging questions in this evolving field.
Case
The patient is a 56-year-old male with a history of hypertension, coronary artery disease and tobacco use. Medications included atenolol, hydrochlorothiazide and aspirin. He developed weight loss with microscopic hematuria, and was found to have a large exophytic mass of 8 centimeters in the left kidney, with small liver and pulmonary nodules suspicious for metastases. He underwent radical nephrectomy and pathology revealed Fuhrman Grade 3 clear cell renal cell carcinoma. He was started on single-agent sunitinib therapy administered in a 4-week on, 2-week off regimen. Before starting sunitinib, his metabolic workup, including serum electrolytes, fasting blood glucose and lipid profile, was unremarkable. His serum creatinine after nephrectomy was 1.3 mg/dL and a 24-hour protein excretion was 270 mg/24hour. An echocardiogram showed mild left ventricular hypertrophy, unchanged over the last 2 years. Over the last year, his office blood pressure (BP) measurements have been in the 120’s/80’smmHg range.
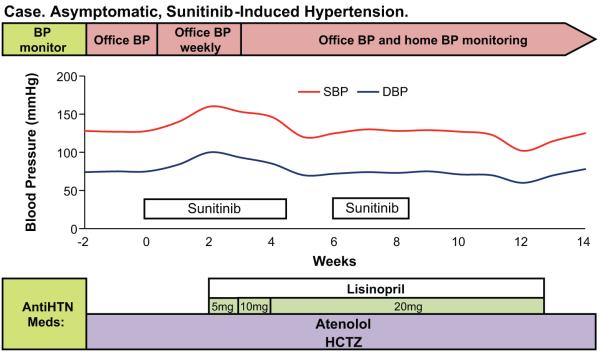
Two weeks after starting sunitinib, his office BP was 160/100mmHg. Manual BPs confirmed this reading. Otherwise, he was asymptomatic, his physical exam was unremarkable, his weight was unchanged and his serum electrolytes and creatinine were unchanged. Lisinopril was begun at 5 mg per day and home BP monitoring initiated. Over the course of the next fourteen days, lisinopril was increased to 20 mg per day at which point his BP was at his baseline. Four weeks later, he developed severe hand-foot skin reaction and sunitinib therapy was held. While off of sunitinib he complained of episodes of lightheadedness upon standing. On evaluation, his office BP was 102/63mmHg. His home BP chart revealed that, during the last week, his BP has been in the 100-110/60’s mmHg range. In this setting, lisinopril dose was decreased. After sunitinib was reinitiated at a lower dose, his BP trended up and lisinopril dose was increased. His BP remained stable in the 120-130’s/80’smmHg thereafter (Figure 1).
Figure 1.
Blood pressure monitoring and therapeutic interventions in a 56 years old male patient with metastatic renal cell carcinoma treated with sunitinib who developed asymptomatic hypertension after sunitinib therapy was started.
Discussion
The management of antiangiogenic therapy-induced hypertension requires a stepwise and individualized approach. First, a thorough evaluation of cardiovascular risk factors should be performed before starting an antiangiogenic agent.9 Once the antiangiogenic agent is started, BP should be monitored throughout therapy. If the patient develops hypertension, prompt intervention is recommended to avoid adverse cardiovascular events and the need for dose reduction or even discontinuation of antiangiogenic therapy. In the following sections, we will review in more detail current antiangiogenic approaches, discuss pathophysiologic mechanisms leading to antiangiogenic therapy induced hypertension and summarize current guidelines to evaluate, monitor and manage antiangiogenic therapy-induced hypertension. We also touch on emerging cardiovascular toxicities such as vascular thrombosis and review the growing interest in the use of hypertension as a marker of therapeutic efficacy.
Antiangiogenic agents
Current antiangiogenic therapies target the VEGF signaling pathway as well as other tyrosine-kinase based signaling pathways. Of the four VEGF members (VEGF A-D), VEGF A is the main pro-angiogenic isoform. It is a soluble protein secreted by tumors to recruit and stimulate endothelial cell proliferation, migration and survival by binding to and activating the VEGF receptor 2 (VEGFR2), which is expressed in endothelial cell membranes, and its downstream pathways.10
VEGF signaling pathway inhibitors target the VEGF molecule, its receptor or downstream pathways. FDA approved antiangiogenic agents include bevacizumab, a recombinant, humanized monoclonal antibody that binds and sequesters the VEGF molecule,11 and multi-targeted tyrosine kinase inhibitors (TKI), small molecules with competitive or allosteric inhibitory activity at the catalytic binding site on the VEGFR2 intracellular domain, such as sunitinib, sorafenib and pazopanib.12 Of note, the latter group of drugs is less specific and targets other tyrosine kinase receptors including platelet derived growth factor receptor (PDGFR) and c-kit.
Pathophysiology of antiangiogenic therapy-induced hypertension
Emerging evidence implicates increased peripheral vascular resistance in the pathophysiology of antiangiogenic therapy-induced hypertension. VEGF binding to VEGFR2 activates its intrinsic tyrosine kinase activity ultimately activating endothelial nitric oxide synthase (eNOS) and increasing nitric oxide (NO) production (Figure 2).13 Experimental evidence that antiangiogenic therapies decrease NO bioavailability is somewhat contradictory. In humans, VEGF inhibition has been associated with decreased urinary nitrite/nitrate excretion and decreased serum levels of NO metabolites,14, 15 but no difference in flow-mediated dilation, a surrogate for NO bioavailability, was observed.15 In swine, NO bioavailability does not appear to contribute to sunitinib-induced hypertension, either.16 In fact, in a swine model of sunitinib-induced hypertension a compensatory increase in NO was measured.16 By contrast, Facemire and colleagues reported that mice treated with an anti-VEGFR2 antibody developed a rise in BP and a reduction in kidney eNOS and nNOS.17 Addition of the NOS inhibitor Nω-nitro-L-arginine methyl ester abolished the difference in BP between anti-VEGFR2 and vehicle, supporting a role for NOS in this model. As sunitinib targets not only VEGFR2 but also other tyrosine kinases, while DC-10 blocks only VEGFR2, one potential explanation for these conflicting results may be that individual antiangiogenic therapies cause hypertension by different mechanisms.
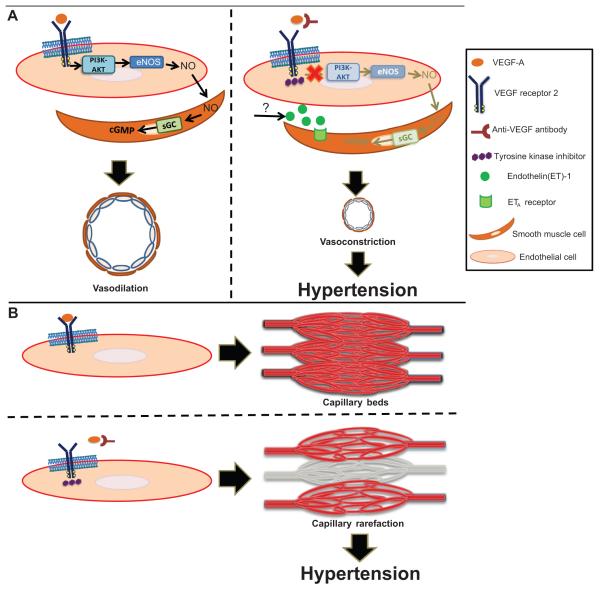
Figure 2.
Proposed mechanisms leading to VEGF signaling pathway inhibitor-induced hypertension. a) Activation of VEGFR-2 by VEGF-A leads to subsequent activation of multiple pathways including phosphatidylinositol-3-kinase (PI3K)-AKT. PI3K-AKT phosphorylates and activates endothelial nitric oxide synthase (eNOS), increasing NO production. NO migrates to adjacent vascular smooth muscle cells, binds soluble guanylate cyclase (sGC) leading to cGMP generation and subsequent vasodilation mediated by cGMP-dependent kinases. When VEGF signaling pathway is inhibited, NO pathway is suppressed and ET-1 pathway is stimulated, promoting vasoconstriction and subsequent hypertension. The source of ET-1 is unknown. b) VEGF maintains capillary network integrity. When VEGF signaling pathway is inhibited, rarefaction, or reduction of the density of capillary beds, may occur.
Evidence implicating endothelin-1 (ET-1), a potent vasoconstrictor, in the etiology of antiangiogenic therapy-induced hypertension is more consistent (Figure 2). Increases in circulating levels of ET-1 have been reported in parallel to a rise in BP in rodents as well as in humans after sunitinib therapy.18 We have reported both a rapid rise in plasma ET-1 in patients receiving regorafenib, a TKI, with rapid normalization of ET-1 levels after therapy is stopped.19 In several preclinical models, the rise in BP induced by antiangiogenic therapy is largely prevented by the co-administration of an endothelin receptor antagonist, providing strong evidence implicating the ET-1 pathway in antiangiogenic therapy induced hypertension.16, 20 The source of ET-1 and the mechanisms linking VEGF inhibition to ET-1 activation remain to be elucidated. VEGF is required for normal endothelial homeostasis, so antiangiogenic therapies may induce endothelial dysfunction which itself is a trigger of ET-1 secretion.21
Antiangiogenic therapies do cause microcapillary rarefaction over time, but the relevance of this observation to the development of hypertension in patients taking these drugs is questionable. Preclinical studies in rodents treated with VEGF inhibitors showed that up to 30% of tracheal mucosal capillary networks regress by 21 days of therapy and reverses with antiangiogenic therapy discontinuation.22 In patients with metastatic colorectal carcinoma receiving bevacizumab, the capillary density of the dorsum of the finger was reduced by ~10% after 6-months of treatment and it was associated with the rise in blood pressure.23 Another small clinical study, reported a reduced capillary density in the mucosal surface of the inner lip of ~20% after 5-weeks of therapy with telatinib, a TKI with similar properties as sunitinib.24 However the time course for capillary rarefaction of several days to weeks does not match the very rapid rise in BP often over hours usually seen in patients who start antiangiogenic therapy.8 Furthermore, as pointed out by Kappers et al, increasing peripheral vascular resistance by 5% requires rarefaction of 40% of the microcapillary bed – more than what has been observed in humans.18, 25
Other vasoactive substances have also been evaluated as potential contributors to antiangiogenic therapy-induced hypertension. However, although in vitro models have suggested a potential interaction between VEGF and some of vasoactive substances (i.e. prostacyclin and oxidative stress),26, 27 animal models and humans have not supported roles of prostacyclin,14, 17 thromboxane-2,17 oxidative stress,15, 16, 20 reninangiotensin system,17, 18, 28 or sympathetic pathways28 in antiangiogenic therapy-induced hypertension. Whether disruption in VEGF-C-induced lymphangiogenesis might be involved in antiangiogenic therapy-induced hypertension is unclear. In rats exposed to high-salt diets, macrophages produce VEGF-C which binds to VEGFR-3 and stimulates lymphangiogenesis which buffers volume overload generated by salt excess.29 Blocking this mechanism could conceivably contribute to the rise in blood pressure associated with TKIs targeting VEGFR 3; however, it would not explain the hypertension observed in patients treated with anti-VEGF-A (i.e. bevacizumab).
Finally, thrombotic microangiopathy (TMA) is an uncommon but important underlying pathology to consider in patients developing antiangiogenic therapy-induced hypertension. A subset of patients that develop antiangiogenic therapy-induced hypertension also develop proteinuria, microangiopathic hemolysis and acute kidney injury.30 This requires immediate discontinuation of the antiangiogenic therapy. When diagnosed in time, our clinical experience indicates that most patients recover and may even tolerate further antiangiogenic therapies from a different class than the one inducing a TMA syndrome.30 Failure to recognize this syndrome with continued administration of the antiangiogenic agent may result in permanent kidney damage.
In summary, most evidence supports increased peripheral vascular resistance as the mechanism leading to antiangiogenic therapy-induced hypertension, mediated primarily by ET-1 with possible contributions from NO pathway suppression (Figure 2).
Antiangiogenic therapy-induced hypertension: Definition
Antiangiogenic therapy-induced hypertension was commonly reported using the Common Terminology Criteria for Adverse Events (CTCAE), a classification system developed to assess the chemotherapeutic toxicities.31 Until recently, the classification of treatment-induced hypertension had no relationship with the standard system used to define hypertension, the Joint National Committee 7 guidelines (JNC-7).32 This discrepancy presented a major challenge for oncologists in defining hypertension and designing therapeutic interventions. Consequently, the classification of hypertension in the most recent version of CTCAE (version 4) was modified to parallel the JNC-7.
Cardiovascular assessment before starting antiangiogenic therapy
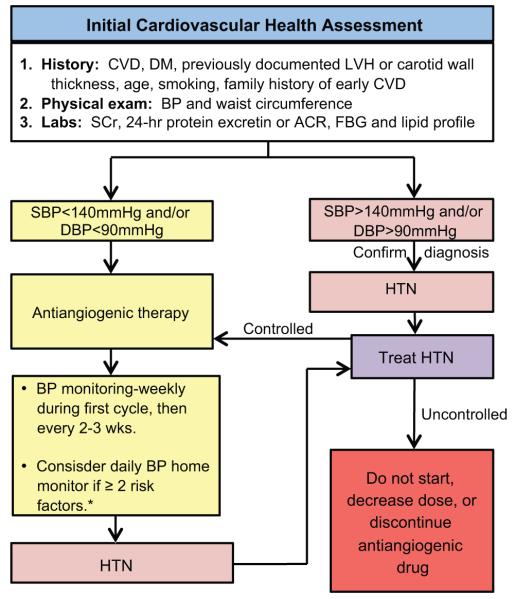
National Cancer Institute (NCI) guidelines on the initial assessment, surveillance and management of BP in patients treated with VEGF signaling pathway inhibitors recommend that, before starting antiangiogenic therapies, all patients should have a full cardiovascular health assessment focusing on ongoing clinical conditions, signs of subclinical end-organ damage and risk factors of cardiovascular disease (Figure 3).9 In fact, these guidelines suggest stratifying patients based on specific risk factors for developing adverse cardiovascular consequencesas follows: low risk= 0 risk factors, high risk = 1 risk factor, or higher risk 2 or more risk factors.9 Major risk factors include systolic blood pressure (SBP) >160mmHg or diastolic blood pressure (DBP) >100mmHg; history of diabetes; history of cardiovascular diseases;established or subclinical renal disease; and subclinical end-organ damage including increased carotid intima media thickness by carotid ultrasound or left ventricular hypertrophy by echocardiogram.9 Also, having three or more of the following characteristics contribute as one of the major risk factors mentioned before: cigarette smoking, dyslipidemia, glucose intolerance, age, family history of early cardiovascular diseases and abdominal obesity.9 Intriguingly, a recent large cohort study showed an increased risk of death from cardiovascular causes and that this increased risk was especially predominant during the first week after diagnosis.33
Figure 3.
Initial cardiovascular evaluation and monitoring of blood pressure in candidates for antinagiogenic therapy. CVD, cardiovascular disease; DM, diabetes mellitus; LVH, left ventricular hypertrophy; BP, blood pressure; SCr, serum creatinine; ACR, albumin to creatinine ratio; FBG, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; HTN, hypertension.
Blood pressure goals and monitoring
The JNC-7 BP goal has been assigned to be <140/90mmHg in the non-diabetic patient without kidney disease, to reduce long-term mortality.32 However, in patients receiving antiangiogenic therapy, life-expectancy is limited by the primary cancer and the primary goal is to decrease the risk of short-term morbidity associated with hypertension (e.g. stroke and myocardial infarction)4, 34 while maintaining effective therapeutic levels of antiangiogenic agents. Nevertheless, given limited BP goal data in the cancer population, NCI guidelines recommend using the JNC-7 BP goal as a reference in this group.9 Additionally, for patients with low-normal BP at baseline in whom increases in BP might not necessarily exceed 140/90mmHg, the BP goal is maintaining DBP pressure <20mmHg from its baseline.9
Before starting antiangiogenic therapy, BP assessment should be performed in the office using standard BP measurement techniques. 35 Ideally, BP evaluation will be repeated on a second visit before starting antiangiogenic therapy. If elevated BP is identified, further evaluations must be undertaken including obtaining repeated BP measurements and assessing for reversible causes of elevated BP such as pain. If hypertension is confirmed, antihypertensive interventions will be required before starting antiangiogenic therapy.9 Once antiangiogenic therapy has been started, NCI protocols recommend weekly BP monitoring during the first cycle, then at least every 2-3 weeks.9 This approach is supported by evidence from clinical trials reporting high BP during the first weeks or even days after starting therapy.36-38 Since frequent 24-hour ambulatory monitoring is impractical in the clinical setting, home monitoring is the preferred technique, as long as appropriate equipment and patient education are provided.9, 39
Therapeutic interventions
Hypertension management should be timely to avoid complications. Initiation of antihypertensive medications should be considered when BP is >140/90mmHg or if there is an increase in DBP ≥20mmHg.9 If SBP >160mmHg, DBP >100mmHg, hypertensive crisis or if antihypertensive interventions do not provide appropriate BP control, antiangiogenic therapy dose should be decreased or held until antihypertensive therapy is effectively titrated.9 With the new-onset hypertension, CBC, urinalysis and serum creatinine should also be obtained as the presence of worsening renal function, proteinuria, hemolytic anemia or thrombocytopenia may point towards an associated thrombotic microangiopathy in which case a referral to a nephrologist is indicated.
Although appropriate BP control is essential, excessive lowering of BP may also have detrimental cardiovascular consequences; thus, careful selection of doses and close BP monitoring are crucial to avoid episodes of hypotension. 9 Moreover, given that most antiangiogenic drug regimens include periods off of the agent, close monitoring during those periods is required as rebound hypotension may also occur.
Multiple antihypertensive drugs are being used to treat antiangiogenic therapy-induced hypertension including calcium channel blockers (CCB), inhibitors of the RAS, beta-blockers and diuretics. At this time, however, no clinical evidence favoring one antihypertensive agent over another is available. Specific antihypertensive agents are primarily selected based on patient’s comorbidities. In our clinical experience, CCB and RAS inhibitors are effective in treating antiangiogenic therapy-induced hypertension.
Dihydropyridine CCB such as amlodipine have great potency reducing arterial smooth muscle cell contractility in the blood vessels,40 making them ideal agents for BP control in this selected population. Clinical studies have reported effective BP control with these antihypertensive agents.36, 41, 42 In a retrospective review of 154 patients treated with bevacizumab, 36% developed bevacizumab-related hypertension. From those, 11% were exclusively treated with CCB, all of them achieving adequate BP control.53 Similar success rates were reported recently by Mir et al.43 Of note, non-dihydropyrdine CCB inhibit CYP3A4 and should be avoided in patients receiving sorafenib and sunitinib.44
Angiotensin converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARBs) are also good choices for the treatment of antiangiogenic therapy-induced hypertension particularly in the setting of proteinuria or when two agents are required. In fact, proteinuria is a relatively common side effect of antiangiogenic therapies45, 46 and regular screening urinalyses should be performed. Although there are no formal recommendations about how frequently these screening urinalyses should be performed, in our practice, we screen at baseline and then every three months after starting therapy. If the screening urinalysis shows ≥ 1+ proteins, further quantification of urinary protein excretion (i.e. spot urine albumin-to-creatinine ratio) is required. If proteinuria is > 1 g/24 hrs and/or is accompanied by acute kidney injury, a referral to a nephrologist for additional evaluation and management is recommended. When to stop anti-angiogenic therapies in the setting of proteinuria is an unresolved question. Some patients can tolerate sub-nephrotic range proteinuria for extended periods and remain on therapy, as long as BP is controlled and glomerular filtration rate remains stable. A detailed discussion of antiangiogenic therapy-induced proteinuria and thrombotic microangiopathy is beyond the scope of this article, but has been reviewed.47
A preclinical study in rats evaluating the efficacy of an ACEi (captopril) and a dihydropyrimidine CCB (nifedipine) in treating hypertension induced by a TKI (cediranib) showed that both agents were effective.48 However, captopril was only effective with mild increases in BP (10-15mmHg), while nifedipine treatment controlled severe increases in BP (30-50mmHg).48 The authors concluded that at very high BPs, RAS was downregulated as a compensatory mechanism to decrease BP; thus, in that setting captopril was not effective in controlling hypertension.48 In support of this hypothesis, they reported reduced renin levels when rats were exposed to higher levels of cediranib.48 Other preclinical studies have also shown suppressed RAS after antiangiogenic therapy.17, 18 In humans, Veronese et al did not observe differences in plasma renin or aldosterone after administration of BAY-43-9006 in patients with advanced renal cell carcinoma,28 while Kappers et al observed decreased levels of plasma renin concentration and activity but no changes in aldosterone after administration of sunitinib.18 Whether these findings in human subjects are a consequence of absolute changes in BP or a drug-specific effect is unclear and requires further evaluation.
Thiazide diuretics are first-line therapies in treating essential hypertension. In our experience, diuretics are less effective than CCB, ACEi or ARBs in treating antiangiogenic therapy-induced hypertension. However, some authors have reported some effectiveness.36, 41 These variable responses may be related to differences in the mechanisms underlying antinagiogenic therapy-induced hypertension.
Whether prophylactic use of antihypertensive agents in non-hypertensive patients might reduce the frequency of antiangiogenic dose reductions or interruptions has been investigated. A prospective clinical study addressed this question in patients treated with cediranib by randomizing subjects (n=126) to one of 4 groups: 30 mg-cediranib with prophylactic low dose CCB, 30 mg-cediranib with no CCB prophylaxis, 45-mg cediranib with CCB prophylaxis, and 45 mg-cediranib with no CCB prophylaxis.49 No specific intervention appeared to be superior to another in avoiding cediranib drug withdrawal. However, less patients developed severe hypertension, defined as SBP >180mmHg or DBP >110mmg, in the groups receiving prophylaxis compared to the groups that did not receive it (1patient vs. 18patients), which certainly decreases the risks of developing cardiovascular complications.49 Whether a similar pattern will be observed with other antihypertensive agents and/or other antiangiogenic drugs is an open question.
Finally, based on the proposed mechanisms contributing to antiangiogenic therapy-induced hypertension, future therapeutic considerations may include NO derivatives and ET receptor antagonists. Agents that increase NO bioavailability such as long-acting nitrates are attractive.44 In fact, adequate response to long-acting nitrates has been reported in cases of patients with antiangiogenic therapy-induced hypertension refractory to ACEi and CCB.50 Further clinical studies evaluating BP control, safety and the effects on antiangiogenic interventions are needed. In fact, preclinical evidence suggest that VEGF-induced angiogenesis is dependent on downstream NO production.51, 52 Furthermore, angiogenesis usually does not occur in eNOS deficient mice.53 Whether nitrate therapy might potentially compromise antiangiogenic benefits is an open question.
Endothelin receptor antagonists (ETA) are also attractive antihypertensive drugs; however, no data on the effects of these agents are available in humans with antiangiogenic therapy-induced hypertension. Clinically, ETAs are currently approved for pulmonary hypertension. Clinical trials evaluating the effect of ETAs in patients with resistant systemic hypertension have not provided strong data supporting their use, especially given main side-effects which include salt and water retention, peripheral edema and high teratogenicity,54 though with the new generation of ETAs, the side effects have become less severe. At this point, careful clinical studies are needed to evaluate the effectiveness and safety of these agents in patients treated with antiangiogenic therapy.
Hypertension as a biomarker for cancer response
As BP elevation with VEGF inhibition appears to be a mechanism-dependent toxicity, the notion that elevations in BP may predict superior tumor outcomes is gaining considerable interest in the oncologic community as a means to optimize dosing and outcomes. In a cohort of patients with colon cancer on bevacizumab, those developing bevacizumab-related hypertension, defined as grade 2-3 hypertension by CTCAE v3, had longer progression-free survival (PFS) (14.5 vs 3.1 months, p=0.04) and an increase in partial remission (PR) by RECIST criteria (p=0.04).55
Similarly, in a cohort of patients with metastatic renal cell carcinoma (mRCC) on sunitinib, those who developed grade 2 or more hypertension had a higher degree of RECIST PR (p=0.009).56 Another study evaluating patients with mRCC on bevacizumab reported that those that did not develop hypertension (defined as BP > 150/100mmHg) had a higher degree of RECIST progressive disease (PD) (p=0.005) and a shorter time to disease progression (4.2 months vs. 8.1 months; p=0.036), though the overall survival was not different between the groups (p=0.22).57 Finally, combined data from five phase II multicenter trials of axitinib in mRCC, non-small cell lung cancer (NSCLC), melanoma, and thyroid cancer, defining hypertension as DBP > 90mmHg, reported an overall reduction in risk of death of 33% in multivariate analyses amongst those developing hypertension (p=0.036) as well as a better objective response rate (ORR) (p<0.001), and a trend toward better PFS (10.2 vs. 7.1 months; p=0.107).58
Although these data suggest that hypertension is a marker for increased VEGF inhibition, findings from other studies have put this theory into question. In a study of NSCLC patients on carboplatin/paclitaxel with either cediranib or placebo, the cediranib armdeveloped more treatment-associated hypertension (68 vs. 45%; p<0.0001), defined as either new-onset or worsening hypertension grade from baseline, or an increase in drug dosage or increase in a new antihypertensive medication.59 However, areduce the risk of death was observed in both cediranib patients who developed hypertension (p=0.06) and patients on placebo who developed hypertension (p=0.0045). Moreover,a better ORR was observed in the placebo group but not in the cediranibgroup. Some proposed mechanisms for this finding are that paclitaxel potentially has some anti-angiogenic properties, or that reduction in tumor burden may reduce systemic VEGF levels and therefore lead to hypertension.
In patients with mRCC on sunitinib, treatment-induced hypertension, defined either as SBP > 140mmHg or DBP > 90mmHg, predicted improved tumor efficacy measured as ORR, PFS, and overall survival (OS) (p<0.001 for all analyses).60 However, in NSCLC patients on cediranib taking taking antihypertensive agents at baseline had improved tumor efficacy, there was no difference in PFS but the median OS was longer in those patients (31.8 vs. 21.4 months; p<0.001). These findings suggest that a host factor predisposing patients to response to these agents, and this host factor may be related to the biology of VEGF blockade.59
Various studies have attempted to explore these mechanisms. In patients withbreast cancer patients receiving paclitaxel with beither bevacizumab or placebo, specific VEGF genotypes were associated with better OS in patients receiving bevacizumab but not in the placebo arm.61 However, the genotypes associated with hypertension were not associated with better OS, and the genotypes associated with better OS were not associated with hypertension. In a study of patients with mRCC on sunitinib, VEGF SNP-634 genotype was associated with the prevalence (p=0.03) and duration (p=0.01) of sunitinib-induced hypertension and the likelihood of having sunitinib-induced hypertension was greater in patients with the GG genotype than in those with the CC genotype (OR: 13.62; 95% CI, 3.71-50.04).62 In terms of clinical outcomes, no single SNP was correlated with response, but the combination of VEGFR2 SNP-889 and VEGF SNP-936 were associated with better OS (p = 0.03). While there may be a host factor involved in determining the tumor efficacy of VEGF inhibition and how it relates to treatment-induced hypertension, the exact mechanisms remain to be elucidated. In addition, studies are ongoing to determine whether treating patients with increasing drug dosages to achieve hypertension will improve outcomes.
Other vascular complications
In addition to hypertension, emerging evidence demonstrates that VEGF inhibitors can lead to vascular thrombosis. Multiple meta-analyses have shown that arterial thrombosis represents an important complication in patients treated with bevacizumab. A pooled analysis of five randomized controlled trials found the addition of bevacizumab to standard chemotherapy leads to a modest increase in the risk of arterial thrombotic events (HR 2.0; 95% CI, 0.66 to 1.20; P=0.44) with risk factors for thrombosis including prior arterial thromboembolic events and age of 65 years or older.63 A similar increase was noted in two follow-up meta-analyses involving a larger number of clinical trials with bevacizumab.64, 65 Increased rates of thrombotic events extend to oral TKIs with anti-VEGF properties where a meta-analysis of over 10,000 patients in clinical trials indicate the relative risk of arterial thrombotic events associated with sunitinib and sorafenib is 3.03 (95% CI, 1.25 to 7.37; p=0.015) compared to control patients.66 Less clear is whether there are increased rates of venous thromboembolic events with the use of VEGF inhibitors. While one initial meta-analysis found a slight increase (RR 1.33, 95% CI, 1.13-1.56, p <0.001) in the risk of venous thrombembolism with the addition of bevacizumab to standard chemotherapy,67 other studies did not confirm this risk.68
In these studies, an arterial thrombotic event was defined as a myocardial infarction, transient ischemic attack (TIA) or cerebrovascular accident (CVA). It is therefore possible that more subtle events were not reported. Importantly, patients were excluded from clinical trials if they had a previous history of atherosclerosis, therefore not reflecting the rates of thrombotic events in the “real world” population. Also, whether the subset of patients with arterial thrombotic events on VEGF inhibitors have significant hypertension after initiation of therapy is not clear. These issues represent important considerations in future clinical trials with “second-generation” VEGF inhibitors and as FDA-approved VEGF inhibitors are being used in the general population.
Perspectives
Antiangiogenic therapies are being increasingly used in the treatment of solid tumors; however, their therapeutic effectiveness may be hindered by the development of hypertension, a common dose-limiting toxicity of these agents. Hence, early identification and treatment of hypertension is essential to maintain an effective therapeutic dose of these agents for the longest period of time. In this regard, NCI recently published useful guidelines on the assessment, surveillance and management of BP in patients receiving antiangiogenic therapy. However, many important questions remain to be answered, such as whether one antihypertensive agent is superior to another in controlling antiangiogenic therapy-induced hypertension, whether different multitargeted tyrosine kinase inhibitors had variable effects on the mechanisms leading to hypertension, and whether a full understanding of the mechanisms causing antiangiogenic therapy-induced hypertension will provide clinically useful biomarkers for both predicting this toxicity and therapeutic efficacy. Future prospective studies focusing on these questions would provide important clinical evidence for the management of patients treated with these promising agents.
Footnotes
Disclosures: Nilka de Jesús-González, none. Emily Robinson, none. Javid Moslehi, none. Benjamin Humphreys, none.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 5.Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: A systematic review and meta-analysis. Lancet Oncol. 2008;9:117–123. doi: 10.1016/S1470-2045(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 6.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: Systematic review and meta-analysis. Acta Oncol. 2009;48:9–17. doi: 10.1080/02841860802314720. [DOI] [PubMed] [Google Scholar]
- 7.An MM, Zou Z, Shen H, Liu P, Chen ML, Cao YB, Jiang YY. Incidence and risk of significantly raised blood pressure in cancer patients treated with bevacizumab: An updated meta-analysis. Eur J Clin Pharmacol. 2010;66:813–821. doi: 10.1007/s00228-010-0815-4. [DOI] [PubMed] [Google Scholar]
- 8.Robinson ES, Matulonis UA, Ivy P, Berlin ST, Tyburski K, Penson RT, Humphreys BD. Rapid development of hypertension and proteinuria with cediranib, an oral vascular endothelial growth factor receptor inhibitor. Clin J Am Soc Nephrol. 2010;5:477–483. doi: 10.2215/CJN.08111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, Ivy SP, Leier CV, Lindenfeld J, Liu G, Remick SC, Steingart R, Tang WH. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604. doi: 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara N, Gerber HP, LeCouter J. The biology of vegf and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-vegf antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 12.Gotink KJ, Verheul HM. Anti-angiogenic tyrosine kinase inhibitors: What is their mechanism of action? Angiogenesis. 2010;13:1–14. doi: 10.1007/s10456-009-9160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hood JD, Meininger CJ, Ziche M, Granger HJ. Vegf upregulates ecnos message, protein, and no production in human endothelial cells. Am J Physiol. 1998;274:H1054–1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 14.Robinson ES, Khankin EV, Choueiri TK, Dhawan MS, Rogers MJ, Karumanchi SA, Humphreys BD. Suppression of the nitric oxide pathway in metastatic renal cell carcinoma patients receiving vascular endothelial growth factor-signaling inhibitors. Hypertension. 2010;56:1131–1136. doi: 10.1161/HYPERTENSIONAHA.110.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer EL, Dallabrida SM, Rupnick MA, Redline WM, Hannagan K, Ismail NS, Burstein HJ, Beckman JA. Contrary effects of the receptor tyrosine kinase inhibitor vandetanib on constitutive and flow-stimulated nitric oxide elaboration in humans. Hypertension. 2011;58:85–92. doi: 10.1161/HYPERTENSIONAHA.110.168120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kappers MH, de Beer VJ, Zhou Z, Danser AH, Sleijfer S, Duncker DJ, van den Meiracker AH, Merkus D. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension. 2012;59:151–157. doi: 10.1161/HYPERTENSIONAHA.111.182220. [DOI] [PubMed] [Google Scholar]
- 17.Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension. 2009;54:652–658. doi: 10.1161/HYPERTENSIONAHA.109.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kappers MH, van Esch JH, Sluiter W, Sleijfer S, Danser AH, van den Meiracker AH. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension. 2010;56:675–681. doi: 10.1161/HYPERTENSIONAHA.109.149690. [DOI] [PubMed] [Google Scholar]
- 19.de Jesus-Gonzalez N, Robinson ES, Penchev RR, von Mehren M, Heinrich MC, Tap W, Demetri GD, George S, Humphreys BD. Regorafenib induces rapid and reversible changes in plasma nitric oxide and endothelin-1. American Journal of Hypertension. 2012 doi: 10.1038/ajh.2012.97. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kappers MH, Smedts FM, Horn T, van Esch JH, Sleijfer S, Leijten F, Wesseling S, Strevens H, Jan Danser AH, van den Meiracker AH. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension. 2011;58:295–302. doi: 10.1161/HYPERTENSIONAHA.111.173559. [DOI] [PubMed] [Google Scholar]
- 21.Bohm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76:8–18. doi: 10.1016/j.cardiores.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, McDonald DM. Cellular changes in normal blood capillaries undergoing regression after inhibition of vegf signaling. Am J Physiol Heart Circ Physiol. 2006;290:H547–559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 23.Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol. 2008;19:927–934. doi: 10.1093/annonc/mdm550. [DOI] [PubMed] [Google Scholar]
- 24.Steeghs N, Gelderblom H, Roodt JO, Christensen O, Rajagopalan P, Hovens M, Putter H, Rabelink TJ, de Koning E. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res. 2008;14:3470–3476. doi: 10.1158/1078-0432.CCR-07-5050. [DOI] [PubMed] [Google Scholar]
- 25.Greene AS, Tonellato PJ, Lui J, Lombard JH, Cowley AW., Jr Microvascular rarefaction and tissue vascular resistance in hypertension. Am J Physiol. 1989;256:H126–131. doi: 10.1152/ajpheart.1989.256.1.H126. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler-Jones C, Abu-Ghazaleh R, Cospedal R, Houliston RA, Martin J, Zachary I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase a2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS Lett. 1997;420:28–32. doi: 10.1016/s0014-5793(97)01481-6. [DOI] [PubMed] [Google Scholar]
- 27.Abid MR, Tsai JC, Spokes KC, Deshpande SS, Irani K, Aird WC. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a rac1-regulated nadph oxidase-dependent mechanism. FASEB J. 2001;15:2548–2550. doi: 10.1096/fj.01-0338fje. [DOI] [PubMed] [Google Scholar]
- 28.Veronese ML, Mosenkis A, Flaherty KT, Gallagher M, Stevenson JP, Townsend RR, O’Dwyer PJ. Mechanisms of hypertension associated with bay 43-9006. J Clin Oncol. 2006;24:1363–1369. doi: 10.1200/JCO.2005.02.0503. [DOI] [PubMed] [Google Scholar]
- 29.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-c-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 30.Patel TV, Morgan JA, Demetri GD, George S, Maki RG, Quigley M, Humphreys BD. A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. J Natl Cancer Inst. 2008;100:282–284. doi: 10.1093/jnci/djm311. [DOI] [PubMed] [Google Scholar]
- 31.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P. Ctcae v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 32.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 33.Fang F, Fall K, Mittleman MA, Sparen P, Ye W, Adami HO, Valdimarsdottir U. Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med. 2012;366:1310–1318. doi: 10.1056/NEJMoa1110307. [DOI] [PubMed] [Google Scholar]
- 34.Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980–982. doi: 10.1056/NEJMc052954. discussion 980-982. [DOI] [PubMed] [Google Scholar]
- 35.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the american heart association council on high blood pressure research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 36.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 37.Maitland ML, Kasza KE, Karrison T, Moshier K, Sit L, Black HR, Undevia SD, Stadler WM, Elliott WJ, Ratain MJ. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009;15:6250–6257. doi: 10.1158/1078-0432.CCR-09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azizi M, Chedid A, Oudard S. Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med. 2008;358:95–97. doi: 10.1056/NEJMc072330. [DOI] [PubMed] [Google Scholar]
- 39.Nazer B, Humphreys BD, Moslehi J. Effects of novel angiogenesis inhibitors for the treatment of cancer on the cardiovascular system: Focus on hypertension. Circulation. 2011;124:1687–1691. doi: 10.1161/CIRCULATIONAHA.110.992230. [DOI] [PubMed] [Google Scholar]
- 40.Abernethy DR, Schwartz JB. Calcium-antagonist drugs. N Engl J Med. 1999;341:1447–1457. doi: 10.1056/NEJM199911043411907. [DOI] [PubMed] [Google Scholar]
- 41.Pande A, Lombardo J, Spangenthal E, Javle M. Hypertension secondary to anti-angiogenic therapy: Experience with bevacizumab. Anticancer Res. 2007;27:3465–3470. [PubMed] [Google Scholar]
- 42.Mir OCR, Ropert S, Cabanes L, Blanchet B, Camps S, Billemont B, Knebelmann B, Goldwasser F. Treatment of bevacizumab-induced hypertension by amlodipine. Invest New Drugs. 2012;30:702–707. doi: 10.1007/s10637-010-9549-5. [DOI] [PubMed] [Google Scholar]
- 43.Mir O, Coriat R, Ropert S, Cabanes L, Blanchet B, Camps S, Billemont B, Knebelmann B, Goldwasser F. Treatment of bevacizumab-induced hypertension by amlodipine. Investigational new drugs. 2012;30:702–707. doi: 10.1007/s10637-010-9549-5. [DOI] [PubMed] [Google Scholar]
- 44.Izzedine H, Ederhy S, Goldwasser F, Soria JC, Milano G, Cohen A, Khayat D, Spano JP. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol. 2009;20:807–815. doi: 10.1093/annonc/mdn713. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 46.Izzedine H, Rixe O, Billemont B, Baumelou A, Deray G. Angiogenesis inhibitor therapies: Focus on kidney toxicity and hypertension. Am J Kidney Dis. 2007;50:203–218. doi: 10.1053/j.ajkd.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 47.Eremina V, Quaggin SE. Biology of anti-angiogenic therapy-induced thrombotic microangiopathy. Semin Nephrol. 2010;30:582–590. doi: 10.1016/j.semnephrol.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Curwen JO, Musgrove HL, Kendrew J, Richmond GH, Ogilvie DJ, Wedge SR. Inhibition of vascular endothelial growth factor-a signaling induces hypertension: Examining the effect of cediranib (recentin; azd2171) treatment on blood pressure in rat and the use of concomitant antihypertensive therapy. Clin Cancer Res. 2008;14:3124–3131. doi: 10.1158/1078-0432.CCR-07-4783. [DOI] [PubMed] [Google Scholar]
- 49.Langenberg MH, van Herpen CM, De Bono J, Schellens JH, Unger C, Hoekman K, Blum HE, Fiedler W, Drevs J, Le Maulf F, Fielding A, Robertson J, Voest EE. Effective strategies for management of hypertension after vascular endothelial growth factor signaling inhibition therapy: Results from a phase ii randomized, factorial, double-blind study of cediranib in patients with advanced solid tumors. J Clin Oncol. 2009;27:6152–6159. doi: 10.1200/JCO.2009.22.2273. [DOI] [PubMed] [Google Scholar]
- 50.Dirix LY, Maes H, Sweldens C. Treatment of arterial hypertension (aht) associated with angiogenesis inhibitors. Ann Oncol. 2007;18:1121–1122. doi: 10.1093/annonc/mdm205. [DOI] [PubMed] [Google Scholar]
- 51.Cooke JP, Losordo DW. Nitric oxide and angiogenesis. Circulation. 2002;105:2133–2135. doi: 10.1161/01.cir.0000014928.45119.73. [DOI] [PubMed] [Google Scholar]
- 52.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Webb DJ. Dorado: Opportunity postponed: Lessons from studies of endothelin receptor antagonists in treatment-resistant hypertension. Hypertension. 2010;56:806–807. doi: 10.1161/HYPERTENSIONAHA.110.160952. [DOI] [PubMed] [Google Scholar]
- 55.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 56.Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of sunitinib activity. Ann Oncol. 2007;18:1117. doi: 10.1093/annonc/mdm184. [DOI] [PubMed] [Google Scholar]
- 57.Bono P, Elfving H, Utriainen T, Osterlund P, Saarto T, Alanko T, Joensuu H. Hypertension and clinical benefit of bevacizumab in the treatment of advanced renal cell carcinoma. Ann Oncol. 2009;20:393–394. doi: 10.1093/annonc/mdn729. [DOI] [PubMed] [Google Scholar]
- 58.Rini BI, Schiller JH, Fruehauf JP, Cohen EE, Tarazi JC, Rosbrook B, Bair AH, Ricart AD, Olszanski AJ, Letrent KJ, Kim S, Rixe O. Diastolic blood pressure as a biomarker of axitinib efficacy in solid tumors. Clin Cancer Res. 2011;17:3841–3849. doi: 10.1158/1078-0432.CCR-10-2806. [DOI] [PubMed] [Google Scholar]
- 59.Goodwin R, Ding K, Seymour L, LeMaitre A, Arnold A, Shepherd FA, Dediu M, Ciuleanu T, Fenton D, Zukin M, Walde D, Laberge F, Vincent M, Ellis PM, Laurie SA. Treatment-emergent hypertension and outcomes in patients with advanced non-small-cell lung cancer receiving chemotherapy with or without the vascular endothelial growth factor receptor inhibitor cediranib: Ncic clinical trials group study br24. Ann Oncol. 2010;21:2220–2226. doi: 10.1093/annonc/mdq221. [DOI] [PubMed] [Google Scholar]
- 60.Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, Figlin RA, Baum MS, Motzer RJ. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–773. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J, Dickler M, Perez EA, Cobleigh M, Shenkier T, Edgerton S, Miller KD. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: Ecog 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JJ, Vaziri SA, Rini BI, Elson P, Garcia JA, Wirka R, Dreicer R, Ganapathi MK, Ganapathi R. Association of vegf and vegfr2 single nucleotide polymorphisms with hypertension and clinical outcome in metastatic clear cell renal cell carcinoma patients treated with sunitinib. Cancer. 2012;118:1946–1954. doi: 10.1002/cncr.26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, Bergsland E, Ngai J, Holmgren E, Wang J, Hurwitz H. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 64.Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis of randomized controlled trials. Acta Oncol. 2010;49:287–297. doi: 10.3109/02841860903524396. [DOI] [PubMed] [Google Scholar]
- 65.Schutz FA, Je Y, Azzi GR, Nguyen PL, Choueiri TK. Bevacizumab increases the risk of arterial ischemia: A large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22:1404–1412. doi: 10.1093/annonc/mdq587. [DOI] [PubMed] [Google Scholar]
- 66.Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: A systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–2285. doi: 10.1200/JCO.2009.27.2757. [DOI] [PubMed] [Google Scholar]
- 67.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA. 2008;300:2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 68.Hurwitz HI, Saltz LB, Van Cutsem E, Cassidy J, Wiedemann J, Sirzen F, Lyman GH, Rohr UP. Venous thromboembolic events with chemotherapy plus bevacizumab: A pooled analysis of patients in randomized phase ii and iii studies. J Clin Oncol. 2011;29:1757–1764. doi: 10.1200/JCO.2010.32.3220. [DOI] [PubMed] [Google Scholar]