Abstract
Background
Whether myocardial recovery occurs more frequently in peripartum cardiomyopathy (PPCM), than in recent onset cardiomyopathies in men and non-peripartum women has not been prospectively evaluated. This was examined through an analysis of outcomes in the Intervention in Myocarditis and Acute Cardiomyopathy 2 (IMAC2) registry.
Methods and Results
IMAC 2 enrolled 373 subjects with recent onset non-ischemic dilated cardiomyopathy. LVEF was assessed at entry and six months, and subjects followed for up to 4 years. Myocardial recovery was compared between men (group1), non-peripartum women (group 2) and subjects with PPCM (group 3).
The cohort included 230 subjects in group 1, 104 in group 2, and 39 in group 3. The mean LVEF at baseline in groups 1, 2, and 3 was 0.23±0.08, 0.24±0.08, and 0.27±0.07 (p=0.04), and at six months was 0.39±0.12, 0.42±0.11, and 0.45±0.14 (p=0.007). Subjects in group 3 had a much greater likelihood of achieving an LVEF >0.50 at 6 months than groups 1 or 2 (19 %, 34%, and 48% respectively, p=0.002).
Conclusions
Prospective evaluation confirms myocardial recovery is greatest in women with PPCM, poorest in men, and intermediate in non-peripartum women. On contemporary therapy, nearly half of women with PPCM normalize cardiac function by six months.
Introduction
Peripartum cardiomyopathy (PPCM) is a form of idiopathic dilated cardiomyopathy (DCM) that presents in women in the in the latter part of pregnancy or the first several months post partum(1, 2). PPCM is a rare complication of pregnancy affecting 1:3,189 live births (3) in the United States, but remains a major cause of maternal morbidity with mortality in published series ranging from 2% to 27% (4, 5, 6, 7). Outcomes in PPCM vary widely and significant recovery of ventricular function occurs in up to 50% of affected women on contemporary therapy (8). Myocardial recovery can occur in all forms of recent onset non-ischemic cardiomyopathy; whether the degree of recovery in PPCM is greater than in non-peripartum women or in men with new onset cardiomyopathy has not been prospectively evaluated. In addition, while factors such as race, hypertension, maternal age and parity appear to impact the risk of PPCM no prospective studies have evaluated their impact on recovery for women presenting with PPCM.
The Intervention in Myocarditis and Acute Cardiomyoathy-2 (IMAC2) registry was an NHLBI funded prospective multicenter investigation of clinical and biologic predictors of outcomes for subjects with the recent onset of non-ischemic cardiomyopathy (ROCM). We compared the recovery of myocardial function in subjects with PPCM in the IMAC2 registry, to that seen in non-peripartum women and men with ROCM.
Methods
IMAC2 (Intervention in Myocarditis and Acute Cardiomyopathy 2) was a prospective multicenter investigation of myocardial recovery in subjects with recent onset (“acute”) non-ischemic dilated cardiomyopathy and myocarditis enrolled at 16 centers (Appendix) from May 2002 through December 2008. All subjects had an LVEF ≤0.40 by echocardiography, less than six months of symptoms, and a clinical evaluation consistent with idiopathic cardiomyopathy or acute myocarditis. All subjects defined as PPCM presented with symptoms in the last month of pregnancy or the first 5 months post partum, however they could be enrolled in the registry more than 5 months post partum as long as they were within 6 months of the onset of symptoms.
Demographic information included self designated race (white, black, Asian, other). A detailed family history was taken from all subjects. Those with a history of idiopathic dilated cardiomyopathy in a primary relative (sibling, parent or child) were excluded from enrollment by protocol. As recovery specifically in peripartum cardiomyopathy was a planned sub-study, a detailed pregnancy history was taken from all women enrolled. Subjects underwent angiography or non-invasive assessment to exclude coronary artery disease, and transthoracic echocardiogram to rule out significant valvular disease. Patients with significant diabetes (requiring therapy with insulin or an oral agent for more than one year), and uncontrolled hypertension (diastolic greater than 95 mmHg or systolic > 160 mmHg) were excluded. Right ventricular endomyocardial biopsy was not required based on current practice guidelines (9, 10).
Subjects were evaluated at 1 month and 6 months post enrollment, and followed at six month interval for up to 48 months. Left ventricular ejection fraction (LVEF) was assessed by transthoracic echocardiogram at enrollment and reassessed at six months. The occurrence of hospitalization, cardiac transplantation, or death was noted. The etiology of all deaths and hospitalizations were adjudicated by an independent events committee. The primary outcome was change in LVEF from baseline to 6 months measured by a core echocardiography laboratory. Secondary outcomes included transplant free survival and survival free from heart failure hospitalization.
Echocardiograms
Echocardiographic studies were reviewed in a blinded fashion by a core laboratory at the University of Pittsburgh. Digital routine grayscale 2-dimensional cine loops were obtained at frame rates of 40 to 90 Hz (mean 60 ± 15 Hz) from standard apical 4-chamber, 2-chamber, and long-axis views, at a depth of 12 to 20 cm during apnea for grayscale imaging. Sector width was optimized to allow for complete myocardial visualization while maximizing frame rate. Gain settings were adjusted for routine clinical grayscale 2-dimensional imaging to optimize endocardial definition. The images were then exported to a personal computer and analyzed offline with customized software. The LV volumes and EF were assessed by biplane Simpson’s rule using manual tracing of digital images. Left ventricular end diastolic dimension (LVEDD) was assessed in the parasternal long axis view.
Statistical Analysis
Subjects enrolled in IMAC were classified for analysis as men (group 1), non-peripartum women (group 2), and PPCM (group 3). Demographic and clinical characteristics were compared using Student t tests for continuous variables and Fisher’s exact test for categorical variables. Baseline LVEF, six month EF and change in LVEF were compared among the three groups. Given differences in baseline values, the 6 month LVEF was compared for the three groups by analysis of covariance (ANCOVA) in a model which controlled for age and baseline LVEF. In addition, within the group with PPCM the impact of medical therapy, parity, and race were evaluated. In order to evaluate the impact of time to presentation (post partum), the recovery of women with PPCM enrolled within 8 weeks post partum was compared to women enrolled more 8 weeks after delivery. The impact of age, as well as diastolic and systolic hypertension, on myocardial recovery was analyzed through a comparison based on median values. For parity, women with multiple pregnancies were compared with those with one pregnancy. For race analysis within PPCM, LVEF at entry, 6 months and change in EF were compared between black and non-black (predominantly white) subjects.
Outcomes analysis evaluated absolute survival and survival free from hospitalization for heart failure. The Kaplan-Meier method was used to estimate event-free survival overall and stratified by group (men, non-PPCM women, and PPCM). Survival curves were compared by use of the log-rank test.
Results
Cohort
The IMAC cohort of 373 subjects included 230 men in group 1, 104 women in group 2, and 39 women group 3. The clinical characteristics and medical therapy at entry are listed in Table 1. The mean age was younger in group 3 than 1 and 2, with a borderline trend toward more black subjects (% black groups 1/2/3=19/21/36, p=0.06). Medical therapy, heart rate, diastolic and systolic blood pressure and NYHA class were comparable in all 3 groups. Subjects in group 3 were less likely to have an implantable cardiac defibrillator (ICD) at entry (% ICD groups 1/2/3= 10/5/0, p=0.04). The increased use of ICDs in groups 1 and 2 did not reflect more chronic disease as symptom duration was similar in all three groups (table 1).
Table 1.
Clinical Characteristics and Medical Therapy at entry
| All (n=373) |
Men (n=230) |
Women Non-PPCM (n=104) |
PPCM (n=39) |
P value | |
|---|---|---|---|---|---|
| Age | 45±14 | 46±14 | 47±13 | 30±7 | 0.001 |
| Race: % black | 21 | 19 | 21 | 36 | 0.06 |
| HR | 83±17 | 83±17 | 81±15 | 86±19 | 0.30 |
| BPsys | 111±20 | 112±21 | 110±19 | 110±18 | 0.53 |
| BPdia | 71±13 | 71±13 | 70±12 | 72±14 | 0.76 |
| NYHA (% 3–4) | 35 | 34 | 40 | 31 | 0.45 |
| Months onset of symptoms | 2.2±1.7 | 2.1±1.6 | 2.5±1.8 | 2.0±1.7 | 0.14 |
| LVEF | 0.24±0.08 | 0.23±0.08 | 0.24±0.08 | 0.27±0.07 | 0.04 |
| ACEI % | 82 | 84 | 76 | 87 | 0.12 |
| ARB% | 9 | 10 | 12 | 0 | 0.07 |
| Beta Blocker % | 82 | 81 | 84 | 85 | 0.75 |
| Aldo RB % | 27 | 29 | 29 | 13 | 0.11 |
| Diuretics | 20 | 72 | 64 | 74 | 0.25 |
| % ICD | 8 | 10 | 5 | 0 | 0.04 |
Legend: BP sys: systolic blood pressure; BP dia: diastolic blood pressure; NYHA: New York Heart Association; ACEI: Angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; AldoRB: aldosterone receptor blocker; ICD: implantable cardiac defibrillator
Left Ventricular Function and Myocardial Recovery
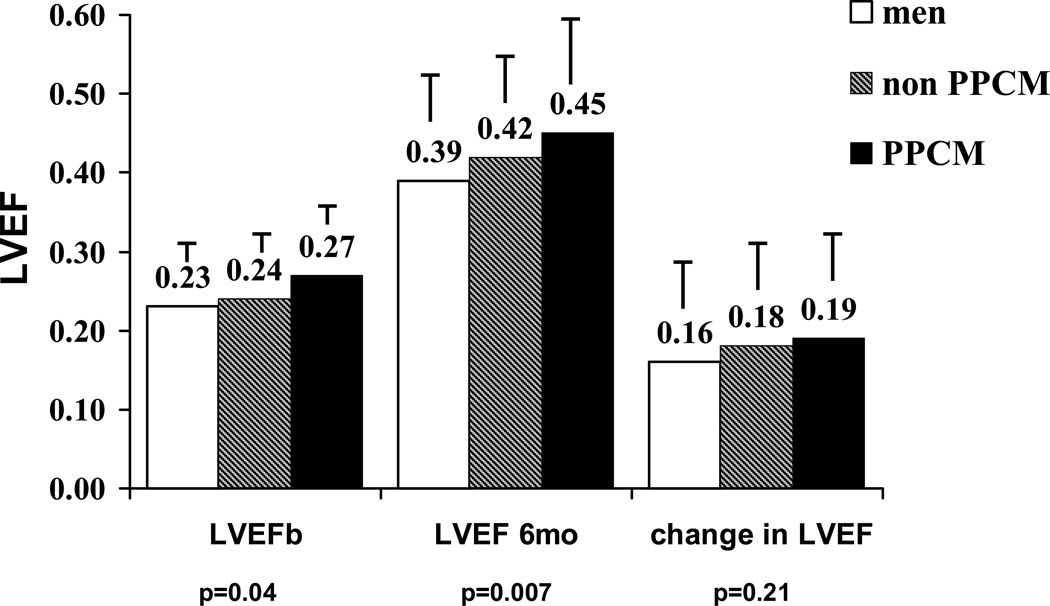
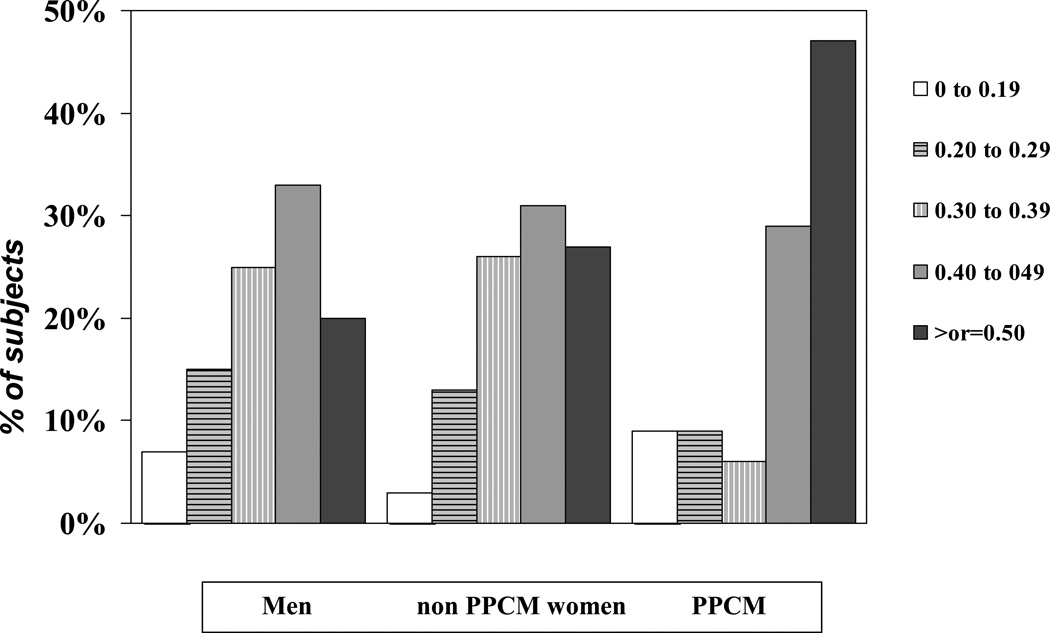
Overall LVEF for all subjects in the IMAC cohort was 0.24 ±0.08 at baseline and 0.40±0.12 at 6 months, with a mean increase of 0.17±0.13. LVEF was highest in group 3 both at entry (LVEF at entry groups 1/2/3=0.23±0.08/ 0.24±0.08/0.27±0.07, p=0.04), and at six months (LVEF 6 months 1/2/3=0.39±0.12 /0.42±0.11/ 0.45±0.14; p=0.007, figure 1). When controlling for baseline LVEF and age, the LVEF at 6 months remained significantly higher in group 3 subjects (p=0.018). The change in LVEF was similar in all groups but tended to be higher in group 3 (ΔLVEF groups 1/2/3=0.16±0.12/ 0.18±0.13/0.19±0.13, p=0.21). The percent of subjects achieving an LVEF ≥ 0.50 was greatest in group 3 (% EF ≥ 0.50 groups 1/2/3= 19/34/48, p=0.002, figure 2). In contrast, the percent of subjects left with a significant cardiomyopathy (LVEF < 30) was similar in all three groups (% EF <0.30 groups 1/2/3= 22/16/18, p=0.41).
Figure 1. LVEF at baseline 6 months and change in LVEF by Group: Men, non-PPCM Women, and PPCM.
Label on each bar represents mean LVEF for the subset. Error bar represents standard deviation. LVEF significantly greater in PPCM at baseline (p=0.04) and 6 month (p=0.007) with a trend toward greater ΔLVEF, with men at all point having the lowest mean LVEF and non–PPCM women being intermediate.
Figure 2. Distribution of values for LVEF and 6 months by Group: Men, non-PPCM Women, and PPCM.
Percent of subjects with LVEF≥0.50 greatest in PPCM (p=0.002), However similar percentage in all groups with LVEF less than 0.30 at 6 months.
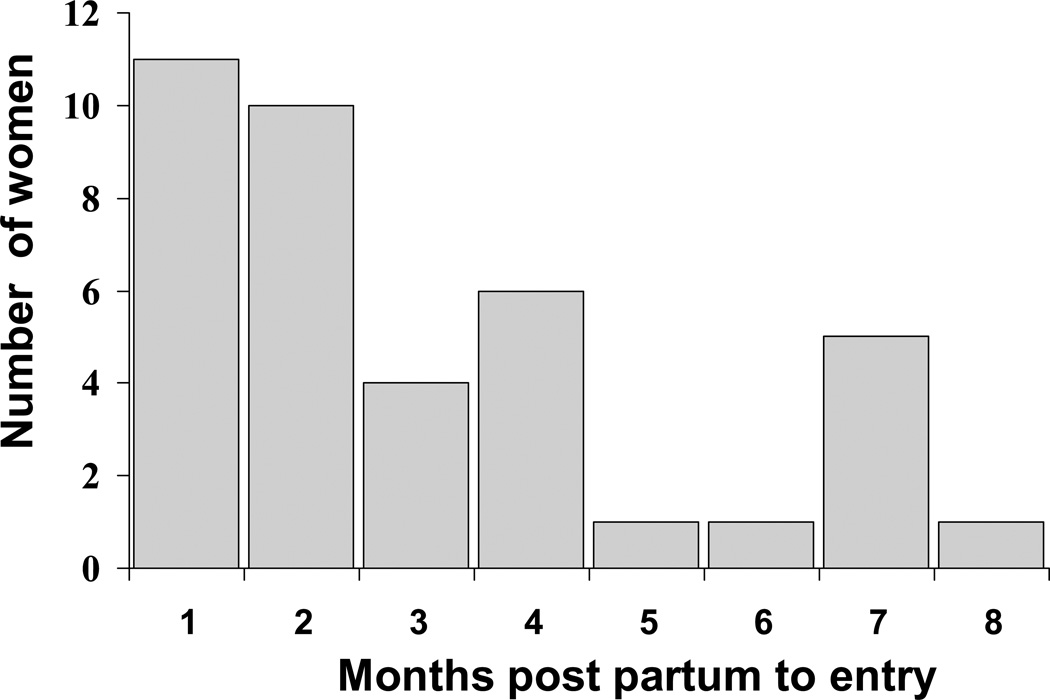
Time post partum to enrollment and Myocardial Recovery for women with PPCM
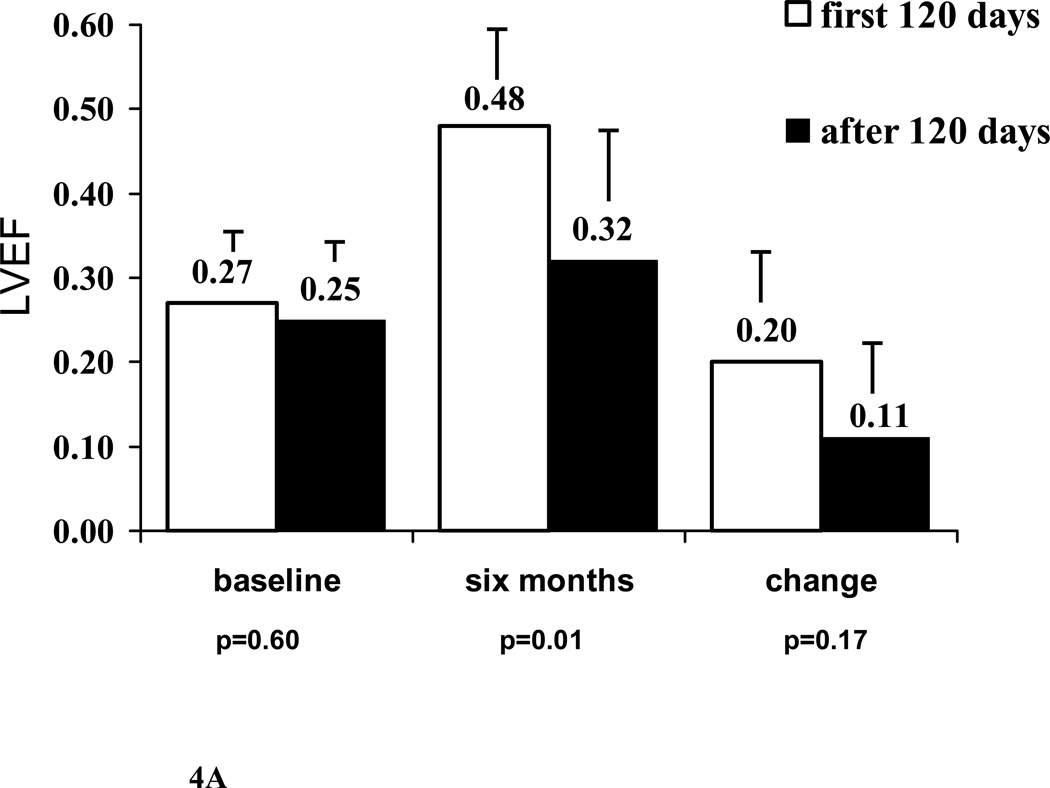
Women with PPCM in IMAC2 were enrolled an average of 77± 63 days post partum (median 56, range 9 to 231, Figure 3). The baseline LVEF, 6 month LVEF and increase in LVEF in subjects presenting early (≤ 60 days post partum, n=21) was similar to those presenting after 60 days (baseline LVEF subjects presenting ≤ 60 days/ > 60 days= 0.28±0.06 / 0.26±0.08, p=0.11; 6 month LVEF = 0.47± 0.14 / 0.42±0.14, p=0.28; ΔLVEF = 0.18± 0.13 / 0.19±0.14, p=0.87). However, for women presenting more than 120 days post partum (n=8), LVEF at 6 months was significant lower with a trend toward a lower ΔLVEF (baseline LVEF subjects presenting ≤ 120 days/ > 120 days= 0.27± 0.07/ 0.25±0.07, p=0.60; LVEF at 6 months = 0.48±0.12/ 0.32±0.16, p=0.01; ΔLVEF= 0.20±0.13/ 0.11±0.12, p=0.17, Figure 4A).
Figure 3. Time post partum.
Timing of enrollment (months post partum) for women with PPCM. Majority enrolled within 2 months post partum.
Figure 4. Myocardial Recovery in PPCM women.
A. Comparison by time to enrollment: Subjects enrolled late (>120 days post partum, n=8) compared with those enrolled less than 120 days (n=31). LVEF at 6 months significantly lower in those enrolled late (p=0.01).
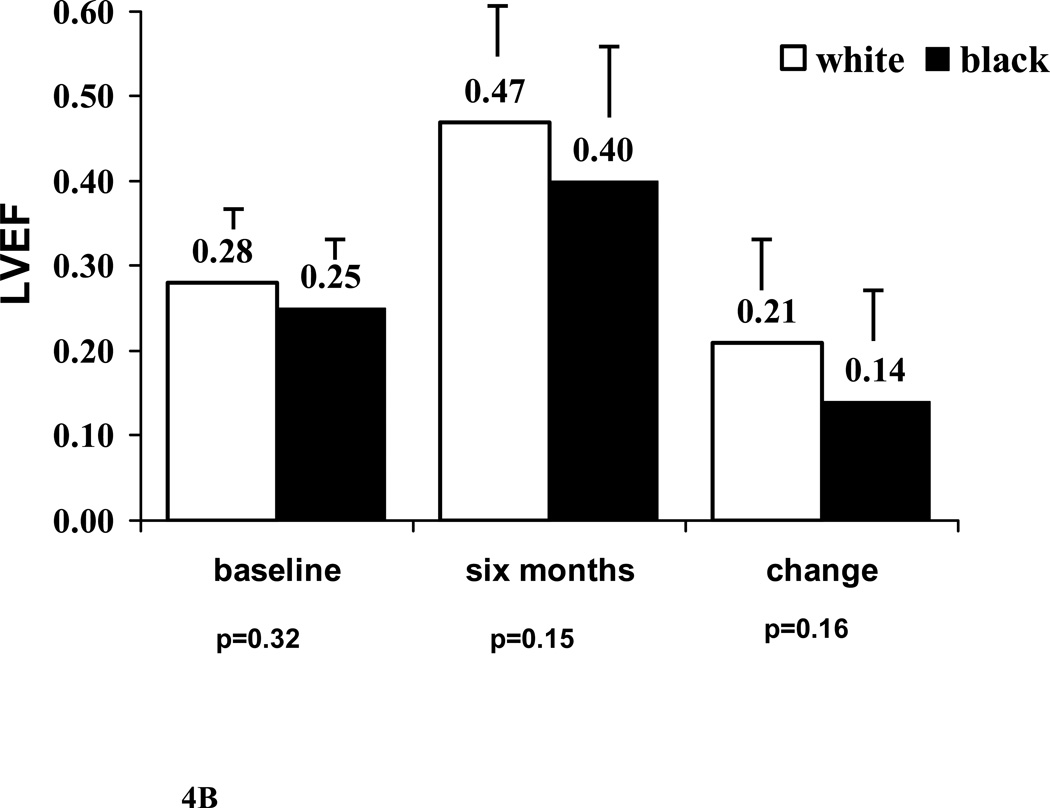
B. Comparison by race: black subjects (n=14) versus white (n=25). Mean values for LVEF at baseline, LVEF at 6 months and change in LVEF all tend to be lower in black subset however no significant differences.
Note: Label on each bar represents mean LVEF for the subset. Error bar represents standard deviation.
Clinical Characteristics and Recovery in PPCM
In terms of clinical predictors, younger women (age 30 or less) presented with more severe LV dysfunction (LVEF at entry age ≤ 30 / > 30) = 0.23±0.07/0.30±0.06, p=0.001). However, no difference was evident by 6 months 0.43±0.17/ 0.46±0.12, p=0.47) as the improvement in LVEF tended to be greater in younger subjects (ΔLVEF age <30/>30=0.22±0.15 / 0.17±0.12, p=0.26). For race, LVEF at entry (white/black=0.28±0.08/ 0.25±0.07), LVEF at 6 months (white/black=0.47±0.13/ 0.39±0.15) and increase in LVEF=0.21±0.13/ 0.14±0.13, figure 4B) all tended to be lower among blacks, but failed to reach significance. Significant myocardial recovery (LVEF ≥0.50) at 6 months was evident in 56% of whites, but only 30% of black subjects. Heart rate, diastolic BP, systolic BP at entry, maternal age and multi-parity had no impact on either 6 month LVEF or ΔLVEF.
Outcomes and PPCM
Overall survival was better in women than men (1/3 year % survival women versus men=100/100 versus 97/92, p=0.01) as no deaths were evident among women (with or without PPCM) during the course of follow up (mean follow up 781±516 days). For survival free from heart failure hospitalization, there was a trend towards better event free survival in women (1/3 year % event free survival women versus men= 90/84 versus 87/78, p=0.11) but no difference was evident among PPCM and non PPCM women.
Discussion
IMAC2 is the first investigation to prospectively compare outcomes in PPCM with other forms of recent onset non-ischemic cardiomyopathy. At both enrollment and six month follow up, subjects with PPCM had significantly higher mean values for LVEF than the subset of men with recent onset cardiomyopathy. The mean values for non-peripartum women were intermediate, suggesting that part of the greater recovery in PPCM may be related to female gender. This study also investigated the influence of PPCM risk factors such as race and blood pressure on subsequent recovery, and found that while recovery appears to be less evident in black subjects, blood pressure, maternal age and multi-parity had little impact on the degree of recovery achieved.
The percentage of women with PPCM achieving an LVEF of ≥0.50 at 6 months was far greater in peripartum women than in either non peripartum women or men. However, the percentage with significant cardiomyopathy at 6 months (LVEF<0.30) did not differ between groups. Indeed, in the PPCM cohort the LVEF 6 months after presentation appeared to have a bimodal distribution, with a majority of patients having marked recovery but a significant minority having persistent severe LV dysfunction. While an autoimmune pathogenesis has long been suspected for PPCM (11), the question remains whether women with persistent cardiomyopathy have a more severe irreversible injury or represent a completely different etiology such as a “latent” cardiomyopathy brought out by pregnancy. Recent investigation in large cohorts of familial dilated cardiomyopathy reveals that genetic etiologies may indeed be identified in a substantial fraction of women presenting with PPCM (12, 13, 14).
The incidence of PPCM varies throughout the world with an incidence observed in Africa and Haiti (15, 16) significantly higher than the incidence observed in the United States. Within the United States, African-American women have an estimated 15.7 fold higher risk of developing PPCM than non- African-American women (17). While the differences by race in the current study failed to reach significance, the trends toward lower LVEF and less recovery in blacks were consistent with previous reports of poorer outcomes in black women with PPCM compared to whites (17, 18). In terms of other risk factors for PPCM neither systolic nor diastolic pressure at presentation was linked to recovery. In a similar fashion multi-parity and higher maternal age also did not affect outcome.
Treatment with beta blockers may assist improvements in LVEF in other forms of non-ischemic cardiomyopathy and may be partially responsible for the excellent recovery noted in the current report. In the absence of an appropriate control group, the current investigation can not address whether medical therapy facilitates recovery in PPCM. In addition, whether the lower degree of recovery in black subjects represents differences in genetic background or a decreased responsiveness to pharmacologic therapy cannot be determined.
The IMAC2 registry was limited by the small number of peripartum subjects, which diminished the ability to fully evaluate the prognostic impact of traditional risk factors. In addition, at enrollment PPCM subjects in IMAC2 were on average more than 2 months postpartum, by which time significant recovery may have already occurred. However this delay would be unlikely to affect the comparison with men and non-peripartum women, for while it is more difficult in these subjects to pinpoint the initiation of the disorder, they likely had similar delays from presentation to enrollment. Finally, enrollment of PPCM subjects was not equal across all centers and we cannot exclude an ascertainment bias in this registry. A subsequent NHLBI funded investigation of myocardial recovery specifically in PPCM, Investigations in Pregnancy Associated Cardiomyopathy (IPAC), was initiated at 30 North American centers in 2010 and is currently underway.
Recently, inhibition of prolactin secretion with bromocriptine has been reported to facilitate recovery in both murine models of PPCM and in clinical reports (19, 20, 21). The degree of spontaneous recovery evident in the IMAC registry demonstrates that anecdotal reports of treatments linked to recovery must be interpreted with caution (22, 23). While the recovery evident is encouraging, the persistence of severe cardiomyopathy in a significant fraction of women warrants further investigation, and demonstrates the need for novel strategies to both delineate and treat women with PPCM destined for poor outcomes on conventional therapy.
Acknowledgments
Supported by NIH contracts HL75038, HL086918, HL102429, HL69912
APPENDIX: IMAC Investigators
University of Pittsburgh Medical Center (80) Dennis M McNamara, MD, Karen Janosko MSN,MBA, Charles McTiernan, Barry London, MD-PhD PhD, Karen Hanley-Yanez BS, John Gorcsan MD, Hidekazu Tanaka MD, Mathew Suffoletto, MD. Cleveland Clinic (51) Randall C Starling, MD; Cynthia Oblak, CCRN. Mayo Clinic (41) Leslie T. Cooper, MD, Annette McNallan, RN, LuAnne Koenig, RN. Jefferson Heart Institute at Jefferson Medical College (34) Paul Mather, MD, Natalie Pierson, BSN, Sharon Rubin, MD, Yanique Bell, RN and Alicia Ervin, RN. Penn State Hershey Medical Center (26) John Boehmer, MD; Patricia Frey, RN, BSN. University of Rochester (21) Jeffrey Alexis MD, Janice Schrack, RN, Pam LaDuke. Methodist Hospital, Houston (20) Guillermo Torre-Amione, MD-PhD, Jeannie Arredondo. University of Florida College of Medicine (20) Daniel F. Pauly, MD-PhD, Pamela C. Smith, LPN. McGill SMBD Jewish General (18) Richard Sheppard, MD; Stephanie Fuoco, RN, BSN. Johns Hopkins Hospital (15) Ilan S. Wittstein, MD; Elayne Breton, RN. Wake Forest University Baptist Medical Center (12) Vinay Thohan, MD; Deborah Wesley, RN, BSN. Massachusetts General Hospital (11) G. William Dec, MD; Diane Cocca-Spofford, RN, BSN, MHA. University of Texas Southwestern Medical Center (11) David W. Markham. MD-MSc, Lynn Fernandez, RN, Colleen Debes, RN. Newark Beth Israel Medical Center (6) Mark J. Zucker MD; Laura Adams, RN. University of Toronto (4) Peter Liu MD, Judith Renton, RN. University of California at Irvine (3) Jagat Narula, MD-PhD, Byron Allen, MD, Elizabeth Westberg, RN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Pearson GD, Veiled JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283:1183–1188. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 2.Sliwa K, Hilfiker-Kleiner D, Petrie MC, Buchmann E, Regitz-Zagrosek V, Schaufelberger M, Tavazzi L, et al. Current state of knowledge on etiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Euro J of Heart Failure. 2010;12:767–778. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 3.Mielniczuk LM, Williams K, Davis DR, Tang AS, Lemery R, Green MS, et al. Frequency of Peripartum Cardiomyopathy. Am J Cardiol. 2006;97:1765–1768. doi: 10.1016/j.amjcard.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 4.Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, Wani OR, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. New England Journal of Medicine. 2001;344(21):1567–1571. doi: 10.1056/NEJM200105243442101. [DOI] [PubMed] [Google Scholar]
- 5.Felker GM, Jaeger CJ, Klodas E, Thiemann DR, Hare JM, Hruban RH, et al. Myocarditis and long-term survival in peripartum cardiomyopathy. Am Heart J. 140:785–789. 200. doi: 10.1067/mhj.2000.110091. [DOI] [PubMed] [Google Scholar]
- 6.Witlin AG, Mabie WC, Sibai BM. Peripartum cardiomyopathy: an ominous diagnosis. Am J Obstet Gynecol. 1997 Jan;176(1 Pt 1):182–188. doi: 10.1016/s0002-9378(97)80033-6. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho A, Brandao A, Martinez EE, Alexopoulos D, Lima VC, Andrade JL, et al. Prognosis in peripartum cardiomyopathy. Am J Cardiol. 1989 Sep 1;64(8):540–542. doi: 10.1016/0002-9149(89)90438-4. [DOI] [PubMed] [Google Scholar]
- 8.Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary therapy. Am Heart J. 2006 Sep;152(3):509–513. doi: 10.1016/j.ahj.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Heart Failure Society of America. Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16(6):e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007 Nov 6;116(19):2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 11.Gleicher N, Elkayam U. Peripartum cardiomyopathy, and autoimmune manifestation of allograft rejection? Autoimmunity Reviews. 2008;12.003:1–4. doi: 10.1016/j.autrev.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 12.van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, van der Werf R, Jongbloed JD, Paulus WJ, et al. Peripartum Cardiomyopathy as a Part of Familial Dilated Cardiomyopathy. Circulation. 2010;121:2169–2175. doi: 10.1161/CIRCULATIONAHA.109.929646. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JL, Horne BD. Birthing the Genetics of Peripartum Cardiomyopathy. Circulation. 2010;121:2157–2159. doi: 10.1161/CIRCULATIONAHA.110.956169. [DOI] [PubMed] [Google Scholar]
- 14.Morales A, Painter T, Li R, Siegfried JD, Li D, Norton N, Hershberger RE. Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation. 2010 May 25;121(20):2176–2182. doi: 10.1161/CIRCULATIONAHA.109.931220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sliwa K, Damasceno A, Mayosi BM. Epidemiology and Etiology of Cardiomyopathy in Africa. Circulation. 2005;112:3577–3583. doi: 10.1161/CIRCULATIONAHA.105.542894. [DOI] [PubMed] [Google Scholar]
- 16.Fett JD, Carraway RD, Dowell DL, King ME, Pierre R. Peripartum cardiomyopathy in the Hospital Albert Schweitzer District of Haiti. Am J Obstet Gynecol. 2002;186:1005–1010. doi: 10.1067/mob.2002.122423. [DOI] [PubMed] [Google Scholar]
- 17.Gentry MB, Dias JK, Luis A, Patel R, Thornton J, Reed GL. African-American Women Have a Higher Risk for Developing Peripartum Cardiomyopathy. J of ACC. 2010;55:654–665. doi: 10.1016/j.jacc.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modi KA, Illum S, Jariatul K, Caldito G, Reddy PC. Poor outcome of indigent patients with peripartum cardiomyopathy in the United States. Am J Obstet Gynecol. 2009;201:171. doi: 10.1016/j.ajog.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 19.Yamac H, Bultmann I, Sliwa K, Hilfiker-Kleiner D. Prolactin: a new therapeutic target in peripartum cardiomyopathy. Heart. 2010;96:1352–1357. doi: 10.1136/hrt.2009.179218. [DOI] [PubMed] [Google Scholar]
- 20.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, et al. A Cathespsin D-Cleaved 16 kDa Form of Prolactin Mediates Postpartum Cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 21.Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, Becker A, McMurray J, et al. Evaluation of Bromocriptine in the Treatment of Acute Severe Peripartum Cardiomyopathy: A Proof-of-Concept Pilot Study. Circulation. 2010;121:1465–1473. doi: 10.1161/CIRCULATIONAHA.109.901496. [DOI] [PubMed] [Google Scholar]
- 22.Hilfiker-Kleiner D, Meyer GP, Schieffer E, Goldmann B, Podewski E, Struman I, et al. Recovery from postpartum cardiomyopathy in 2 patients by blocking prolactin release with bromocriptine. JACC. 2007;50(24):2354–2355. doi: 10.1016/j.jacc.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Bozkurt B, Villanueva FS, Holubkov R, Tokarczyk T, Alvarez RJ, Jr, MacGowan GA, et al. Intravenous Immune Globulin in the Therapy of Peripartum Cardiomyopathy. JACC. 1999;34:177–180. doi: 10.1016/s0735-1097(99)00161-8. [DOI] [PubMed] [Google Scholar]