Abstract
Mucopolysaccharidosis IIIA (MPS IIIA or Sanfilippo disease) is a neurodegenerative disorder caused by a deficiency in the lysosomal enzyme sulfamidase (SGSH), catabolizing heparan sulfate (HS). Affected children present with severe behavioral abnormalities, sleep disturbances, and progressive neurodegeneration, leading to death in their second decade. MPS I, a similar neurodegenerative disease accumulating HS, is treated successfully with hematopoietic stem cell transplantation (HSCT) but this treatment is ineffectual for MPS IIIA. We compared HSCT in MPS IIIA mice using wild-type donor cells transduced ex vivo with lentiviral vector-expressing SGSH (LV-WT-HSCT) versus wild-type donor cell transplant (WT-HSCT) or lentiviral-SGSH transduced MPS IIIA cells (LV-IIIA-HSCT). LV-WT-HSCT results in 10% of normal brain enzyme activity, near normalization of brain HS and GM2 gangliosides, significant improvements in neuroinflammation and behavioral correction. Both WT-HSCT and LV-IIIA-HSCT mediated improvements in GM2 gangliosides and neuroinflammation but were less effective at reducing HS or in ameliorating abnormal HS sulfation and had no significant effect on behavior. This suggests that HS may have a more significant role in neuropathology than neuroinflammation or GM2 gangliosides. These data provide compelling evidence for the efficacy of gene therapy in conjunction with WT-HSCT for neurological correction of MPS IIIA where conventional transplant is ineffectual.
Introduction
Mucopolysaccharidosis IIIA (MPS IIIA or Sanfilippo type A) is a neurodegenerative lysosomal storage disease resulting from a deficiency in the enzyme sulfamidase (N-sulfoglucosamine sulfohydrolase, SGSH, EC 3.10.1.1), caused by mutations in the SGSH gene.1 The enzyme deficiency leads to accumulation of heparan sulfate (HS) in cells, leading to cellular and organ dysfunction, particularly in the brain.1 Patients present with progressive failure to achieve developmental milestones, severe behavioral changes including hyperactivity and sleep disturbances, later cognitive and motor function decline and a markedly shortened lifespan.1-3 The age of presentation and severity of symptoms varies significantly. Disease neuropathology is poorly understood, with several factors probably contributing to the onset of disease including primary HS storage in the brain, secondary storage of GM gangliosides, amongst other lipids,4,5 and severe neuroinflammation.6-8 There are no current treatments for MPS III.
Intravenous enzyme replacement therapy is a successful treatment for attenuated MPS diseases storing HS, such as MPS I Hurler-Scheie, which has limited neurological involvement due to residual enzyme activity in the brain. In this case, delivered recombinant enzyme is taken up by mannose-6-phosphate receptors and cross-corrects residual enzyme-deficient recipient cells. However, the presence of antibodies against the recombinant enzyme may limit the effectiveness of this therapy.9 Since enzyme is unable to cross the blood brain barrier, intravenous enzyme replacement therapy is ineffective in neuronopathic MPS diseases including MPS I Hurler (IH) and MPS IIIA.
Patients with MPS IH usually receive hematopoietic stem cell transplantation (HSCT).10,11 Donor cells repopulate the recipient's hematopoietic system and engrafted donor leukocytes secrete enzyme that can cross-correct cells in the periphery. In addition, monocytes traffic from the bone marrow into the brain where they differentiate into microglial cells and mediate cross-correction in the recipient central nervous system.12 As long as treatment is delivered early in life, this results in significant beneficial effects on cognitive outcomes, lifespan, and peripheral bone and joint disease in MPS IH patients.10,11,13
In contrast, MPS IIIA patients show increased lifespan but no significant neurological improvements after HSCT, despite storage of very similar substrates in the brain.13,14,15 Following unrelated cord blood transplants, one year patient survival rates are similar (77% MPS IH, 79% MPS III) but 3-year patient survival is markedly different (75% MPS IH, 56% MPS III), suggesting that engraftment is successful but that transplant is not curative for MPS III.15 We have recently reported that metabolic correction (expressed as reduction of glycosaminoglycan (GAG) substrate), of MPS I patients receiving transplants from heterozygote donors with one enzyme gene copy, is less complete than those receiving unrelated transplants from homozygous donors with two enzyme gene copies.16 HSCT failure in MPS IIIA patients could therefore be due to insufficient enzyme being produced by donor-derived microglia in the brain,13,14 while gene therapy could be an approach to increase secreted enzyme in the brain beyond that achieved by wild-type transplantation.
A clinically relevant gene therapy approach for MPS IIIA and the clinically indistinguishable MPS IIIB, is direct brain delivery of recombinant AAV.6,17,18 However, this approach is very invasive and has potential scale-up issues with limited distribution of vector from the injection sites in the brain,19,20 as well as the potential for immune responses in patients exposed directly to vector or enzyme.21
The alternative approach of ex vivo gene delivery to HSCs, using a lentiviral vector (LV-HSCT), has become progressively more clinically achievable for neurodegenerative metabolic diseases in recent years. This is due to vastly improved HSCT survival rates, of over 90% for MPS IH,10 and several studies showing the potential for correction of neurodegenerative diseases via HSC modification.22-25 Ex vivo LV-HSCT was used to replace the arylsulfatase A enzyme in a mouse model of metachromatic leukodystrophy, and achieved 10% of normal brain enzyme and neuronal correction,23 which has resulted in an ongoing clinical trial. In MPS I, erythroid-specific LV-HSCT resulted in neurological correction of mice,26 while another LV-HSCT approach has resulted in 4.5-fold increases in brain enzyme and significant improvements in peripheral disease in MPS I mice.22 In mouse models of MPS IIIA and IIIB, HSCT alone is unable to correct the neurological phenotype.17,27 However, an oncoretroviral HSCT approach in MPS IIIB mice resulted in 25% of normal brain enzyme levels in two cases; although copy numbers were not stated and behavioral analysis was not performed.28 No data are published on LV-HSCT in MPS IIIA.
To evaluate the efficacy of LV-HSCT against normal HSCT in MPS IIIA mice we compared MPS IIIA mice receiving either WT or MPS IIIA cells that were ex vivo transduced with an SGSH expressing lentiviral vector, against mice receiving normal WT-HSCT. We have demonstrated that LV-HSCT in WT cells can significantly increase the SGSH enzyme activity in the brain, normalize brain HS, reduce secondary pathology, and correct behavior of MPS IIIA mice where normal HSCT fails.
Results
Transduction with SGSH lentiviral vector results in significant gene expression without toxicity
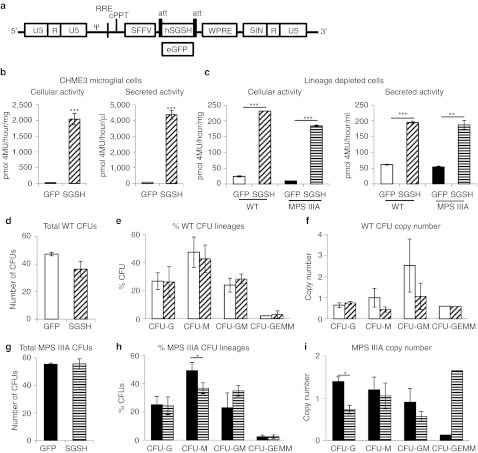
We constructed a lentiviral vector-expressing human SGSH or enhanced green fluorescent protein (eGFP) under the internal spleen focus-forming virus (SFFV) promoter (Figure 1a). To confirm that microglia could over-express SGSH without toxicity, the human microglial cell line, CHME3, was transduced at an multiplicity of infection of 10 with the lentiviral vector containing eGFP or SGSH. A 73-fold increase in cellular SGSH activity (Figure 1b) and a 37-fold increase in secreted SGSH activity was observed without cellular toxicity. We then transduced WT and MPS IIIA lineage depleted (lin-) bone marrow at an multiplicity of infection of 25 with the lentiviral vector containing eGFP or SGSH. A 15- and 12-fold increase over WT, in cellular SGSH activity (Figure 1c) was present in SGSH transduced WT and MPS IIIA HSCs respectively and a 18- and 19-fold increase in secreted SGSH activity. We then assessed the effect of transduction on the number of colony-forming units using methylcellulose culture. There was no difference in the total number of colonies with WT lin– cls (Figure 1d) or MPS IIIA lin– cells (Figure 1g). There was also no difference in the proportion of different colony types with WT lin– cells (Figure 1e), however, with MPS IIIA lin– cells there was a reduction in the proportion of colony-forming unit-M after SGSH transduction (P = 0.036) (Figure 1h). There was a trend towards higher vector copies in the eGFP transduced colonies compared to SGSH but this was only significantly elevated in colony-forming unit-G with MPS IIIA lin– cells (Figure 1f,i).
Figure 1.
Lentiviral N-sulfoglucosamine sulfohydrolase (SGSH) transduced microglia and hematopoietic stem cells (HSCs) have improved SGSH activity. (a) The lentiviral vector plasmid pHR'SIN-cPPT-SEW40 was converted to pHRsin.SFFV.hSGSH.att.wpre and pHRsin.SFFV.eGFP.att.wpre. These plasmids were used to produce lentiviral vector containing human SGSH or enhanced green fluorescent protein (eGFP) under the internal spleen focus-forming virus (SFFV) promoter. (b) The human microglial cell line CHME3 was transduced at an multiplicity of infection (MOI) of 10 with LV-SGSH or LV-eGFP and enzyme activity measured in cells and media. (c) Lineage depleted wild-type (WT) and mucopolysaccharidosis IIIA (MPS IIIA) bone marrow was transduced at an MOI of 25 with a lentiviral vector-expressing eGFP or SGSH, after 60 hours the enzyme activity was measured in cells and media. (d) Lineage depleted WT bone marrow was transduced at an MOI of 25 with a lentiviral vector-expressing eGFP or SGSH, after 14 days of culture in methylcellulose the number of colonies were counted. (d) The total number of colonies, (e) the percentage of the different colony types, and (f) the vector copy number was determined. (g) Lineage depleted MPS IIIA bone marrow was transduced at an MOI of 25 with a lentiviral vector-expressing eGFP or SGSH, after 14 days of culture in methylcellulose the number of colonies were counted. (g) The total number of colonies, (h) the percentage of the different colony types, and (i) the vector copy number was determined. Error bars represent the standard error of the mean (SEM) and significant difference is demonstrated with *P <0.05, **P <0.01 and ***P <0.001.
LV-HSCT results in significantly increased SGSH activity in the MPS IIIA brain
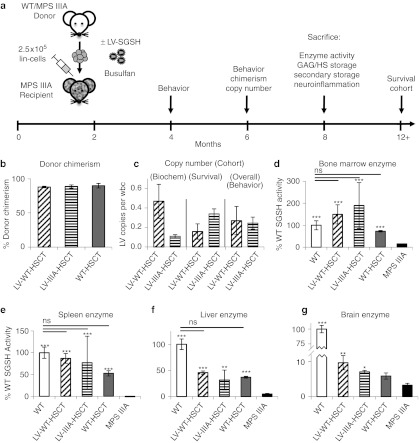
In order to evaluate whether ex vivo transduction of HSCs using LV-SGSH could improve treatment outcomes in MPS IIIA mice, WT, or MPS IIIA lin– bone marrow was either mock-transduced or transduced with LV-SGSH before transplant into busulfan myeloablated 6–8-week-old MPS IIIA mice (n = 16 per group) (Figure 2a). Age-matched control groups of WT to WT transplant, untransplanted WT and MPS IIIA were used for comparison (n = 16 per group). At 16 weeks post-transplant, peripheral blood chimerism was 88, 89, and 90% in the LV-WT-HSCT, LV-IIIA-HSCT, and WT-HSCT groups, respectively (Figure 2b). The average copy number of the biochemistry/histology group (Figure 2–4 and Supplementary Figure S1) was 0.47 integrations per white blood cell for LV-WT-HSCT and 0.11 integrations for LV-IIIA-HSCT groups (Figure 2c), however, the copy number for the LV-WT-HSCT survival group (Figure 6) was only 0.16 and for LV-IIIA-HSCT was 0.34. Overall for the behavioral group (survival and histology cohorts combined, Figure 5 and Supplementary Figure S2) the copy number was 0.27 for LV-WT-HSCT and 0.25 for LV-IIIA-HSCT. No adverse events or leukemic blasts have been observed on blood smears from all mice including the survival cohort, kept for over 1 year post-transplant (n = 6 per group).
Figure 2.
Lentiviral (LV)-N-sulfoglucosamine sulfohydrolase (SGSH) improves enzyme activity in the brain of mucopolysaccharidosis IIIA (MPS IIIA) mice. (a) Lineage depleted bone marrow was transduced or untransduced with a lentiviral vector-expressing human SGSH under the SFFV promoter and transplanted into busulfan conditioned recipients at 2 months (8 weeks) of age. Groups were; wild-type (WT) to WT transplant, WT untreated, MPS IIIA untreated, WT donor cells transduced with LV-SGSH into MPS IIIA recipients (LV-WT-HSCT), MPS IIIA donor cells transduced with LV-SGSH into MPS IIIA recipients (LV-IIIA-HSCT) and WT to MPS IIIA (WT-HSCT) (n = 16 per group). An open-field behavioral test was performed at 4 and 6 months (16 and 24 weeks) of age, chimerism and copy number were determined at 6 months of age and at 8 months of age (32 weeks of age, 24 weeks post-transplant), mice were sacrificed for biochemical and histological analysis and a cohort was kept for survival and sacrificed at their humane endpoint. (b) Donor chimerism was determined using flow cytometry at 16 weeks post-transplant. (c) Lentiviral vector copy number in white blood cells was determined by quantitative PCR (QPCR) at 16 weeks post-transplant. The average for the biochemistry/histology group (Figures 2–4 and Supplementary Figure S1), survival group (Figure 6) and overall/behavior group (Figure 5 and Supplementary Figure S2) has been shown. SGSH enzymatic activity was measured in (d) bone marrow (e) spleen, (f) liver, and (g) brain. Error bars represent the SEM. Significant difference to MPS IIIA is demonstrated with *P < 0.05, **P < 0.01, and ***P < 0.001. Where treatments result in significant improvement to MPS IIIA and there is no significant difference to WT, this is shown by a line and ns. Groups were; WT untreated (WT), WT donor cells transduced with LV-SGSH into MPS IIIA recipients (LV-WT-HSCT), MPS IIIA donor cells transduced with LV-SGSH into MPS IIIA recipients (LV-IIIA-HSCT), WT to MPS IIIA (WT-HSCT), and MPS IIIA untreated (MPS IIIA).
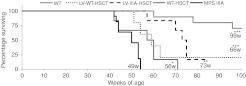
Figure 6.
Lentiviral (LV)-N-sulfoglucosamine sulfohydrolase (SGSH) increases lifespan. A cohort of 6–10 mice from each group were kept to 92 weeks of age. Mice were sacrificed when they reached their humane end point, frequently caused due to urine retention. The estimated mean survival in weeks (w) for each group is shown at the bottom of the curve. The estimated mean survival was calculated by Mantel–Cox log rank pairwise comparisons, where data points were censored the largest survival time was used. Significant difference to mucopolysaccharidosis IIIA (MPS IIIA) is demonstrated with *P < 0.05, **P < 0.01, and ***P < 0.001 Groups were: wild-type (WT) untreated (WT), WT donor cells transduced with LV-SGSH into MPS IIIA recipients (LV-WT-HSCT), MPS IIIA donor cells transduced with LV-SGSH into MPS IIIA recipients (LV-IIIA-HSCT), WT to MPS IIIA (WT-HSCT) and MPS IIIA untreated (MPS IIIA). The copy number of the survival cohort was 0.16 copies per white blood cell for LV-WT-HSCT and 0.34 for LV-IIIA-HSCT.
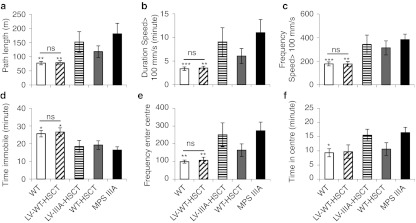
Figure 5.
Lentiviral (LV)-wild-type (WT)-HSCT corrects behavior. The open-field behavior test was performed for one hour at the same point of the circadian rhythm at 6 months (24 weeks) of age (n = 10 female mice per group). The measures of hyperactivity were: (a) path length, (b) duration spent moving over 100 mm/s, (c) frequency spent moving over 100 mm/s, (d) duration immobile, while (e) frequency of centre entries and (f) duration in centre measures thigmotaxis and may be a measure of sense of danger. Error bars represent the SEM. Significant difference to mucopolysaccharidosis IIIA (MPS IIIA) is demonstrated with *P < 0.05, **P < 0.01, and ***P < 0.001. Where treatments result in significant improvement to MPS IIIA and there is no significant difference to WT this is shown by a line and ns. Groups were; WT untreated (WT), WT donor cells transduced with LV-SGSH into MPS IIIA recipients (LV-WT-HSCT), MPS IIIA donor cells transduced with LV-SGSH into MPS IIIA recipients (LV-IIIA-HSCT), WT to MPS IIIA (WT-HSCT), and MPS IIIA untreated (MPS IIIA). The copy number of the behavioral cohort was 0.27 copies per white blood cell for LV-WT-HSCT and 0.25 for LV-IIIA-HSCT.
To determine the outcome of therapy 5 mice per group were sacrificed at 8 months of age and perfused with Tyrode's to flush blood from organs. SGSH enzyme activity was measured in the bone marrow, spleen, liver, and brain (Figure 2d–g). Untreated MPS IIIA mice expressed ~3% of WT activity.4 All treatments significantly increased bone marrow SGSH activity to 150, 189, and 73% of WT levels by LV-WT-HSCT, LV-IIIA-HSCT, and WT-HSCT, respectively, these were not significantly different from each other or WT (Figure 2d). All treatments significantly increased SGSH activity in the spleen to 87, 77, and 53% of WT levels by LV-WT-HSCT, LV-IIIA-HSCT, and WT-HSCT, respectively (Figure 2e). In the liver, SGSH activity was significantly increased to 46, 32, and 37% of WT activity by LV-WT-HSCT, LV-IIIA-HSCT, and WT-HSCT, respectively (Figure 2f). In the brain, SGSH activity was significantly increased to 10% of WT levels (P = 0.007) by LV-WT-HSCT and just significantly to 7% of WT levels (P = 0.05) by LV-IIIA-HSCT. WT-HSCT increased activity to 6% of WT levels, but this was not significantly elevated over residual MPS IIIA brain enzyme activity of 3% (P = 0.2) (Figure 2g).
LV-WT-HSCT normalizes primary HS storage and sulfation in the MPS IIIA brain
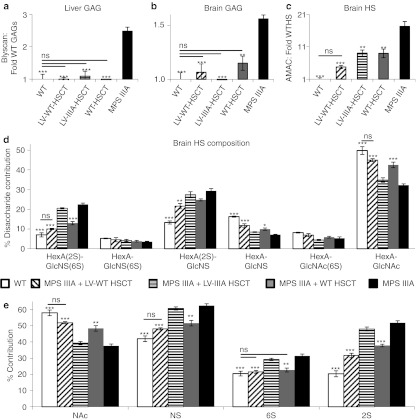
Total sulfated GAGs, including HS, were measured in the liver and brain (Figure 3a,b). In the liver, GAGs were normalized to WT levels by LV-WT-HSCT and WT-HSCT, with near normalization by LV-IIIA-HSCT. In the brain, LV-WT-HSCT and LV-IIIA-HSCT both normalized GAGs to WT levels, whilst WT-HSCT significantly reduced GAG levels. Total brain HS levels were measured following 2-aminoacridone (AMAC)-labeled disaccharide analysis (Figure 3c), revealing that all transplants significantly decreased total brain HS. WT-HSCT and LV-IIIA-HSCT both decreased HS storage by 51%, whereas LV-WT-HSCT normalized brain HS levels to those of WT with a 77% reduction.
Figure 3.
Lentiviral (LV)-N-sulfoglucosamine sulfohydrolase (SGSH) reduces primary storage in mucopolysaccharidosis IIIA (MPS IIIA) mice. At 8 months of age the level of sulfated glycosaminoglycans was determined using the Blyscan assay in the (a) liver and (b) brain. (c) The level of HS storage in the brain was determined by 2-aminoacridone (AMAC). (d) The different HS disaccharides were quantified. (e) The relative proportion of HS that was N-acetylated (NAc), N-sulfated (NS), 6-O-sulfated (6S), and 2-O-sulfated (2S) was also determined by AMAC. Error bars represent the SEM. Significant difference to MPS IIIA is demonstrated with *P < 0.05, **P < 0.01 and ***P < 0.001. Where treatments result in significant improvement to MPS IIIA and there is no significant difference to wild-type (WT), this is shown by a line and ns. Groups were; WT untreated (WT), WT donor cells transduced with LV-SGSH into MPS IIIA recipients (LV-WT-HSCT), MPS IIIA donor cells transduced with LV-SGSH into MPS IIIA recipients (LV-IIIA-HSCT), WT to MPS IIIA (WT-HSCT) and MPS IIIA untreated (MPS IIIA). The average copy number of the biochemistry/histology group was 0.47 integrations per white blood cell for LV-WT-HSCT and 0.11 integrations for LV-IIIA-HSCT groups.
Six characteristic HS disaccharides are revealed by AMAC analysis (Figure 3d). Overall, MPS IIIA mouse brains displayed significant increases in the four most highly sulfated disaccharides HexA(2S)-GlcNS(6S) (P = 5.6 × 10−7) and HexA(2S)-GlcNS (P = 7.8 × 10−5), and commensurate reductions in HexA-GlcNS (P = 1.2 × 10−5) and HexA-GlcNAc (P = 2.7 × 10−5). This led to overall significant increases in N- (P = 0.00002), 2-O- (P = 4.3 × 10−7) and 6-O-sulfation (P = 0.0007) and a commensurate reduction in N-acetylated (unsulfated, P = 0.00002) regions over WT (Figure 3e). LV-IIIA-HSCT was unable to change this abnormal profile, while WT-HSCT significantly improved outcomes in 3 out of 4 abnormal disaccharides (P < 0.03–0.00001) and LV-WT-HSCT further improved all 4 abnormal disaccharide profiles (P < 0.01–1 × 10−6) and normalized HexA(2S)-GlcNS(6S) and HexA-GlcNAc to WT proportions (Figure 3d). Both WT-HSCT and LV-WT-HSCT significantly improved abnormal HS sulfation patterning towards normal WT levels, but only LV-WT-HSCT was able to fully normalize this in the case of NAc, N- and 6-O-sulfation to WT levels (P = 0.0001, P = 0.0001, P = 0.0006, Figure 3e). 2-O-sulfation remained significantly increased over WT (P = 0.002). LV-IIIA-HSCT had no significant effect on HS sulfation patterning.
LV-WT-HSCT and WT-HSCT reduce secondary storage and neuroinflammation equally
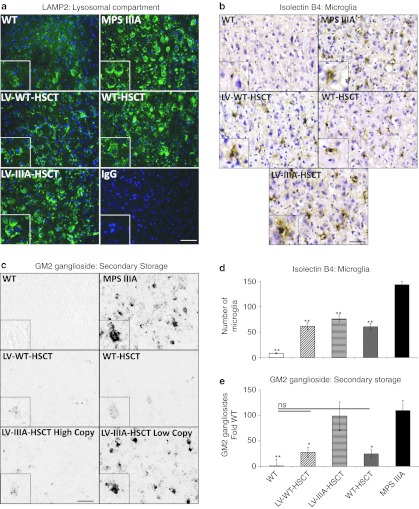
In order to determine whether treatment had an effect on lysosomal swelling, we stained the cerebral cortex for lysosomal associated membrane protein 2 (LAMP2) (green) and a nuclear stain, DAPI (blue) (Figure 4a). MPS IIIA mice exhibited cells with large, dense LAMP2 staining around the nucleus, while WT mice displayed discrete punctate LAMP2 staining in a perinuclear location. LV-IIIA-HSCT did not appear to have an effect on abnormal MPS IIIA lysosomal morphology while WT-HSCT was able to reduce some of this pathology and LV-WT-HSCT resulted in cells with substantial reduction of abnormal LAMP2 staining.
Figure 4.
Neuropathology is improved by all treatments. (a) Representative sections of brain cortex (layer IV/V) from –0.84 mm relative to bregma were stained with LAMP2 (green) to demonstrate lysosomal compartment size and DAPI (blue) to highlight nuclei. (Bar = 50 µm in low power and 100 µm in high power insert). (b) Representative images of cerebral cortex layer IV/V from around –0.46 mm relative to bregma showing isolectin B4-positive microglia (brown) and nuclei (blue) and (c) GM2 ganglioside (black). Two images of lentiviral (LV)-IIIA HSCT are shown, one with a high copy and one with a low copy to demonstrate the variable response in this group (Bar = 50 µm in low power and 100 µm in high power insert). (d) The number of microglia were counted, and (e) GM2 ganglioside storage was quantified using Image J from two fields of view (×20 objective) per brain section, four sections per mouse (n = 5 mice per group). Error bars represent the SEM. Significant difference to mucopolysaccharidosis IIIA (MPS IIIA) is demonstrated with *P < 0.05, **P < 0.01, and ***P < 0.001. Where treatments result in significant improvement to MPS IIIA and there is no significant difference to wild-type (WT) this is shown by a line and ns. Groups were; WT untreated (WT), WT donor cells transduced with LV-N-sulfoglucosamine sulfohydrolase (SGSH) into MPS IIIA recipients (LV-WT-HSCT), MPS IIIA donor cells transduced with LV-SGSH into MPS IIIA recipients (LV-IIIA-HSCT), WT to MPS IIIA (WT-HSCT) and MPS IIIA untreated (MPS IIIA). The representative high copy LV-IIIA-HSCT had a copy of 0.14 copies per white blood cell and the low copy contained 0.08 copies per white blood cell. The average copy number of the biochemistry/histology group was 0.47 integrations per white blood cell for LV-WT-HSCT and 0.11 integrations for LV-IIIA-HSCT groups.
Severe neuroinflammation in MPS IIIA mice is measurable by the significant infiltration of Isolectin B4-positive microglial cells into the cerebral cortex (Figure 4b,d). WT mice have virtually no microglia in this location. Microglial numbers were significantly reduced by 61% in LV-WT-HSCT (P = 1 × 10−7), 50% in LV-IIIA-HSCT (P = 3 × 10−6) and 61% in WT-HSCT (P = 1 × 10−7), but there was no significant difference between treatments and all remained significantly elevated above WT.
MPS IIIA mouse brains also displayed significant secondary storage of GM2 gangliosides, particularly in lamina II/III and V/VI of the primary motor, somatosensory, and parietal areas of the cerebral cortex (Figure 4c,e) while no staining was detected in these areas in WT mice.5 A significant decrease in GM2 gangliosides of 76% (P = 0.02) was detected after LV-WT-HSCT and 78% (P = 0.02) after WT-HSCT, normalizing GM2 gangliosides to WT levels. Treatment with LV-IIIA-HSCT did not significantly reduce GM2 gangliosides. However, there was significant variability between mice with some almost completely corrected and others hardly corrected at all (Figure 4c). More effective GM2 ganglioside clearance correlated with higher lentiviral copy number. GM2 ganglioside storage was observed in the amygdala in MPS IIIA mice, but did not appear to be reduced by any treatment (Supplementary Figure S1).
MPS IIIA behavior is fully corrected by LV-WT-HSCT but unchanged by WT-HSCT or LV-IIIA-HSCT
At 4 and 6 months of age a 60-minute open-field test was performed to determine the effect of treatment on mouse behavior.29 In order to control for the effect of busulfan conditioning we initially compared age-matched untransplanted WT mice with WT to WT transplanted mice, and found their behavior to be indistinguishable (data not shown). In our hands, MPS IIIA mice present a phenotype of hyperactive behavior with increased path length, duration and frequency of rapid exploratory behavior, while immobility time is reduced. MPS IIIA mice also display reduced thigmotaxis which we interpret as a reduced sense of danger, with increased frequency and duration of centre entries.30 At 4 months of age, MPS IIIA mice showed increased hyperactive behavior30 with significantly increased path length (P = 0.034, Supplementary Figure 2a) but no significant change in duration or frequency of rapid exploratory behavior or immobility (Supplementary Figure 2b–d). Frequency and duration of centre entries are significantly increased at 4 months (Supplementary Figure 2e,f). Although no significant reductions are seen with any treatment at this age, LV-IIIA-HSCT and WT-HSCT appear to have little effect on behavior while LV-WT-HSCT shows a trend towards normalization of every parameter to WT levels except centre entry duration.
At 6 months of age, MPS IIIA behavior diverges further from the WT control mice30 to give highly significant differences in path length (Figure 5a, P = 0.002), duration (Figure 5b, P = 0.001), and frequency of speed over 100 mm/s (Figure 5c, P = 0.002), immobility time (Figure 5d, P = 0.02), frequency of centre entries (Figure 5e, P = 0.001), and duration in the centre (Figure 5f, P = 0.04). These behaviors can be observed in Supplementary Video S1 and strongly suggest increases in hyperactivity and a reduced sense of danger.30
Neither LV-IIIA-HSCT nor WT-HSCT were able to significantly improve any of these abnormal behaviors, although WT-HSCT did show a trend towards correction in sense of danger measures (frequency and duration of centre entries, Figure 5e,f). Hyperactivity in particular, was not corrected by either treatment.
In contrast LV-WT-HSCT significantly improved path length (Figure 5a, P = 0.01), duration (Figure 5b, P = 0.004), and frequency of speed over 100 mm/s (Figure 5c, P = 0.009), immobility time (Figure 5d, P = 0.04), and frequency of centre entries (Figure 5e, P = 0.007), with a nonsignificant trend to reduction in centre duration (Figure 5f, P = 0.11). All behaviors were normalized to WT levels and none were significantly different from the WT group. In the behavior cohorts (all mice) average copy number was 0.27 for LV-WT-HSCT and 0.25 for LV-IIIA-HSCT.
LV-HSCT increases the lifespan of MPS IIIA mice
A cohort of 5–10 randomly selected mice per group were kept to assess the effect of treatment on survival (Figure 6). WT and WT-WT transplanted groups were pooled as survival is not significantly affected in this group. Three WT mice reached the humane end point by week 100, the end of the study. Untreated MPS IIIA mice all died between weeks 42–54 with a mean survival of 49 weeks. WT-HSCT-treated mice all died between weeks 42–71 with significantly improved survival (P = 0.03). LV-WT-HSCT delayed death to weeks 51–>100 (P = 0.01) with one mouse from this group still surviving at week 100, the estimated mean survival (66) was 10 weeks longer than WT-HSCT (56). Interestingly, LV-IIIA-HSCT delayed death to weeks 57–>85 (P = 0.0002, estimated mean 73), with one mouse still surviving from this group at week 85, and had significantly better survival than WT HSCT (P = 0.01). The lentiviral copy number was determined from blood taken at 6 months of age. The copy number of the survival cohort of LV-IIIA-HSCT mice was 0.34 copies/cell, which was significantly higher than the biochemistry cohort (0.11 copies) and 0.16 for LV-WT-HSCT which was significantly lower than the biochemistry cohort (0.47 copies).
Discussion
This study is the first to compare a lentiviral vector-based HSCT gene therapy approach against normal transplantation in Sanfilippo disease. Although WT-HSCT is able to significantly improve many pathological markers in the brain of MPS IIIA mice it was unable to significantly improve brain enzyme levels or significantly improve abnormal behavior. In contrast, augmentation of brain enzyme by transduction of WT HSCs with a lentiviral vector expressing the SGSH gene before transplant (LV-WT-HSCT), improved all pathological markers as well as, or better than WT-HSCT and normalized several of these markers to WT levels. In particular, LV-WT-HSCT was able to significantly improve brain enzyme levels to 10% of normal and fully correct behavior of MPS IIIA mice. We also transduced MPS IIIA HSCs with a lentiviral vector expressing the SGSH gene (LV-IIIA-HSCT) and observed a phenotype somewhere between WT-HSCT and LV-WT-HSCT treatments.
HSCT in MPS IIIA patients was discontinued in the UK more than a decade ago as it was found to have no significant effect on neurological outcomes, despite some improvement in lifespan.13,14 Our data on WT-HSCT outcomes in MPS IIIA mice are consistent with these clinical observations and are also consistent with similar data obtained after whole bone marrow transplant of irradiated MPS IIIA mice.27 More recently, cord blood transplantation has been performed in 19 MPS IIIA and IIIB patients,15 of which 12 survived and 9 had disease stabilization, but there was limited impact on cognitive function. In two patients that were transplanted before 2 years of age, modest gains in cognitive functions have been reported. Overall the transplanted patients appear to sleep better and have fewer behavioral problems, but behavior is not corrected and this treatment remains controversial.
Our lentiviral vector is capable of effective gene expression in human microglial cells and we have previously shown it to be expressed in all murine and human hematopoietic lineages31 producing therapeutic protein for over a year in mice.32 In the periphery, very significant improvements in normal enzyme activity are clearly present, but in the perfused brain, only LV-WT-HSCT (10%) (P < 0.01) and LV-IIIA-HSCT (7%) (P < 0.05), were able to significantly increase enzyme activity above baseline levels of 3%, with WT-HSCT close, but not significant at 6%, suggesting that SGSH activity from donor WT microglial cells is probably around 3%, while gene therapy is providing around 4% for copy numbers of 0.11–0.5 in the biochemistry cohort. The copy number achieved in vivo with LV-SGSH is lower than that of LV-eGFP, which we speculated could be due to SGSH toxicity in hematopoietic stem or progenitor cells. However, when lin− cells are transduced with SGSH or eGFP-expressing lentiviral vectors, similar numbers of colony-forming units are obtained and no lineage skewing is observed (Figure 1) suggesting that this is not the case.
Total HS and HS composition were significantly altered in MPS IIIA mouse brains. We have recently shown that similar increases in amount of HS and sulfation composition, particularly in 2-O sulfation of HS are also present in MPS I.33 In the same work we also show that HS is present in significant excess in nonlysosomal and extracellular locations,33 and in unpublished work, show a functional role for abnormal HS sulfation in altered cell-to-cell signaling. Only LV-WT-HSCT was able to normalize both the amount of HS and the NAc, N- and 6-O-S sulfation patterning. Despite significant reductions in 2-O sulfation of HS by LV-WT-HSCT this was not normalized, suggesting that treatment could probably be improved further. Total GAG measurements based on the Blyscan assay are much less sensitive than the AMAC method of HS analysis and this may be why we are unable to see differences between LV-WT-HSCT and WT-HSCT in the brain using this method. LV-IIIA-HSCT significantly reduced total HS but did not alter composition. LV-IIIA-HSCT was the most variable treatment and this discrepancy could just reflect variable copy numbers between mice. This was particularly evident in GM2 ganglioside analysis where some mice were corrected by LV-IIIA-HSCT, even in the amygdala region, and others completely uncorrected (Supplementary Figure S1). This correlated with copy number.
Both LV-WT-HSCT and WT-HSCT reduced the number of infiltrating microglial cells in the cortex by similar amounts, while only LV-WT-HSCT was able to correct behavior. Microglial pathology was probably the least well corrected of all of the measured markers. This could suggest a relatively minor role for microglial infiltration in the neuropathology of MPS IIIA behavior. This is supported by work demonstrating that early neurodegeneration occurs independently of neuroinflammation in MPS IIIB mice.34
GM2 and GM3 gangliosides could have significant roles in neurodegeneration in MPS IIIA as they are components of lipid rafts and glycosynaptic microdomains, while increased GM2 gangliosides may affect signaling, adhesion, motility and growth in the brain.5 However, the ability to clear cerebral cortex GM2 ganglioside storage with WT-HSCT while LAMP2 lysosomal storage and behavior remain uncorrected, suggests that GM2 ganglioside may be less important than HS in neuropathology. Notably, GM2 ganglioside storage in the amygdala was not reduced by most treatments (except high copy number LV-IIIA-HSCT).
Neither WT-HSCT nor LV-IIIA-HSCT were able to significantly improve behavior. Although LV-WT-HSCT fully corrected most behaviors, the copy number achieved was low (0.27 copies per cell) and could be improved significantly. LV-IIIA-HSCT had a variable outcome in behavior and other parameters and this likely reflects the relatively variable copy number (0.08–0.55, average 0.25). This problem is most evident in the survival curves, where, against all expectation, the biggest improvement in survival was observed in the LV-IIIA-HSCT group, followed by LV-WT-HSCT and then WT-HSCT. The average copy number in the randomly selected LV-IIIA-HSCT survival cohort was 0.34, compared to 0.11 for the biochemistry cohort and the copy number of the LV-WT-HSCT survival group was lower, 0.16 compared to 0.47 for the biochemistry group.
The LV-HSCT approach has been applied to several other mouse models of disease. In a similar outcome to our own, 10% of normal ARSA brain expression was achieved in the mouse model of metachromatic leukodystrophy, which was associated with behavioral correction at 6 months of age.23 A clinical trial has now begun using this approach. In the mouse model of MPS I, up to 450% of normal brain enzyme levels were achieved and ~200% was associated with brain and peripheral disease correction.22 In globoid cell leukodystrophy ~16% of normal brain enzyme levels were achieved which improved survival of the mice, however comparing the mice that had <16% and those that had 16–25% reveals that better survival is achieved with higher enzyme levels.24 It is interesting to note the difference in achievable enzyme expression in the brain in MPS I against metachromatic leukodystrophy, MPS IIIA, and globoid cell leukodystrophy. The difference between the level of enzyme produced and the level required for correction in the brain for different enzymes could be due to the different normal expression levels, the amount that is actually required to fulfill normal catabolism and the amount of storage.
MPS IIIA still lacks any clinically approved treatment but there are three approaches in clinical development of significance. Intrathecal enzyme replacement therapy is currently being evaluated in the clinic having shown efficacy in MPS IIIA mice35 and dogs.36 This therapy is based on the premise that the spinal cerebrospinal fluid can distribute drug throughout the central nervous system. It may be hampered by a requirement for repeated, long-term delivery and adequate distribution within the brain cerebrospinal fluid. Another approach is direct injection of an AAV vector coexpressing SUMF1 and SGSH, successfully evaluated in mice,6 and now in clinical trial. It is unclear how well this treatment will scale from the 0.5 cm3 mouse brain to a 1,500 cm3 human brain. Finally, we have recently shown that a substrate reduction therapy approach using high dose genistein in MPS IIIB mice was able to improve neuropathology and behavior.37 Any of these approaches could potentially be used in combination with LV-HSCT to improve disease outcomes.
This study suggests two possible clinical strategies for lentiviral delivery of SGSH in allogeneic normal or autologous MPS IIIA transplants. Given the improved 1 year survival after HSCT in the similar disease MPS IH, of 93% in our centre38 and 91% in several European centres,10 a clinical approach using unrelated CD34+ cord blood donor cells for lentiviral transduction is potentially feasible and is likely to yield higher enzyme levels. The increased risk of allogeneic transplant, by using LV-WT-HSCT may be offset by the ability to achieve neuropathological and behavioral correction with copy numbers of 0.3 per cell, thus mitigating the risk of proto-oncogene transactivation. Currently, for clinical efficacy we would either require improved copy numbers or improved expression of SGSH to apply lentiviral-SGSH delivery in autologous MPS IIIA cells (LV-IIIA-HSCT).
In this study, we used an internal viral SFFV promoter; however, this is unlikely to receive regulatory approval as cellular promoters such as PGK have a lower risk of transformation.39 Therefore, to translate either the LV-WT-HSCT or LV-IIIA-HSCT approach to the clinic, mammalian promoters and methods of improving SGSH expression will need to be evaluated. SUMF1 modifies sulfatases such as SGSH by producing a formyl-glycine residue that is required for sulfatase activity, and overexpression of SUMF1 alongside SGSH has been shown to increase enzymatic activity by 1.5-fold.6 There have been reports that the level of SUMF1 expression is critical to achieve significantly improved SGSH expression but codon-optimization of SGSH could also improve expression.
In summary, the LV-WT-HSCT approach significantly improved brain SGSH activity to 10% of normal levels, normalizing HS storage, N-, 6-O sulfation, and unsulfated HS composition, GM2 ganglioside storage and was the only approach to correct behavior in mice with MPS IIIA. LV-WT-HSCT significantly reduces 2S HS composition and neuroinflammation with improved survival. Although both WT-HSCT and LV-IIIA-HSCT showed improvements in many of these parameters, neither treatment was able to significantly change abnormal behavior. Given the unmet clinical need, we believe lentiviral mediated hematopoietic stem cell gene therapy is a clinically relevant and viable approach to treat MPS IIIA and similar neurodegenerative disorders.
Materials and Methods
Construction and testing of SGSH lentiviral vector. The lentiviral vector plasmid pHR'SIN-cPPT-SEW40 was modified by replacing the eGFP gene with a Gateway conversion cassette (Invitrogen Life Technologies, Paisley, UK # 11828-019) producing pHRsin.SFFV.Gateway. The human SGSH cDNA sequence including 5′ and 3′ UTR flanked by attB sites from image clone #5226903 (Geneservice, Paisley, UK) was recombined in a Gateway cloning reaction with BP Clonase into a donor vector (pDonor221) containing attP sites and then into the lentiviral vector using LR Clonase to create pHRsin.SFFV.hSGSH.att.wpre as described41 (Invitrogen Life Technologies).
To confirm the ability of microglia to produce active enzyme from the lentiviral construct, the human microglial cell line CHME3 was transduced at an multiplicity of infection of 10 with lentiviral vector. Twenty four hours later the media was changed and 48 hours after that the media and cells were collected for analysis of SGSH activity.
Total bone marrow mononuclear cells from WT and MPS IIIA mice were lineage depleted using the murine hematopoietic progenitor enrichment cocktail (Stem Cell Technologies, Grenoble, France) according to manufacturer's instructions. Cells were resuspended in X-Vivo10 (BioWhittaker, Cologne, Germany), transduced as previously described31 at an multiplicity of infection of 25 and set up in MethoCult culture (Stem Cell Technologies) as previously described31 to assess lineage development. A further 48 hours later the cells and media were collected for SGSH activity analysis. After 14 days MethoCult colonies were counted and colonies collected for quantitative PCR analysis.
Lentiviral vector production and titration. HEK 293T cells were seeded in 15-cm tissue culture plates (Corning, Amsterdam, the Netherlands) in DMEM/10%FCS/2 mmol/l L-glutamine (Lonza, Basel, Switzerland) and cultured overnight at 37 °C until 40–70% confluent. VSV-G pseudotyped lentiviral vector was produced by the transient transfection of HEK 293T cells with pHRsin.SFFV.hSGSH.att.wpre, pMD2G, and pδ8.91gag/pol32 in a 3:1:2 ratio using a total of 15 µg of plasmid DNA per dish, 44.5 µl of Fugene 6 (Roche, Burgess Hill, UK) in Optimem (Gibco, Paisley, UK) as per manufacturer's instructions. The media was replaced 4 hours and 24 hours after transduction and harvested at 36 and 60 hours. Cells were removed by centrifugation at 262g for 15 minutes at 4 °C, and filtered through a 0.45-µm low protein-binding filter (Nalgene, Roskilde, Denmark). Lentiviral vector particles were concentrated by centrifugation at 21,191g for 150 minutes at 4 °C, resuspended in phosphate-buffered saline and stored at –80 °C. Lentiviral vector was titred using a similar method to Kutner et al.42 1 × 105 murine lymphoma (EL4) cells (ATCC Number TIB-39; Sigma-Aldrich, Gillingham, Dorset) were cultured in RPMI 1640 (Lonza)/10% FCS/2 mmol/l L-glutamine and transduced with five dilutions of concentrated lentiviral vector. Four days later, genomic DNA was extracted from the cells using the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich) and analyzed by quantitative PCR to determine the number of integrated lentiviral genomes per cell. The infectious titre was calculated as the number of cells at transduction multiplied by the number of lentiviral copies per cell divided by the volume of lentiviral vector added. Titres of 1.1 × 109 TU/ml (integrated transducing units) were normally reached.
Quantitative PCR copy number determination. The WPRE copy number of lentiviral vectors in genomic DNA from murine cell lines, bone marrow, blood, and spleen samples was determined by Quantitative Real Time PCR using the Applied Biosystems 7500 Real Time PCR System. A primer and probe set against wpre (TAMRA) were used as previously described43 and standardized against rodent gapdh primer and probe set (VIC) (Applied Biosystems, Paisley, UK). Samples were run in duplicate 25-µl reactions using the cycling parameters 50 °C for 2 minutes, 95 °C for 10 minutes, then 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute. The copy number was determined using a standard curve generated by dilutions of genomic DNA from an EL4 cell line clone (ALS EL4 eGFP 2.2) containing 2 integrated copies/cell of pHRsin.SFFV.eGFP.att.wpre. The EL4 cell clone (ALS EL4 eGFP 2.2) was made by transducing EL4 cells with the pHRsin.SFFV.eGFP.att.wpre lentiviral vector-expressing eGFP, followed by two consecutive rounds of single cell cloning. The lentiviral copy number per cell was determined by Southern blot of whole genomic DNA as previously described.44
Mice and transplant procedures. All in vivo procedures were ethically approved in accordance with UK Home Office regulations. MPS IIIA mice4 were backcrossed for 10 generations onto the C57BL/6J background (B6.Cg-Sgshmps3a/6J), maintained by heterozygote breeding and genotyped as previously described.30,45 Littermate controls were used throughout. MPS IIIA mice were also backcrossed onto the PEP3 CD45.1 congenic background (B6.SJL-PtprcaPepcb/BoyJ) to distinguish donor and recipient cells.
Total bone marrow mononuclear cells were isolated from femur, tibia, and ilium of WT CD45.1 or MPS IIIA/CD45.1 mice and lineage depleted using the murine hematopoietic progenitor enrichment cocktail (Stem Cell Technologies) according to manufacturer's instructions. Cells were resuspended in X-Vivo10 (BioWhittaker) and transduced as previously described.31
Seven-to-eight-week-old mice housed in individually ventilated cages were myeloablated with 125 mg/kg Busulfan (Busilvex; Pierre Fabre, Castres, France,) in five daily doses via intraperitoneal injection. 1 week before myeloablation and for a further 7 weeks, mice received acidified water (pH 2.8), irradiated food, mash, and sugar free jelly to prevent gastrointestinal infections and to encourage fluid uptake. Within 24 hours of the final injection of Busulfan, 1.5–2.5 × 105 lineage depleted transduced (LV-SGSH) or un-transduced hematopoietic stem cells were delivered intravenously.
Flow cytometry for chimerism analysis. Hematopoietic engraftment was assessed at 6-16 weeks post-transplant in peripheral blood by staining with anti-mouse CD45.1-PE for donor and CD45.2-FITC for recipient hematopoietic cells (BD Pharmingen, Oxford, UK) in a 5% solution of ToPro3 Iodide (Molecular Probes, Paisley, UK) on a BD FACS Canto II flow cytometer.
Behavior. At 4 and 6 months (16 and 24 weeks) of age 10 female mice were assessed for open-field behavior over 60 minutes at the same circadian time point as previously described29,30,37 and data analyzed using TopScan suite software version 2.0 (Clever System, Reston, VA).
Sample processing. At 8 months of age, anesthetized mice were transcardially perfused with 37 °C Tyrode's. Brains were removed; one hemisphere frozen at –80 °C and one fixed in 4% paraformaldehyde for 24 hours then 30% sucrose 2 mmol/l MgCl2/phosphate-buffered saline for 48 hours before freezing at –80 °C. For biochemical assays the snap frozen hemisphere was homogenized so that all assays represent an average of each hemisphere. Liver and spleen were frozen at –80 °C. For SGSH and GAG assays, samples of brain, liver, or spleen were homogenized and sonicated in homogenization buffer (0.5 mol/l NaCl, 0.02 mol/l Tris pH 7–7.5), then centrifuged at 2,200g for 15 minutes at 4 °C and the supernatant collected. Protein concentration was determined using the BCA (Thermo Scientific, Loughborough, UK) assay according to manufacturer's instructions.
SGSH enzyme activity. SGSH enzyme activity was measured in a two-step protocol using a fluorescent substrate MU-αGlcNS (Moscerdam, Oegstgeest, the Netherlands) as per manufacturer's instructions46 with minor modifications. The amount of starting material was standardized to 40 µg of total protein for liver and spleen and 60 µg for brain.
Blyscan for total sulfated GAGs. The total amount of sulfated GAGs (GAG) in 100 µg of liver and brain was determined using the Blyscan Kit (Biocolor, Carrickfergus, UK).47 Actinase E (200 µg) was added to the sample, incubated at 55 °C for 20 hours before heating at 100°C for 5 minutes and centrifuging at 3,000g for 10 minutes. The supernatant was incubated with 1,9-dimethymethylene blue for 30 minutes with vigorous shaking. After centrifuging at 10,000g for 15 minutes at 4 °C the pellet was incubated with dye dissociation reagent for 15 minutes and the color quantified using a spectrophotometer at 656 nm. The quantity was determined against a known concentration GAG standard.
AMAC-labeled disaccharide analysis of HS. Analysis of the quantity and sulfation state of HS was determined as previously described.33 Total HS was calculated by summing the peak area and applying a labeling efficiency correction factor as described.33 Three randomly assigned mice from each group were analyzed.
Immunohistochemistry. Four brain sections per mouse taken from bregma 0.26, –0.46, –1.18, and –1.94 mm were stained for Isolectin B4 and GM2 gangliosides and quantified as previously described.37,48 Isolectin B4 stained sections were counterstained with Mayer's hematoxylin before mounting. A section from –0.84 mm relative to bregma (2 mice per group) was stained for LAMP2 to demonstrate the size of the lysosomal compartment. Sections were blocked in 5% goat serum, 1 mg/ml bovine serum albumin, 0.1% Triton X-100 in TBS for 1 hour and incubated overnight at 4 °C with rat anti-LAMP2 immunoglobulin G (2 µg/ml; developed by August, JT, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) in blocking solution, washed 4× in TBS and stained with Alexa 488 goat anti-rat immunoglobulin G (1:1,000; Invitrogen) in blocking solution for 1 hour in the dark. After washing with TBS, sections were counterstained with 300 nmol/l DAPI (Invitrogen, Paisley, UK) for 15 minutes. Sections were mounted using ProLong Gold Anti-fade mounting medium (Invitrogen, Paisley, UK). Representative sections from cortex layer IV/V have been displayed.
Statistical analysis. JMP software version 8 (SAS Institute, Cary, NC) was used with one way ANOVA and Tukey post-hoc test to analyze data. Data were log transformed where they failed normality tests. Significance was assumed for probabilities of 0.05 or lower. Survival analysis was performed in SPSS version 19 (IBM, Armonk, NY) using Kaplan–Meier analysis with Mantel–Cox log rank pairwise comparisons. The estimated mean survival was calculated by Mantel–Cox log rank pairwise comparisons, where data points were censored the largest survival time was used.
SUPPLEMENTARY MATERIAL Figure S1. GM2 ganglioside storage in whole brain. Figure S2. Four-month open-field behavior. Video S1. Behavioral correction of MPS IIIA mice by LV-WT-HSCT but not other treatments.
Acknowledgments
This work was funded by The Sanfilippo Children's Research Foundation, The United Kingdom Society for Mucopolysaccharide Diseases, The Irish Society for Mucopolysaccharide Diseases, and the Biotechnology and Biological Sciences Research Council. The sponsors did not play a role in study design or execution. The authors gratefully acknowledge the help and assistance of the staff of the Manchester BSU and the Manchester Biomedical Research Centre. A.L.-S., F.L.W., K.J.L.-S. A.S., R.J.H., S.J.H., C.L.R.M., R.F.W., and B.W.B., have no conflict of interest to disclose. S.A.J. and J.E.W. are involved in a phase I/II clinical trial for intrathecal enzyme replacement therapy in MPS IIIA with Shire Human Genetic Therapies Inc. (NCT01299727, NCT01155778).
Supplementary Material
GM2 ganglioside storage in whole brain.
Four-month open-field behavior.
Behavioral correction of MPS IIIA mice by LV-WT-HSCT but not other treatments.
References
- Valstar M, Ruijter G, van Diggelen O, Poorthuis B, Wijburg F. Sanfilippo syndrome: A mini-review. J Inher Metabol Dis. 2008;31:240–252. doi: 10.1007/s10545-008-0838-5. [DOI] [PubMed] [Google Scholar]
- Héron B, Mikaeloff Y, Froissart R, Caridade G, Maire I, Caillaud C.et al. (2011Incidence and natural history of mucopolysaccharidosis type III in France and comparison with United Kingdom and Greece Am J Med Genet A 155A58–68. [DOI] [PubMed] [Google Scholar]
- Meyer A, Kossow K, Gal A, Mühlhausen C, Ullrich K, Braulke T.et al. (2007Scoring evaluation of the natural course of mucopolysaccharidosis type IIIA (Sanfilippo syndrome type A) Pediatrics 120e1255–e1261. [DOI] [PubMed] [Google Scholar]
- Bhaumik M, Muller VJ, Rozaklis T, Johnson L, Dobrenis K, Bhattacharyya R.et al. (1999A mouse model for mucopolysaccharidosis type III A (Sanfilippo syndrome) Glycobiology 91389–1396. [DOI] [PubMed] [Google Scholar]
- McGlynn R, Dobrenis K., and, Walkley SU. Differential subcellular localization of cholesterol, gangliosides, and glycosaminoglycans in murine models of mucopolysaccharide storage disorders. J Comp Neurol. 2004;480:415–426. doi: 10.1002/cne.20355. [DOI] [PubMed] [Google Scholar]
- Fraldi A, Hemsley K, Crawley A, Lombardi A, Lau A, Sutherland L.et al. (2007Functional correction of CNS lesions in an MPS-IIIA mouse model by intracerebral AAV-mediated delivery of sulfamidase and SUMF1 genes Hum Mol Genet 162693–2702. [DOI] [PubMed] [Google Scholar]
- Arfi A, Richard M, Gandolphe C, Bonnefont-Rousselot D, Thérond P., and, Scherman D. Neuroinflammatory and oxidative stress phenomena in MPS IIIA mouse model: the positive effect of long-term aspirin treatment. Mol Genet Metab. 2011;103:18–25. doi: 10.1016/j.ymgme.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Savas PS, Hemsley KM., and, Hopwood JJ. Intracerebral injection of sulfamidase delays neuropathology in murine MPS-IIIA. Mol Genet Metab. 2004;82:273–285. doi: 10.1016/j.ymgme.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Saif MA, Bigger BW, Brookes KE, Mercer J, Tylee KL, Church HJ.et al. (2012Hematopoietic stem cell transplantation ameliorates the high incidence of neutralizing allo-antibodies observed in MPSI-Hurler after pharmacological enzyme replacement therapy Haematologica [DOI] [PMC free article] [PubMed]
- Boelens JJ, Rocha V, Aldenhoven M, Wynn R, O'Meara A, Michel G, EUROCORD, Inborn error Working Party of EBMT and Duke University et al. Risk factor analysis of outcomes after unrelated cord blood transplantation in patients with hurler syndrome. Biol Blood Marrow Transplant. 2009;15:618–625. doi: 10.1016/j.bbmt.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Wynn RF, Mercer J, Page J, Carr TF, Jones S., and, Wraith JE. Use of enzyme replacement therapy (Laronidase) before hematopoietic stem cell transplantation for mucopolysaccharidosis I: experience in 18 patients. J Pediatr. 2009;154:135–139. doi: 10.1016/j.jpeds.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Krivit W, Sung JH, Shapiro EG., and, Lockman LA. Microglia: the effector cell for reconstitution of the central nervous system following bone marrow transplantation for lysosomal and peroxisomal storage diseases. Cell Transplant. 1995;4:385–392. doi: 10.1177/096368979500400409. [DOI] [PubMed] [Google Scholar]
- Shapiro EG, Lockman LA, Balthazor M., and, Krivit W. Neuropsychological outcomes of several storage diseases with and without bone marrow transplantation. J Inherit Metab Dis. 1995;18:413–429. doi: 10.1007/BF00710053. [DOI] [PubMed] [Google Scholar]
- Sivakumur P., and, Wraith JE. Bone marrow transplantation in mucopolysaccharidosis type IIIA: a comparison of an early treated patient with his untreated sibling. J Inherit Metab Dis. 1999;22:849–850. doi: 10.1023/a:1005526628598. [DOI] [PubMed] [Google Scholar]
- Prasad VK, Mendizabal A, Parikh SH, Szabolcs P, Driscoll TA, Page K.et al. (2008Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes Blood 1122979–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn RF, Wraith JE, Mercer J, O'Meara A, Tylee K, Thornley M.et al. (2009Improved metabolic correction in patients with lysosomal storage disease treated with hematopoietic stem cell transplant compared with enzyme replacement therapy J Pediatr 154609–611. [DOI] [PubMed] [Google Scholar]
- Heldermon CD, Ohlemiller KK, Herzog ED, Vogler C, Qin E, Wozniak DF.et al. (2010Therapeutic efficacy of bone marrow transplant, intracranial AAV-mediated gene therapy, or both in the mouse model of MPS IIIB Mol Ther 18873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressant A, Desmaris N, Verot L, Bréjot T, Froissart R, Vanier MT.et al. (2004Improved behavior and neuropathology in the mouse model of Sanfilippo type IIIB disease after adeno-associated virus-mediated gene transfer in the striatum J Neurosci 2410229–10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wang CM, Clark KR., and, Sferra TJ. Recombinant AAV serotype 1 transduction efficiency and tropism in the murine brain. Gene Ther. 2003;10:1528–1534. doi: 10.1038/sj.gt.3302011. [DOI] [PubMed] [Google Scholar]
- Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L.et al. (2007Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain Hum Gene Ther 18195–206. [DOI] [PubMed] [Google Scholar]
- Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N.et al. (2008Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA Hum Gene Ther 19463–474. [DOI] [PubMed] [Google Scholar]
- Visigalli I, Delai S, Politi LS, Di Domenico C, Cerri F, Mrak E.et al. (2010Gene therapy augments the efficacy of hematopoietic cell transplantation and fully corrects mucopolysaccharidosis type I phenotype in the mouse model Blood 1165130–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Capotondo A, Fasano S, del Carro U, Marchesini S, Azuma H.et al. (2006Gene therapy of metachromatic leukodystrophy reverses neurological damage and deficits in mice J Clin Invest 1163070–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner B, Visigalli I, Hiramatsu H, Lechman E, Ungari S, Giustacchini A.et al. (2010Identification of hematopoietic stem cell-specific miRNAs enables gene therapy of globoid cell leukodystrophy Sci Transl Med 258ra84. [DOI] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I.et al. (2009Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy Science 326818–823. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhang W, Kalfa TA, Grabowski G, Davies S, Malik P.et al. (2009Reprogramming erythroid cells for lysosomal enzyme production leads to visceral and CNS cross-correction in mice with Hurler syndrome Proc Natl Acad Sci USA 10619958–19963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AA, Hannouche H, Rozaklis T, Hassiotis S, Hopwood JJ., and, Hemsley KM. Allogeneic stem cell transplantation does not improve neurological deficits in mucopolysaccharidosis type IIIA mice. Exp Neurol. 2010;225:445–454. doi: 10.1016/j.expneurol.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Ryazantsev S, Ohmi K, Zhao HZ, Rozengurt N, Kohn DB.et al. (2004Retrovirally transduced bone marrow has a therapeutic effect on brain in the mouse model of mucopolysaccharidosis IIIB Mol Genet Metab 82286–295. [DOI] [PubMed] [Google Scholar]
- Langford-Smith A, Malinowska M, Langford-Smith KJ, Wegrzyn G, Jones S, Wynn R.et al. (2011Hyperactive behaviour in the mouse model of mucopolysaccharidosis IIIB in the open field and home cage environments Genes Brain Behav 10673–682. [DOI] [PubMed] [Google Scholar]
- Langford-Smith A, Langford-Smith KJ, Jones SA, Wynn RF, Wraith JE, Wilkinson FL.et al. (2011Female mucopolysaccharidosis IIIA mice exhibit hyperactivity and a reduced sense of danger in the open field test PLoS ONE 6e25717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapati EK, Bigger BW, Miskin J, Chipchase D, Parsley KL, Mitrophanous K.et al. (2005Comparison of HIV- and EIAV-based vectors on their efficiency in transducing murine and human hematopoietic repopulating cells Mol Ther 12537–546. [DOI] [PubMed] [Google Scholar]
- Bigger BW, Siapati EK, Mistry A, Waddington SN, Nivsarkar MS, Jacobs L.et al. (2006Permanent partial phenotypic correction and tolerance in a mouse model of hemophilia B by stem cell gene delivery of human factor IX Gene Ther 13117–126. [DOI] [PubMed] [Google Scholar]
- Holley RJ, Deligny A, Wei W, Watson HA, Niñonuevo MR, Dagälv A.et al. (2011Mucopolysaccharidosis type I, unique structure of accumulated heparan sulfate and increased N-sulfotransferase activity in mice lacking a-l-iduronidase J Biol Chem 28637515–37524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausseil J, Desmaris N, Bigou S, Attali R, Corbineau S, Vitry S.et al. (2008Early neurodegeneration progresses independently of microglial activation by heparan sulfate in the brain of mucopolysaccharidosis IIIB mice PLoS ONE 3e2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley KM, Beard H, King BM., and, Hopwood JJ. Effect of high dose, repeated intra-cerebrospinal fluid injection of sulphamidase on neuropathology in mucopolysaccharidosis type IIIA mice. Genes, Brain and Behavior. 2008;7:740–753. doi: 10.1111/j.1601-183X.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- Crawley AC, Marshall N, Beard H, Hassiotis S, Walsh V, King B.et al. (2011Enzyme replacement reduces neuropathology in MPS IIIA dogs Neurobiol Dis 43422–434. [DOI] [PubMed] [Google Scholar]
- Malinowska M, Wilkinson FL, Langford-Smith KJ, Langford-Smith A, Brown JR, Crawford BE.et al. (2010Genistein improves neuropathology and corrects behaviour in a mouse model of neurodegenerative metabolic disease PLoS ONE 5e14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn RF, Mercer J, Page J, Carr TF, Jones S., and, Wraith JE. Use of enzyme replacement therapy (Laronidase) before hematopoietic stem cell transplantation for mucopolysaccharidosis I: experience in 18 patients. J Pediatr. 2009;154:135–139. doi: 10.1016/j.jpeds.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Modlich U, Navarro S, Zychlinski D, Maetzig T, Knoess S, Brugman MH.et al. (2009Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors Mol Ther 171919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C.et al. (2002High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter Hum Gene Ther 13803–813. [DOI] [PubMed] [Google Scholar]
- Hartley JL, Temple GF., and, Brasch MA. DNA cloning using in vitro site-specific recombination. Genome Res. 2000;10:1788–1795. doi: 10.1101/gr.143000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner RH, Zhang XY., and, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- Lizée G, Aerts JL, Gonzales MI, Chinnasamy N, Morgan RA., and, Topalian SL. Real-time quantitative reverse transcriptase-polymerase chain reaction as a method for determining lentiviral vector titers and measuring transgene expression. Hum Gene Ther. 2003;14:497–507. doi: 10.1089/104303403764539387. [DOI] [PubMed] [Google Scholar]
- Themis M, Waddington SN, Schmidt M, von Kalle C, Wang Y, Al-Allaf F.et al. (2005Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice Mol Ther 12763–771. [DOI] [PubMed] [Google Scholar]
- Langford-Smith K, Arasaradnam M, Wraith JE, Wynn R., and, Bigger BW. Evaluation of heparin cofactor II-thrombin complex as a biomarker on blood spots from mucopolysaccharidosis I, IIIA and IIIB mice. Mol Genet Metab. 2010;99:269–274. doi: 10.1016/j.ymgme.2009.10.175. [DOI] [PubMed] [Google Scholar]
- Karpova EA, Voznyi YaV, Keulemans JL, Hoogeveen AT, Winchester B, Tsvetkova IV.et al. (1996A fluorimetric enzyme assay for the diagnosis of Sanfilippo disease type A (MPS IIIA) J Inherit Metab Dis 19278–285. [DOI] [PubMed] [Google Scholar]
- Malinowska M, Wilkinson FL, Bennett W, Langford-Smith KJ, O'Leary HA, Jakobkiewicz-Banecka J.et al. (2009Genistein reduces lysosomal storage in peripheral tissues of mucopolysaccharide IIIB mice Mol Genet Metab 98235–242. [DOI] [PubMed] [Google Scholar]
- Canal MM, Wilkinson FL, Cooper JD, Wraith JE, Wynn R., and, Bigger BW. Circadian rhythm and suprachiasmatic nucleus alterations in the mouse model of mucopolysaccharidosis IIIB. Behav Brain Res. 2010;209:212–220. doi: 10.1016/j.bbr.2010.01.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GM2 ganglioside storage in whole brain.
Four-month open-field behavior.
Behavioral correction of MPS IIIA mice by LV-WT-HSCT but not other treatments.