Abstract
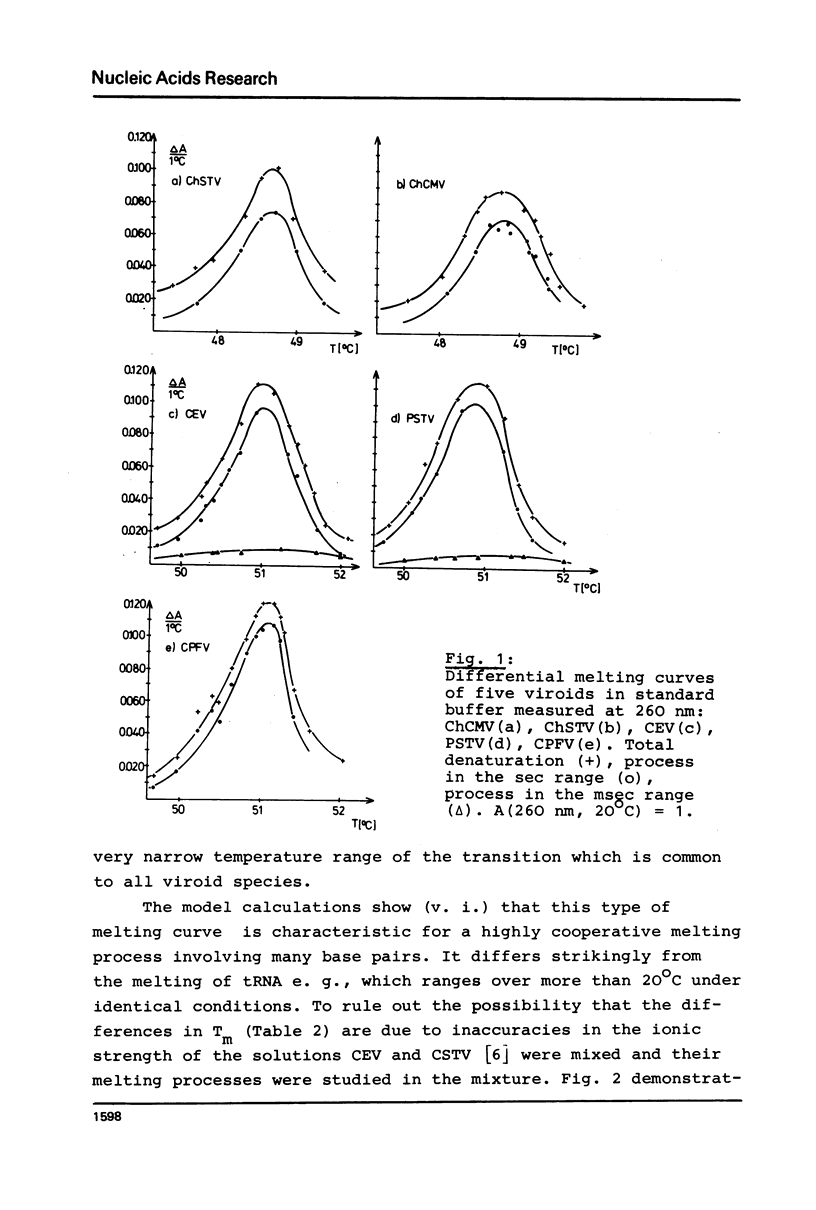
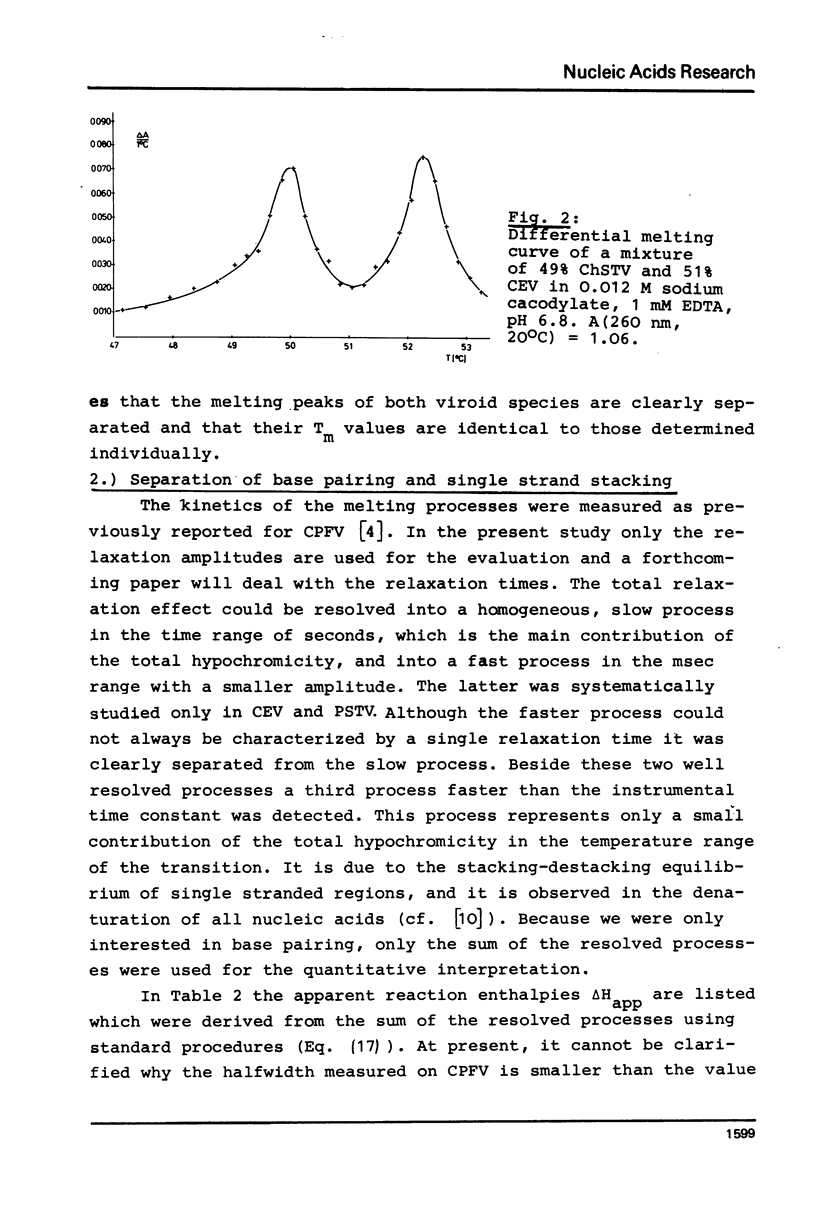
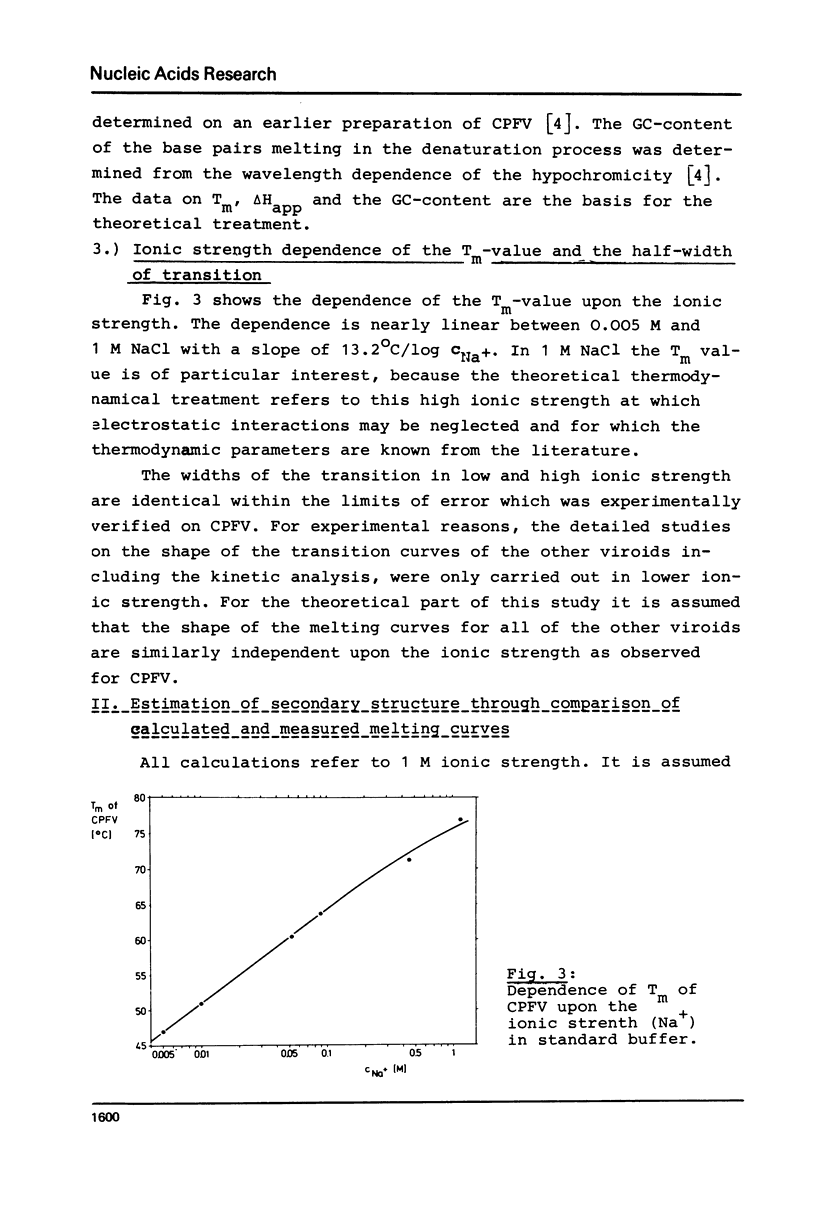
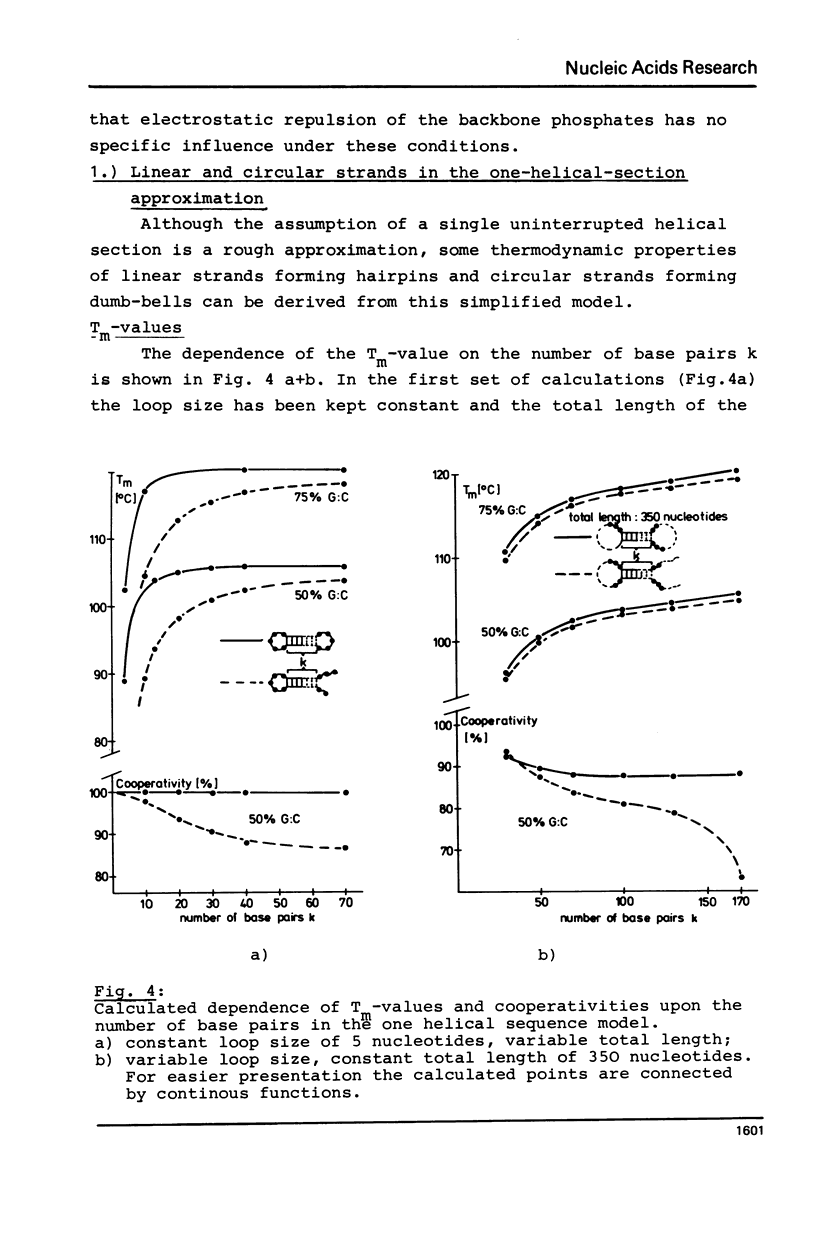
The thermodynamic parameters of five different highly purified viroid "species" were determined by applying UV-absorption melting analysis and temperature jump methods. Their thermal denaturation proved to be a highly cooperative process with midpoint-temperatures (Tm) between 48.5 and 51 degrees C in 0.01 M sodium cacodylate, 1 mM EDTA, pH 6.8. The values of the apparent reaction enthalpies of the different viroid species range between 3,140 and 3,770 kJ/mol. Although the cooperativity is as high as found in homogeneous RNA double helices the Tm-value of viroid melting is more than 30 degrees C lower than in the homogeneous RNA. In order to explain this deviation, melting curves were simulated for different models of the secondary structure of viroids using literature values of the thermodynamic parameters of nucleic acids. Our calculations show that the following refinement of our earlier model is in complete accordance with the experimental data: In their native conformation viroids exist as an extended rodlike structure characterized by a series of double helical sections and internal loops. In the different viroid species 250-300 nucleotides out of total 350 nucleotides are needed to interprete the thermodynamic behaviour.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Coutts S. M., Riesner D., Römer R., Rabl C. R., Maass G. Kinetics of conformational changes in tRNA Phe (yeast) as studied by the fluorescence of the Y-base and of formycin substituted for the 3'-terminal adenine. Biophys Chem. 1975 Oct;3(4):275–289. doi: 10.1016/0301-4622(75)80020-2. [DOI] [PubMed] [Google Scholar]
- Cox R. A. A spectrophotometric study of the secondary structure of ribonucleic acid isolated from the smaller and larger ribosomal subparticles of rabbit reticulocytes. Biochem J. 1970 Mar;117(1):101–118. doi: 10.1042/bj1170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisi C., Crothers D. M. Electrostatic contributions to oligonucleotide transitions. Biopolymers. 1971 Nov;10(11):2323–2343. doi: 10.1002/bip.360101123. [DOI] [PubMed] [Google Scholar]
- Dickson E., Prensky W., Robertson H. D. Comparative studies of two viroids: analysis of potato spindle tuber and citrus exocortis viroids by RNA fingerprinting and polyacrylamide-gel electrophoresis. Virology. 1975 Dec;68(2):309–316. doi: 10.1016/0042-6822(75)90274-3. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Viroids: the smallest known agents of infectious disease. Annu Rev Microbiol. 1974;28(0):23–39. doi: 10.1146/annurev.mi.28.100174.000323. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Wells R. D. Synthesis and thermal melting behavior of oligomer-polymer complexes containing defined lengths of mismatched dA-dG and dG-dG nucleotides. Biochemistry. 1977 May 31;16(11):2367–2374. doi: 10.1021/bi00630a009. [DOI] [PubMed] [Google Scholar]
- Elson E. L., Scheffler I. E., Baldwin R. L. Helix formation by d(TA) oligomers. 3. Electrostatic effects. J Mol Biol. 1970 Dec 28;54(3):401–415. doi: 10.1016/0022-2836(70)90118-x. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. 3. Small internal loops resulting from mismatches. J Mol Biol. 1973 Aug 5;78(2):301–319. doi: 10.1016/0022-2836(73)90118-6. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Sänger H. L. Comparative oligonucleotide fingerprints of three plant viroids. Nucleic Acids Res. 1977 Jun;4(6):2021–2028. doi: 10.1093/nar/4.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henco K., Riesner D., Sanger H. L. Conformation of viroids. Nucleic Acids Res. 1977 Jan;4(1):177–194. doi: 10.1093/nar/4.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder J. W., Lingrel J. B. Determination of secondary structure in rabbit globin messenger RNA by thermal denaturation. Biochemistry. 1975 Sep 23;14(19):4209–4215. doi: 10.1021/bi00690a009. [DOI] [PubMed] [Google Scholar]
- Inners L. D., Felsenfeld G. Conformation of polyribouridylic acid in solution. J Mol Biol. 1970 Jun 14;50(2):373–389. doi: 10.1016/0022-2836(70)90199-3. [DOI] [PubMed] [Google Scholar]
- Kallenbach N. R. Theory of thermal transitions in low molecular weight RNA chains. J Mol Biol. 1968 Nov 14;37(3):445–466. doi: 10.1016/0022-2836(68)90114-9. [DOI] [PubMed] [Google Scholar]
- Klump H., Riesner D., Sänger H. L. Calorimetric studies on viroids. Nucleic Acids Res. 1978 May;5(5):1581–1587. doi: 10.1093/nar/5.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomant A. J., Fresco J. R. Polynucleotides. 13. Stoichiometric and thermodynamic studies of polynucleotide helices with non-complementary residues. Biopolymers. 1973;12(8):1889–1903. doi: 10.1002/bip.1973.360120815. [DOI] [PubMed] [Google Scholar]
- Sanger H. L., Klotz G., Riesner D., Gross H. J., Kleinschmidt A. K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler I. E., Elson E. L., Baldwin R. L. Helix formation by d(TA) oligomers. II. Analysis of the helix-coli transitions of linear and circular oligomers. J Mol Biol. 1970 Feb 28;48(1):145–171. doi: 10.1016/0022-2836(70)90225-1. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Morris T. J., Weathers L. G., Rodorf B. F., Kearns D. R. Physical properties of a minimal infectious RNA(viroik) associated with the exocortis disease. Virology. 1975 Jan;63(1):160–167. doi: 10.1016/0042-6822(75)90381-5. [DOI] [PubMed] [Google Scholar]
- Van N. T., Holder J. W., Woo S. L., Means A. R., O'Malley B. W. Secondary structure of ovalbumin messenger RNA. Biochemistry. 1976 May 18;15(10):2054–2062. doi: 10.1021/bi00655a005. [DOI] [PubMed] [Google Scholar]



