Abstract
The goal of this work was to characterize the in-vivo behavior of [18F]mefway as a suitable positron emission tomography (PET) radiotracer for the assay of 5-hydroxytryptamine1A (5-HT1A) receptor density (Bmax). Six rhesus monkeys were studied using a multiple-injection (M-I) protocol consisting of three sequential bolus injections of [18F]mefway. Injection times and amounts of unlabeled mefway were optimized for the precise measurement of Bmax and specific binding parameters koff and kon for estimation of apparent KD. The PET time series were acquired for 180 minutes with arterial sampling performed throughout. Compartmental analysis using the arterial input function was performed to obtain estimates for K1, k2, koff, Bmax, and KDapp in the cerebral cortex and raphe nuclei (RN) using a model that accounted for nontracer doses of mefway. Averaged over subjects, highest binding was seen in the mesial temporal and dorsal anterior cingulate cortices with Bmax values of 42±8 and 36±8 pmol/mL, respectively, and lower values in the superior temporal cortex, RN, and parietal cortex of 24±4, 19±4, and 13±2 pmol/mL, respectively. The KDapp of mefway for the 5-HT1A receptor sites was 4.3±1.3 nmol/L. In conclusion, these results show that M-I [18F]mefway PET experiments can be used for the in-vivo measurement of 5-HT1A receptor density.
Keywords: 5-HT1A, Bmax, KD, [18F]mefway, multiple-injection PET
Introduction
The serotonin 5-hydroxytryptamine1A (5-HT1A) receptor subtype is known to be a critical regulator of the 5-HT system and is believed to have a pivotal role in the pathophysiology of many neuropsychiatric illnesses. 5-hydroxytryptamine1A receptors are expressed both postsynaptically, throughout the forebrain (neocortex and hippocampus), and as a major somatodendritic autoreceptor in the 5-HT neurons of the raphe nuclei (RN). Alterations in the density of 5-HT1A receptors have been implicated in a wide range of neuropsychiatric diseases, based on postmortem histology and animal models. During neurodevelopment, prenatal alcohol exposure has been shown to alter 5-HT1A receptor expression (Druse et al, 1991). Positron emission tomography (PET) imaging with 5-HT1A-specific radioligands has a role for providing in-vivo assay of receptor density (Bmax).
The 5-HT1A-specific antagonists currently being used for PET investigation of the 5-HT1A system include [11C]WAY-100635 (Pike et al, 1996), [18F]MPPF (Le Bars et al, 1998), [18F]FCWAY (Carson et al, 2000), and [18F]mefway (Saigal et al, 2006), all of which are structurally related to WAY-100635. In-vitro measurements of Bmax have been reported with [11C]WAY-100635 and validated by comparison with its tritiated form (Hall et al, 1997), finding high levels of 5-HT1A receptor expression throughout the frontal, temporal, parietal, and cingulate cortices with the highest receptor density in the hippocampus (including regions CA1, subiculum, and uncus). In-vivo measurements of 5-HT1A Bmax in humans have been measured using [18F]MPPF, finding similar rank order in 5-HT1A receptor expression across brain regions as in-vitro studies (Costes et al, 2002). The in-vivo PET assay of Bmax in humans involves increased experimental complexity compared with typical ‘tracer' PET studies, requiring multiple injections of nontracer doses of ligand.
The multiple-injection (M-I) PET technique involves performing serial injections with varying levels of unlabeled ligand, with the intent of occupying a significant fraction of the receptors (Delforge et al, 1990). This strategy permits the estimation of Bmax, separated from the ligand receptor-specific binding parameters (kon and koff). The M-I method provides a measure of receptor density that is independent of the characteristics of the radioligand, unlike the ‘binding potential' metric obtained with tracer-only PET studies, which represent a composite function of Bmax and ligand-receptor affinity (Innis et al, 2007). The M-I methods have also been used for the measurement of Bmax in the peripheral benzodiazepine (Delforge et al, 1996), β-adrenergic (Muzic et al, 2000; Salinas et al, 2007), dopamine transporter (Morris et al, 1996; Poyot et al, 2001), dopamine D2 (Delforge et al, 1999; Christian et al, 2004; Mauger et al, 2005; Vandehey et al, 2010), and nicotinic acetylcholine (Gallezot et al, 2008) receptor systems.
The goal of this work was two-fold: (1) to characterize the in-vivo kinetic behavior of [18F]mefway and (2) to show its utility for in-vivo measurement of 5-HT1A Bmax using the M-I approach. In-vitro and in-vivo validation studies with [18F]mefway have shown its high selectivity for 5-HT1A receptors (Saigal et al, 2006) and favorable imaging characteristics (Wooten et al, 2011a). A more complete characterization of [18F]mefway, measuring radioligand delivery and clearance (K1 and k2) and the binding and dissociation rate constants (kon and koff), will provide the necessary information for future experimental design in gauging the detection sensitivity of [18F]mefway to subtle alterations in the 5-HT1A system by disease or mechanistic perturbations in animals and humans. The rhesus monkey serves as a powerful model for studying 5-HT related function, due to its similarities with humans in neurochemical development, anatomy, receptor pharmacology, genetic polymorphisms, neurobehavior, and social structure. In this work, we report the in-vivo KD (KDapp) of [18F]mefway and 5-HT1A Bmax in the rhesus monkey. An M-I protocol was used with injections of varying cold masses to decouple the kinetic parameters necessary for estimation of KDapp and 5-HT1A Bmax.
Materials and methods
Chemical Synthesis
The synthesis of [18F]mefway (N-{2-[4-(2-methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(4-trans-[18F]-fluoromethylcyclohexane)carboxamide) was performed according to previously reported methods (Saigal et al, 2006). The average specific activity at the time of first injection of the PET scan was 77 GBq/μmol. The unlabeled mefway used for the M-I studies was purchased from a commercial vendor (Huayi Isotopes, Toronto, ON, Canada) as an 85:15 isomeric mixture of trans-mefway to cis-mefway. The injected mass for the partial saturation administrations (injection #2) was calculated based on the fractional mass of the trans-mefway and the pharmacological effects of the cis-mefway component were assumed to be negligible based on our previously reported findings (Wooten et al, 2011b).
Design of the Multiple-Injection Experiments
The M-I experiments were designed to optimize the precision of the parameter estimates of Bmax and the ligand-specific binding parameters (kon and koff). A requirement was placed on the experimental design to limit the first injection to tracer-only doses of [18F]mefway and a duration of 90 minutes before the subsequent injection. This design permits the comparison of [18F]mefway BPND with other subjects using single-injection protocols acquired at our center. Experimental designs were investigated for 2- and 3-injection studies to select the amount of unlabeled mefway and the injection time for each administration. The experimental design was selected using the D-optimal criterion, which minimizes the correlation between the kinetic parameters (described in Salinas et al, 2007). The inverse determinant of the reduced Hessian (det(HR)−1) is proportional to the volume of the indifference region for the parameter estimates. In minimizing this region, the parameter precision is increased. Only kon, koff, and Bmax were considered for the optimization. The experimental designs were simulated using the COMKAT software (Muzic and Cornelius, 2001) based on previously measured arterial input functions (Wooten et al, 2011a) and a range of Bmax (40 to 100 pmol/mL), koff (0.01 to 0.1 per minute), and kon (0.004 to 0.04 per minute) values estimated from our previous studies and the literature values with comparable ligands (Gunn et al, 1998; Hall et al, 1997). As a validation of the selected experimental designs, simulated noise was added to each data point of the simulated regions of interest (ROIs) similar to a reported method (Logan et al, 2001) using the equation:
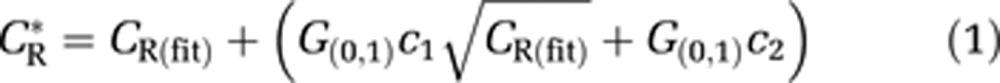 |
where CR* is the noise-added time-activity curves (TACs) in the different brain regions, CR(fit) is the noise-free TACs, G(0,1) is the noise addition that was a pseudorandom number from a normal distribution with a mean of zero and a standard deviation of 1, and the constants c1(1.5 to 2.5) and c2(0 to 60) represent scaling factors to approximate noise found in the original TACs (using 50 realizations). Parameter estimates were performed on these simulated TACs to examine the parameter identifiability. The optimization was repeated after the first several experiments to incorporate the newly measured parameters in the design of the later experiments.
Positron Emission Tomography Scans
The M-I PET experiments were conducted on a total of six Macaca mulatta (rhesus) subjects (2 male and 4 female; 8.2±1.6 kg; 13.0±3.9 years). The subjects were anesthetized for the scans using ketamine (10 mg/kg intramuscularly) and maintained under anesthesia using 0.75% to 2% isoflurane during the course of the experiment. Atropine sulfate (0.27 mg) was administered to reduce secretions. Body temperature, heart rate, breathing rate, and oxygen saturation (SpO2) levels were monitored and recorded. Catheters were placed in the saphenous vein for administration of ligand and the femoral artery for arterial sampling. Subjects were housed at The Harlow Primate Laboratory at the University of Wisconsin in Madison, which is governed by and strictly adheres to stringent federal statutes and regulations regarding the care and ethical use of laboratory animals. The NIH and USDA oversee the policies and statutes governing the care and ethical use of these subjects. All experimental procedures were approved by the University of Wisconsin Institutional Animal Care and Use Committee.
The PET scans were acquired using a Concorde microPET P4 scanner (Tai et al, 2001), which has a detection sensitivity of ∼1.2% and in-plane resolution of 2.8 mm as measured using the reconstruction methods for this protocol. Subjects were placed in the prone position in a custom-built stereotaxic headholder. Before injection, a 518-second transmission scan was performed using a 57Co point source. The collection of 3 hours of emission data was initiated with the first bolus injection of [18F]mefway. On the completion of the scan, the subjects were removed from the scanner and returned to their cage when swallowing reflexes were restored and monitored continuously until fully alert.
Measurement of the Arterial Input Function
Arterial samples were collected to provide an input function for kinetic modeling of [18F]mefway. Arterial samples were collected in volumes of 0.5 mL every 10 to 15 seconds for 2 minutes immediately after each injection to every 10 to 20 minutes toward the end of each injection segment. The 0.5-mL whole blood samples were mixed with 50 μL heparinized saline and assayed for radioactivity with a well counter that was cross-calibrated with the PET scanner. The hematocrit was measured to correct for the volume of the heparinized saline present in the extracted plasma. The whole blood samples were then centrifuged for 5 minutes to allow the extraction of 250 μL of plasma, which was added to 50 μL of sodium bicarbonate and assayed for radioactivity. The parent compound was removed from the plasma using two liquid-liquid ethyl acetate extractions as previously reported (Wooten et al, 2011a).
As implemented, the M-I model requires the [18F]mefway arterial time course to be separated and uniquely defined for each of the three injections. This requires the mathematical removal of radioactivity of the first injection from the second and third injections (and the second injection from the third injection). The first injection was parameterized by fitting the data from 5 to 90 minutes after injection to a biexponential function and extrapolating the curve to the end of the study (3 hours) for subtraction from the subsequent injections. The same method was used to parameterize the second injection, which was then subtracted from the third injection. The individual time courses of [18F]mefway were then decay corrected and divided by the specific activity (at the time of each injection) to yield units of molar concentration (pmol/mL) of mefway that are unaffected by radioactive decay.
Data Analysis
Image Reconstruction
The dynamic list mode emission data were binned into 2 minute sinograms and reconstructed with a filtered back projection algorithm using a 0.5 per cm ramp filter with corrections applied for attenuation, scatter, and scanner normalization. The final matrix size was 128 × 128 × 63, with voxel dimensions of 1.90 × 1.90 × 1.21 mm3.
Regions of Interest Selection
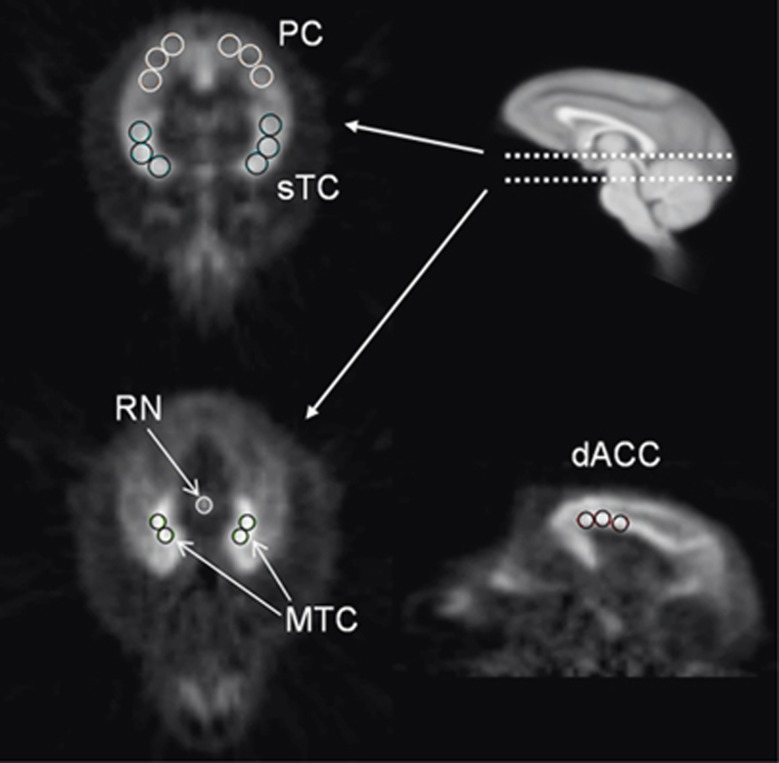
Regions of interest were selected from the PET images in cortical areas with uniform 5-HT1A binding. Multiple circular ROIs were placed within the regions of the mesial temporal cortex (MTC) (0.28 cm3), superior temporal cortex (sTC) (0.91 cm3), parietal cortex (PC) (0.91 cm3), dorsal anterior cingulate cortex (dACC) (0.73 cm3), and the cerebellum (CB) (0.92 cm3) to extract TACs. The ROIs were also applied to the subcortical region of the RN (0.08 cm3), guided by the focal uptake of [18F]mefway binding. A denoising algorithm was applied to the dynamic PET images before the extraction of the TACs (Christian et al, 2010). The ROIs for these regions are shown in Figure 1.
Figure 1.
Regions of interest (ROIs) in the areas of the mesial temporal (MTC), dorsal anterior cingulate (dACC), superior temporal (sTC), parietal (PC) cortices, and raphe nuclei (RN) drawn on summed dynamic frames (20 to 90 minutes). The top right sagittal magnetic resonance image illustrates the transaxial planes shown in the two positron emission tomography (PET) images in the left panel and is in the same space as the sagittal PET image (bottom right).
Parameter Estimation
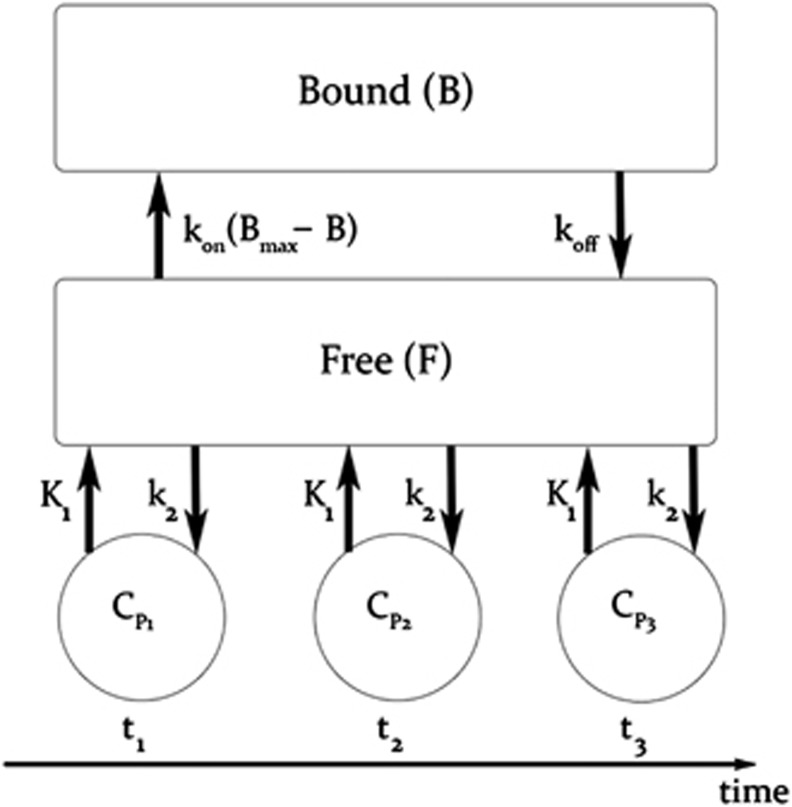
The in-vivo kinetic behavior of [18F]mefway was analyzed using a two-compartment model, as shown in Figure 2, to account for ligand in the free (F) (pmol/mL), including unbound and nondisplaceably bound, and specifically bound (B) (pmol/mL) states. Cpi represents the molar concentration of mefway in the plasma (pmol/mL) and serves as the input function. For studies with multiple injections, B and F compartments are created for each injection (i) (Delforge et al, 1990; Muzic et al, 2000; Christian et al, 2004; Morris et al, 2004), as described by the differential equations:
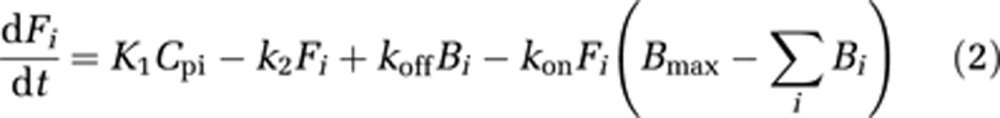 |
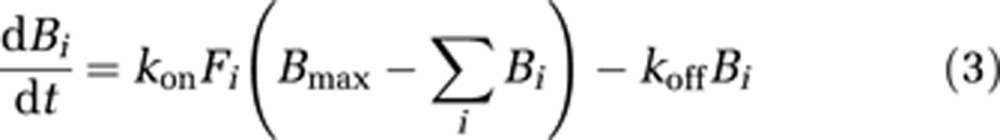 |
Figure 2.
Two-compartment model describing a three-injection protocol. t1−3 represent the injection times, CP1−3 represent the molar ligand concentration (pmol/mL) in the arterial plasma and bidirectionally exchanged between the free (F) and bound (B) states.
The bidirectional transport of radiotracer across the blood brain barrier, represented by K1 (mL/min per mL) and k2 (1/min), dissociation rate constant (koff) (1/min), association rate constant (kon) (1/min), and receptor density (Bmax) (pmol/mL) are common between each of the injections. The modeled PET signal is obtained by converting the absolute molar concentrations (Fi and Bi) into Bq/cm3 by multiplication with the time decaying specific activities (sai(t)) of each injection. The data are then normalized by the frame duration and can be expressed by the following model:
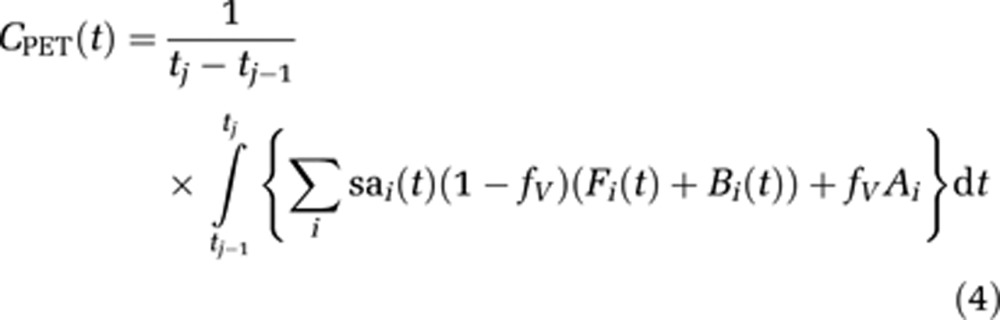 |
The fractional blood volume (fV) accounts for the vascular component of radioactivity measured in the decaying whole blood (Ai) and was fixed to a value of 0.04 for all brain regions. Thus, the model sums the radioactivity concentration for each injection (i) to obtain the PET signal, CPET, which is not corrected for radioactive decay to be consistent with the acquired PET data.
The parameter estimation for each subject was performed using COMKAT (Muzic and Cornelius, 2001). The following assumptions were implemented to permit the simultaneous estimation of all the regional parameters for [18F]mefway throughout the brain:
Nondisplaceable distribution volume, VND=K1/k2, is uniform across all ROIs, whereas K1 can vary between ROIs to account for regional differences in blood flow.
The specific binding association and dissociation rate constants, kon and koff, are uniform across all ROIs, whereas Bmax can vary between ROIs.
The blood flow within each ROI does not change throughout the course of the experiment, so the radiotracer influx and efflux constants, K1 and k2, are time invariant.
With these assumptions, estimates of kon, koff, and VND were made uniform for all ROIs and estimates of K1 and Bmax were unique for each ROI. For the CB, the compartment for specific binding (B) was not included and only K1 was uniquely measured for this region. Thus for each subject, a total of 14 parameters were simultaneously estimated from the time series data of 6 brain regions (MTC, sTC, PC, dACC, RN, and CB). The apparent equilibrium dissociation constant, KDapp, was calculated as: KDapp=koff/kon. The parameter estimates were obtained by minimizing the least squares objective function (o) between CPET(t) and the corresponding ROI measurement (r) for each time frame (j) of the PET scan scaled by a weighting factor (w). Uniform weighting was selected for these data as it has been shown to minimize bias for parameter estimates in PET compartment analysis (Muzic and Christian, 2006). The objective function is described as:
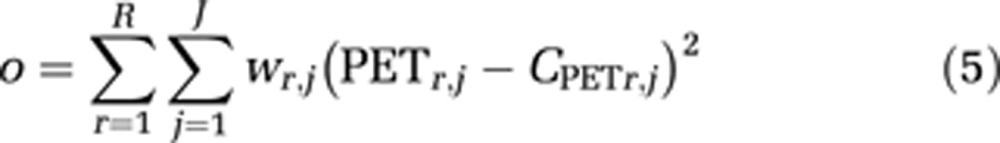 |
with six ROIs (R=6) and 180 minutes of PET data in 2 minutes time frames (J=90).
Estimation of uncertainties in the parameter estimates were performed similarly to a method used by Salinas et al (2007) and Vandehey et al (2010). The parameter estimates obtained from the PET data were used to generate a noise-free simulated data set for each subject. Noise was then added to the simulated data using the noise model described in equation (1), with 50 noise trials for each subject. Parameter estimates were then obtained for each trial and the standard deviation of each parameter was calculated from the population obtained from the 50 noise trials. The coefficient of variation (cov=s.d./mean × 100%) was then measured for each parameter and mean coefficients of variation were reported for each parameter as the average cov over the six subjects.
For comparison with the Bmax estimates, measurements were also made for nondisplaceable binding potential, BPND (Innis et al, 2007), using the data from 0 to 90 minutes, consisting of only the high specific activity first injection. BPND was estimated using the CB as a reference region with the Logan distribution volume ratio (DVR) method and calculated as BPND=DVR−1 (Logan et al, 1996), using a period of linearization of t*=40 minutes and a k2 value obtained from the compartmental modeling.
Results
Optimization of Multiple-Injection Protocol
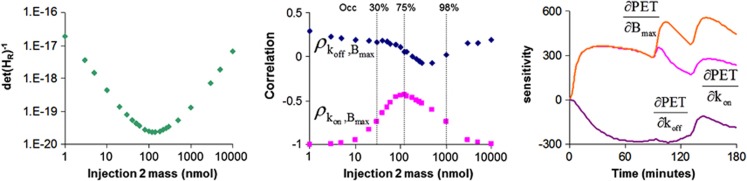
The optimization of the M-I protocol focused on selecting the unlabeled dose of mefway for the second and third injections and the timing of the third injection. It was found that identifiability of Bmax, kon, and koff was most sensitive to the mefway dose for the second injection and much less sensitive to the timing and dose of the third injection (see Discussion and Figure 3). Figure 3 illustrates the dependence of the det(HR)−1 on the mefway dose for the second injection, suggesting the optimal dose is ∼100 to 200 nmol. Also shown is the Bmax correlation with kon and koff, showing that the high correlation is reduced at an optimal range of unlabeled mass doses for the second injection. The sensitivity curves in the region of the MTC for Bmax, kon, and koff (shown in Figure 3) illustrate the decoupling of the parameters over the duration of the experiment.
Figure 3.
Experimental design—effects of mass in the second injection. (Left) D-optimal inverse determinant of the reduced Hessian (det(HR)−1) criterion as a function of injected mass. (Middle) Correlation between Bmax with kon and koff as a function of injected mass. (Right) Sensitivity curves for kon, koff, and Bmax in the mesial temporal cortex (MTC) in which the second injection consists of unlabeled mefway mass that occupies ∼75% of the available receptors. PET, positron emission tomography.
The experimental protocols for subjects M1 to M6 are shown in Table 1. It should be noted that the initial optimizations were based on a KDapp that was ∼70% lower than the newly measured values. Analysis from the first subject showed that optimal parameter estimation needed only a high mass dose in injection 2 and low mass in injection 3, which was then implemented in the following studies. As the experiments progressed, the most recent parameter estimates were incorporated into the optimization and resulted in increasing the mefway mass for the second injection.
Table 1. Injection parameters for multiple-injection (M-I) experiments.
| Injection# | Parameter |
Subject |
|||||
|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | M6 | ||
| 1 | Time (minutes) | 0 | 0 | 0 | 0 | 0 | 0 |
| Activity (MBq) | 58.1 | 66.2 | 63.3 | 61.8 | 64.8 | 57.7 | |
| Mass (nmol) | 2.4 | 0.5 | 0.8 | 0.8 | 1.2 | 0.6 | |
| 2 | Time (minutes) | 91 | 90 | 90 | 90 | 91 | 90 |
| Activity (MBq) | 60.3 | 61.0 | 57.4 | 60.0 | 51.4 | 58.8 | |
| Mass (nmol) | 19.2 | 30.6 | 175.5 | 164.1 | 141.1 | 134.6 | |
| 3 | Time (minutes) | 150 | 120 | 130 | 130 | 131 | 130 |
| Activity (MBq) | 46.3 | 65.9 | 61.0 | 55.1 | 59.6 | 58.1 | |
| Mass (nmol) | 113.6 | 1.1 | 1.7 | 1.5 | 2.4 | 1.4 | |
Measurement of kon, koff, and Bmax
The parameter estimates for all of the subjects are given in Table 2, including a single estimate for kon, koff, and VND across all regions and individual ROI estimates for K1 and Bmax in the MTC, sTC, PC, dACC, and RN. For these regions, the highest 5-HT1A receptor density was found in the MTC and dACC, with intermediate levels in the sTC and RN, and lower receptor expression in the PC. The average (over brain regions) cov in Bmax was 17.9% across the six subjects. Of the estimated parameters, kon displayed the lowest variability across this group of subjects, with a cov of 14%. There was slightly higher variability in koff (cov=17%), which resulted in a cov=29% in KDapp due to the propagation of errors. The average KDapp of [18F]mefway for the 5-HT1A receptor site was 4.3±1.3 pmol/mL.
Table 2. Parameter measurements across regions.
| Region | Parameter | Units | M1 | M2 | M3 | M4 | M5 | M6 | Mean | s.d. |
|---|---|---|---|---|---|---|---|---|---|---|
| All ROIs excluding CB | kon | 1/min | 0.0082 | 0.0066 | 0.0057 | 0.0079 | 0.0076 | 0.0059 | 0.0070 | 0.0010 |
| koff | 1/min | 0.024 | 0.028 | 0.029 | 0.024 | 0.028 | 0.039 | 0.029 | 0.005 | |
| KD | pmol/mL | 3.0 | 4.2 | 5.1 | 3.1 | 3.6 | 6.6 | 4.3 | 1.3 | |
| All ROIs | VND | unitless | 3.7 | 3.3 | 3.5 | 2.4 | 2.2 | 2.2 | 2.9 | 0.6 |
| CB | K1 | mL/mL per minute | 0.62 | 0.36 | 0.68 | 0.28 | 0.80 | 0.29 | 0.50 | 0.20 |
| MTC | K1 | mL/mL per minute | 0.70 | 0.59 | 0.83 | 0.50 | 0.46 | 0.55 | 0.60 | 0.13 |
| Bmax | pmol/mL | 42.3 | 29.3 | 54.8 | 36.6 | 38.0 | 47.8 | 41.5 | 8.2 | |
| sTC | K1 | mL/mL per minute | 1.01 | 0.67 | 0.95 | 0.51 | 0.51 | 0.48 | 0.69 | 0.22 |
| Bmax | pmol/mL | 21.6 | 21.0 | 29.8 | 21.3 | 20.2 | 28.1 | 23.7 | 3.8 | |
| PC | K1 | mL/mL per minute | 1.03 | 0.65 | 0.69 | 0.40 | 0.47 | 0.35 | 0.60 | 0.23 |
| Bmax | pmol/mL | 14.2 | 11.2 | 13.3 | 11.7 | 10.9 | 15.5 | 12.8 | 1.7 | |
| dACC | K1 | mL/mL per minute | 1.16 | 0.68 | 1.01 | 0.48 | 0.50 | 0.42 | 0.71 | 0.28 |
| Bmax | pmol/mL | 33.8 | 30.4 | 50.1 | 30.1 | 28.6 | 43.9 | 36.1 | 8.0 | |
| RN | K1 | mL/mL per minute | 1.00 | 0.80 | 1.05 | 0.51 | 0.48 | 0.63 | 0.74 | 0.22 |
| Bmax | pmol/mL | 18.8 | 17.6 | 18.2 | 14.8 | 19.0 | 26.6 | 19.2 | 3.6 |
MTC, mesial temporal cortex; sTC, superior temporal cortex; PC, parietal cortex; dACC, dorsal anterior cingulate cortex; CB, cerebellum; RN, raphe nucleus; ROI, region of interest.
The coefficients of variation (cov=s.d./mean × 100) for each estimated parameter found using Monte Carlo methods, resulted in uncertainties of: kon (4%), koff (4%), KD (6%), VND (2%), K1 in the CB (4%), MTC (4%), sTC (4%), PC (5%), dACC (4%), RN (4%), Bmax in the MTC (3%), sTC (4%), PC (4%), dACC (3%), and RN (4%).
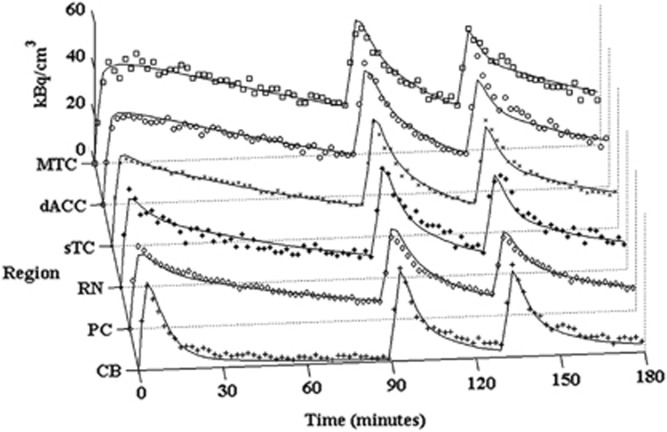
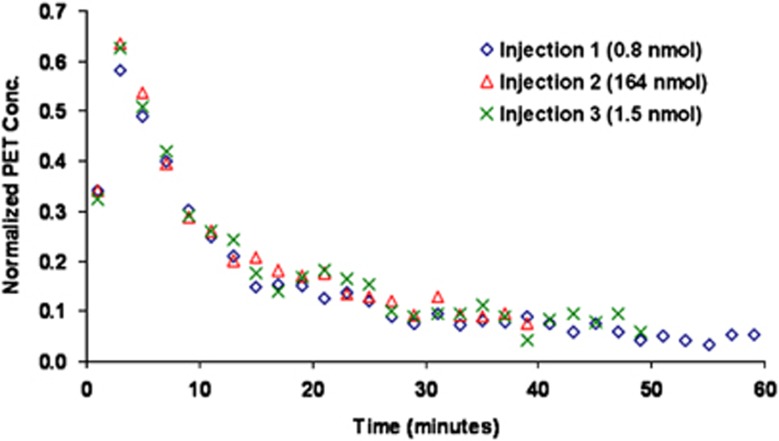
Figure 4 displays the PET measured time courses and the model output for the regions of the MTC, dACC, sTC, RN, PC, and CB in one subject. The CB is also shown separately in Figure 5 to illustrate the absence of measurable 5-HT1A binding in this region, with each of the three injections superimposed over a single time course after correction for residual activity from previous injection(s).
Figure 4.
Measured time-activity curves (not corrected for decay) and model predictions (solid line) for subject M4 in the regions of the MTC (□), dACC (○), sTC ( × ), RN (*), PC (◊), and CB(+). MTC, mesial temporal cortex; sTC, superior temporal cortex; PC, parietal cortex; dACC, dorsal anterior cingulate cortex; CB, cerebellum; RN, raphe nucleus.
Figure 5.
Cerebellum time-activity curves (not corrected for decay) for three injections in subject M4 (Bq/mL/i.d. × 1,000). The data for injection 2 and injection 3 were corrected for residual activity present from the previous injection(s) by subtracting the extrapolated activity using a biexponential function. PET, positron emission tomography.
Discussion
In our previous work, we reported a direct comparison of the in-vivo behavior of [18F]mefway with two other 5-HT1A receptor antagonists, [11C]WAY-100635 and [18F]MPPF, under single-bolus injection conditions (Wooten et al, 2011a), thus these experiments were not designed for the direct measurement of KD. In the work presented herein, a KDapp of 4.3±1.3 nmol/L was measured when averaged over the six subjects, which is similar to in-vivo measures for other related 5-HT1A PET antagonists. Farde and colleagues (1997) performed a PET study of [11C]WAY-100635 in a single cynomolgus monkey using varying mass injections with competing drug and reported a KDapp of ∼1 to 2 nmol/L (estimated from the figure). A recent study was conducted using [18F]FPWAY in 21 rhesus monkeys and reported apparent KDapp values ranging from 1 to 4 nmol/L (cov∼30% using s.e.m.) in rhesus monkeys (Spinelli et al, 2010). For [18F]MPPF, M-I studies were performed in humans (n=5) and reported KDapp=2.8 nmol/L (cov∼50%) (Costes et al, 2002), yielding comparable results with [18F]mefway. This similarity in the 5-HT1A affinity of [18F]MPPF and [18F]mefway is not consistent with our previous results, which suggested that the KDapp for [18F]mefway is approximately three-fold lower compared with [18F]MPPF based on BPND comparisons (Wooten et al, 2011a) and in-vitro measures (Khawaja et al, 1995; Saigal et al, 2006). This discrepancy in magnitude between the compounds may be due to differences in species, nonspecific binding in the brain (fND), or experimental design. However, the lower KDapp values reported for [18F]MPPF were accompanied by reduced estimates of Bmax (discussed below), indicating a degree of compatibility with our results within the context of the ratio of Bmax/KDapp.
In-Vivo Measurement of 5-HT1ABmax
The highest values of Bmax were measured in the regions of the MTC and dACC. Due to the limited resolution of PET assay and the lack of accompanying magnetic resonance imaging scans, only large areas with uniform binding of each specific brain region were selected for analysis. In-vitro autoradiography measurements in the hippocampus of the cynomolgus monkey reveal a heterogeneous distribution of 5-HT1A receptors across the hippocampal subfields, with highest binding in the area dentata, subiculum, and parasubiculum in the range of 50 to 100 fmol/mg of protein (Köhler et al, 1986). In humans, post mortem in-vitro studies using [3H]WAY-100635 have shown high levels of receptor density in the hippocampus areas ranging from 31 fmol/mg of wet tissue in the parahippocampus to 113 fmol/mg of wet tissue in the hippocampus CA1 pyramidal layer (Hall et al, 1997). A separate study also using [3H]WAY-100635 found 5-HT1A receptor densities of 200 to 300 fmol/mg of wet tissue in the CA1 of the hippocampus (Burnet et al, 1997). We measured an average Bmax value of ∼40 pmol/mL in the MTC, which included regions of the hippocampus. These in-vivo measurements of Bmax are significantly lower than those obtained with the in-vitro assay. The direct comparison of in-vitro and in-vivo PET assay is challenging due to high degree of spatial averaging with PET, which will result in an underestimation of Bmax for structures with cross-sectional areas that are comparable to the scanner resolution (<2 to 4 mm). An example of this effect is most profound when considering the small focal structure of the RN. Reported in-vitro receptor densities in the dorsal raphe in humans range from 50 fmol/mg of wet tissue to 230 fmol/mg of wet tissue (Burnet et al, 1997; Hall et al, 1997) compared with our measured densities in the raphe of 18.1±4.3 pmol/mL. The in-vivo measurements we report are not specific to just the dorsal region of the RN, but selected as the focal region in the superior PET images of the midbrain region. There was no attempt to correct for the resolution related partial volume effects of this region and we acknowledge that the reported Bmax in the RN is likely underestimates of those that would be obtained with autoradiography. However, we do not believe that this invalidates an intersubject comparison of (in vivo) PET measures in the region of the RN, assuming that the partial volume correction is consistent for all subjects. Because this correction accounts not only for regional size of brain structures but also for the spill over (and spill in) of the radioligand signal from surrounding regions, consideration must be given to variations in brain and body size of the subjects being compared. The subjects in this work were all fully grown adults of comparable weight (8.2±1.6 kg) and age (13.0±3.9 years), however, partial volume correction may be needed in experimental designs having greater variability in brain size (e.g., comparing early adolescent with adults subjects).
There have been several studies reporting in-vivo PET measures of 5-HT1A receptor densities using a variety of equilibrium and M-I techniques with PET radioligands. In a cynomolgus monkey, Bmax values of ∼16 and 5 pmol/mL were found in the neocortex and RN, respectively, using a 2-injection protocol with [11C]WAY-100635 (Farde et al, 1997). A more recent study in juvenile rhesus monkeys (n=21) found Bmax values of 5 to 13 pmol/mL (cov∼26% using s.e.m.) and 1 to 2 pmol/mL (cov∼34% using s.e.m.) in the hippocampus and RN, respectively, using a 2-point bolus plus constant infusion equilibrium protocol with [18F]FPWAY (Spinelli et al, 2010). Both of these previous studies used scatchard graphical analysis to estimate Bmax, which has been shown to be a valid technique with PET measurements, although high uncertainty in Bmax estimation can be seen if there is insufficient receptor occupancy for the partial saturation injection (Holden and Doudet, 2004). This may explain some of the variation between results, with experiments using [18F]FPWAY achieving only 40% occupancy. In humans, M-I PET studies were performed with [18F]MPPF, finding Bmax values of 2.9 pmol/mL (cov∼50%) in the hippocampus (Costes et al, 2002) using a 2-injection (nonequilibrium) protocol. This is a 10-fold difference in measured Bmax compared with the values reported herein. This discrepancy in Bmax may be attributed in part to species differences; however, it may also be the result of methodological differences with the level of receptor saturation achieved for the experiments, which is discussed in the section below.
Considerations in Experimental Design
Careful attention must be given to the experimental design in M-I studies to ensure identifiability of each individual parameter, particularly between kon and Bmax. Generally, parameter estimation algorithms will yield combinations of Bmax and kon estimates that accurately model the experimental data, even in the case of a faulty experimental M-I design. In such situations, it is the product, kon·Bmax, that is identified and not the independent measures of either parameter. For this work, the D-optimal criterion (which minimizes the indifference region and increases parameter precision) was used to assess the measured precision of the binding parameters kon, Bmax, and koff and to determine the required experimental design for identifying each. It should be noted that the 3-injection design implemented in this protocol was not selected based on the criteria of experimental simplicity (i.e., the shortest scan with fewest injections). The requirement was enforced that the first injection of each study consist of high specific activity [18F]mefway and 90 minutes of scanning. It has been shown that for other applications, it is possible to measure Bmax with a 2-injection protocol with partial saturating doses of the ligand for the first injection (Salinas et al, 2007). Such a design foregoes the ability to measure BPND for comparison with radiotracer-only scans and was therefore not investigated for this work. Figure 3 illustrates the relation between one of the experimental variables (unlabeled mefway mass for the second injection) with the D-optimal metric and the effects on the measured output parameters and sensitivity curves. The correlation of koff with Bmax ρkoff, Bmax remains small (<0.3) across all values of injected mass, suggesting their relation is not exclusively dependent on the mefway dose, but improvement in decoupling these parameters could be achieved over a range of competing doses of mefway. The variability of the measure koff across subjects was relatively low, 0.030±0.005 per minute (cov=17%), which is attributed to its high identifiability for this experimental design. The correlation between kon and Bmax (ρkon, Bmax) revealed much greater dependence on the amount of competing mefway. As expected from radiotracer studies and illustrated in Figure 3, there is complete correlation between the parameters (ρkon, Bmax = − 1) in the absence of significant mefway dose. This correlation can be significantly reduced by increasing the competing mefway in the second injection, with a minima in correlation corresponding to receptor occupancy of ∼75%.
An examination of the sensitivity curves, ∂PET(t)/∂θi, where θi represents an estimated parameter, graphically illustrates the decoupling of the parameters. Figure 3 shows the scaled sensitivity curves for Bmax, kon, and koff. Complete coupling between kon and Bmax can be seen in the sensitivity curves before injection 2 where curves overlay. In the case where partial receptor saturation occurs (receptor occupancy ∼75%), there is a divergence of the sensitivity curves for kon and Bmax, indicating a reduction in the covariance. The sensitivity curves illustrate that identification of kon and Bmax comes primarily from injection 2, and additional decoupling of koff from kon and Bmax comes from the third injection.
Examination of the D-optimal criteria, as seen in Figure 3, shows that the decoupling of kon and Bmax is increased with increasing mefway mass (up to a limit) with an occupancy range optimal for decoupling being 50% to 96% of the 5-HT1A receptor sites. This finding that higher receptor occupancy is optimal to uncouple kon and Bmax is comparable to that of a previous 2-injection M-I study using [18F]-(S)-fluorcarazalol for measuring Bmax of myocardial β-adrenergic receptors, which found receptor occupancies of ∼90% were optimal for precise estimation of receptor concentration (Salinas et al, 2007). For our implementation with three injections, we found maximum decoupling of kon and Bmax occurred with a saturating dose of ∼75% receptor occupancy, corresponding to a dose, averaged over the six subjects, of ∼12 nmol/kg. The peak occupancies observed for subjects M1 to M6 were 95, 43, 93, 93, 91, and 73% respectively, from the high dose mefway injections. We compare these occupancies with an M-I study in humans using [18F]MPPF (Costes et al, 2002), which administered MPPF doses of ∼20-fold (estimated) lower than doses used in this work. Based on our simulations with mefway, unlabeled dose at this level would not be adequate to decouple kon, koff, and Bmax, resulting in instability of the parameter estimates. This large difference in occupancy levels may explain the discrepancies in the 5-HT1A Bmax estimates between methods.
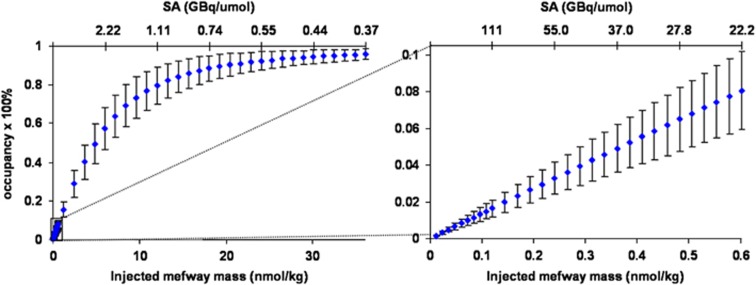
The peak transient occupancy of the 5-HT1A receptors as a function of mefway dose is shown in Figure 6. This relation is based on the measured in-vivo parameters of the six subjects and the error bars represent the standard deviation accounting for the variations in the parameters as well as the differences in [18F]mefway observed in the measured input function. This information can be used as a guide for examining mass effects in radiotracer-only studies. For a [18F]mefway synthesis yielding a specific activity of 111 to 185 GBq/μmol (3,000 to 5,000 mCi/μmol) and a 111-MBq (3 mCi) injection (typical of our tracer-only single-bolus injection studies), the 5-HT1A receptor occupancy will range from 1.0% to 1.6%. Under these conditions, it will be possible to perform two sequential radiotracer studies on different subjects, from the same batch (i.e., synthesis) of [18F]mefway, without exceeding a threshold of 4% to 5% receptor occupancy from unlabeled mefway at ‘tracer' levels.
Figure 6.
Receptor occupancy as a function of injected mass (nmol/kg) and specific activity when using the average parameter values. The intersubject standard deviation is shown by the error bars, which illustrate the difference seen among subjects due to the individual parameters and subject weights. The bottom axis shows the injected mass in nmol/kg and the top axis shows the specific activity (SA) in GBq/μmol (assuming a 111-MBq (3 mCi) injection typical to our tracer-only single-bolus injection studies). The left image shows the receptor occupancy in the full range from high specific activity to near saturation. The right image shows the receptor occupancy in the mass range of a typical high specific activity injection.
The analysis for the parameter estimations involved several assumptions that require consideration. The blood flow was assumed to be constant over the course of the 3-hour experiment. Potential alterations in blood flow would change the rate of mefway delivery (via K1) and efflux (via k2) and bias the measurement of these parameters. The heart rates of the subjects were monitored throughout the studies and showed a gradual decreasing trend during the course of the experiment, with a total decrease of ∼20±5% for the 3-hour scan. There was no observable change in heart rate, SpO2 levels, or breathing rate when the saturating dose of mefway was delivered. Although this does not discount the possibility of changes to cerebral blood flow, it does alleviate the concern that global changes were induced by the mefway drug. The model included a separate K1 for each region to account for regional differences in ligand delivery; however, the assumption was made that the nondisplaceable volume of distribution (VND) remains constant across all examined brain regions. This assumption is consistent with reference region methods of analysis for single injection PET studies. Further, this constraint can accommodate regional perfusion changes, as it has been shown that VND remains constant under conditions of changing blood flow (Logan et al, 1994). The assumption of uniform VND across regions was examined by fitting each region independently and allowing k2 to float with the other parameters to determine the effects on the resulting Bmax and VND estimates. Minimal change in Bmax (∼1%) and VND (∼5%) was observed in the high density 5-HT1A receptor region of the MTC. However, in the lower density 5-HT1A receptor region of the PC, a negative correlation between VND and Bmax was observed leading to an increase of ∼19% for Bmax and a decrease of ∼18% for VND when averaged over all subjects. Thus, any potential bias introduced by the assumption of constant VND would surface primarily in the low 5-HT1A receptor density regions.
As configured, implementation of the M-I model required separate arterial input functions for each injection, defined in units of molar concentration of mefway (pmol/mL). Separation of the input functions involved extrapolating data from previous injection(s) and subtracting from the subsequent injection(s), with a biexponential function
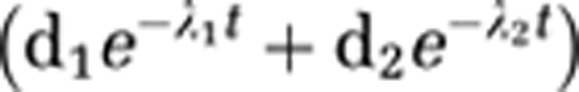 |
used for the extrapolation. This was necessary to account for residual activity from previous injections. At 30 minutes after injection 2, the radioactivity from injection 1 still accounted for ∼15% of the total parent radioactivity in the plasma and at 30 minutes after injection 3, injections 1 and 2 accounts for ∼27% of the total plasma activity. The fitting of the second injection to a biexponential function was problematic because it consisted of only 40 minutes of arterial sampling time before the administration of the third injection. We initially explored the use of a scaled (by injected activity) version of the first injection for the subsequent injections based on the assumption that each injection would have similar a time course. However, close examination of the input functions revealed the second injection possessed a broader peak (i.e., smaller λ1), suggesting an alteration in the radiotracer delivery between injections. It is speculated that the addition of pharmacological doses of unlabeled mefway increased the bioavailability of [18F]mefway in the system, possibly by releasing previously bound [18F]mefway from the receptors and by preventing new radiotracer from binding. It was initially assumed that small inaccuracies in the extrapolated function fitted to 40 minutes or less of arterial sampling data, coming from the second injection, would result in large variation in the binding parameter estimates due to the presence of the unlabeled mefway in this injection. To examine the effects of potential inaccuracies in the input function of the second injection, the data were analyzed with two different extrapolation schemes. The range of variation was determined from the input function of injection 1, first using the entire 90-minute time course and a second using only the data out to 40 minutes. The percent difference in the biexponential decay parameters (λ1 and λ2) was typically <20% between methods; however, in one study, there was a 44% difference in λ2 (the slow decay term). This difference was then incorporated into the extrapolated data for the second injection to examine the effects of inaccurate extrapolation on the outcome variables Bmax and KDapp. It was found that Bmax varied by <1.5% in all areas and KDapp varied by <1.3%, suggesting that the experimental design is relatively insensitive to the concentration of mefway and its time course once the third injection is administered, as illustrated by the sensitivity curves for Bmax and kon.
The unlabeled mefway used in these experiments was a commercially purchased reference standard consisting of an isomeric mixture of cis- and trans-mefway (15:85). We have recently shown that cis-[18F]mefway exhibits low, but significant 5-HT1A binding compared with trans-[18F]mefway, with a BPND in the MTC of 0.58 and 7.70 for cis- and trans-[18F]mefway, respectively (Wooten et al, 2011b). This profound difference in the binding affinity of isomeric pairs has been reported for other 5-HT1A PET radiotracers (Lang et al, 1999; Wilson et al, 1999). In this work, the reported injected masses are based only on the measured trans-mefway mass and exclude the cis-mefway mass fraction. The presence of unlabeled cis-mefway in the high dose injections of mefway are assumed to cause negligible effect. It can be approximated, from our previously reported binding potentials for cis- and trans-[18F]mefway (Wooten et al, 2011b), that for equal cis- and trans-concentrations, the cis-mefway would represent 7.6% (0.58/7.70) of the bound mefway, which suggests that only ∼1% of the receptor sites are occupied by the cis-isomer for the mixture used in this work.
BPND as an Index for Bmax
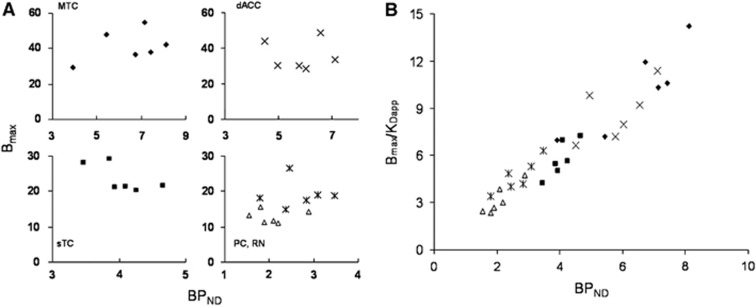
The first 90 minutes of each study was used as a baseline to extract BPND estimates for comparison with direct measurements of Bmax and KDapp. The outer cortex in the lobes of the CB was used as the reference region for the BPND estimation. We have previously reported on the use of the CB as a region with negligible 5-HT1A binding for radiotracer [18F]mefway studies (Wooten et al, 2011a). The lack of measurable specific binding was confirmed in this study, revealing no measurable displacement of [18F]mefway in the CB as illustrated in Figure 5. Shown in Figure 7A are scatter plots of the measured Bmax values with the BPND for the ROI data across subjects. Regression analysis found no significant correlation between Bmax and BPND for any of the regions: MTC (P=0.3), dACC (P=0.98), sTC (P=0.14), PC (P=0.83), and RN (P=0.99). However, significant correlation was found between BPND and Bmax/KD in all regions (MTC (P=0.014), sTC (P=0.017), PC (P=0.0065), and RN (P=0.023)) with the exception of the dACC (P=0.15). These data are shown in Figure 7B with all of the regions plotted on the same axis (although they were analyzed separately). A similar lack of correlation between the dopamine transporter Bmax and a composite binding parameter (BPP) has been reported using [11C]cocaine and serial equilibrium PET studies (Logan et al, 1997). The authors attributed the absence of correlation to a lack of robustness in their estimation of Bmax. A significant positive correlation has been reported between BPND and Bmax values as well as BPND and Bmax/KDapp in extrastriatal dopamine D2 receptors using [11C]FLB457 and a scatchard type multiscan session (Olsson et al, 2004). In this particular work, however, a correlation was also observed between Bmax and KDapp that may contribute to the correlation observed between Bmax and BPND. Additionally, the data were regressed across subjects and across regions whereas our analysis is not regressed across brain regions. It has been suggested that nonequilibrium experiments, such as M-I designs which decouple Bmax and kon, may improve the identifiability of Bmax (Morris et al, 1999). For the data reported herein with six subjects, the mean cov of Bmax across the ROIs was 17.9% which was similar to the mean cov of the BPND values of 17.3%, suggesting that the same variability is seen in the binding indices and neither exhibits an advantage in precision of parameter estimation. The data in Figure 7 suggest that BPND is not a representative proxy of the receptor density (Bmax) across subjects, but can serve as an index of Bmax/KDapp. We neither attempt to generalize this statement for all PET neuroligands and neuroreceptor systems nor attempt to explain this relation of Bmax and BPND based on the methodological approaches that were used, as these issues have been discussed in the literature (Morris et al, 1999; Olsson et al, 2004) and remain largely unresolved.
Figure 7.
(A) Comparison of Bmax with BPND in the regions of the MTC (♦), dACC ( × ), sTC (▪), RN ( ), and PC (▵). (B) Comparison of Bmax/KDapp with BPND for all subjects across brain regions. MTC, mesial temporal cortex; sTC, superior temporal cortex; PC, parietal cortex; dACC, dorsal anterior cingulate cortex; RN, raphe nucleus.
), and PC (▵). (B) Comparison of Bmax/KDapp with BPND for all subjects across brain regions. MTC, mesial temporal cortex; sTC, superior temporal cortex; PC, parietal cortex; dACC, dorsal anterior cingulate cortex; RN, raphe nucleus.
All in-vivo PET methods for Bmax and KDapp measurement, equilibrium and nonequilibrium, use assumptions that can potentially bias the outcome measures. In this work, KDapp was fixed as a constant across all ROIs by estimating only a single kon and koff for each subject. This was performed primarily to reduce the number of estimated parameters and in turn enhance the identifiability of the others. KDapp is sensitive to several factors that may challenge this assumption, such as endogenous 5-HT competition within the vicinity of the receptors. The effects of competing neurotransmitter on the measured KDapp are given as (KDapp)est=(KDapp)0(1+F5HT/KDen), where (KDapp)est is the measured KDapp in the presence of endogenous 5-HT (as measured in these experiments), (KDapp)0 is the measured KDapp in the absence of 5-HT, F5HT is the synaptic concentration of 5-HT, and KDen is the equilibrium dissociation constant of 5-HT (Delforge et al, 2001). This relation suggests that 50% occupancy of the receptors by endogenous 5-HT will result in a doubling of (KDapp)est compared with conditions under 5-HT depletion. By imposing a single KDapp across all brain regions (i.e., ROIs) for each subject, we assume uniform 5-HT occupancy across regions. We also assume no temporal change in endogenous 5-HT due to anesthesia during the course of the experiments. Although no effects on endogenous 5-HT due to ketamine have been measured (Bacopoulos et al, 1979), isoflurane has been shown to reduce endogenous 5-HT levels (Tokugawa et al, 2007). To minimize potential variations in 5-HT across the cohort, all subjects were anesthetized with the same methodology and consistent timing was used for the injections. The vital signs were examined for evidence of physiological changes. SpO2 levels remained constant throughout the scanning procedure and were relatively similar between subjects with mean and standard deviation of 98.6±0.6% saturation. Breathing rate was within a range of 10 to 20 breaths/minute, and did not reveal a noticeable trend in variation from beginning to end of the scanning procedure. The average breathing rate among all subjects was 15.1±2.3 breaths/minute.
Further, 5-HT depletion and competition experiments will be required to fully validate the use of a uniform KDapp and the potential effects of isoflurane. Similar studies with PET ligands have been performed for the dopamine system to closely examine the effects of endogenous neurotransmitter on radioligand binding (Delforge et al, 2001). Without these experiments, we are left to speculate that the effects of competing 5-HT will induce only small changes in these results based on the similarities in in-vivo behavior between [18F]mefway with [11C]WAY-100635 (Wooten et al, 2011a, 2011b). Studies with [11C]WAY-100635 and [3H]WAY-100635 have shown negligible changes in binding due to increases and decreases in endogenous serotonin levels (Maeda et al, 2001; Rice et al, 2001). Further, our previous in-vitro work revealed that inhibition by 5-HT was similar for mefway (Saigal et al, 2006) and WAY-100635 (Khawaja et al, 1995).
In summary, we have made in-vivo measurements of K1, VND, kon, koff, and Bmax for [18F]mefway in various 5-HT1A regions of the rhesus monkey brain using PET. Experiment design optimized the identifiability of the binding parameters kon, koff, and Bmax to improve precision in parameter estimates. These results show that M-I [18F]mefway PET experiments can be used to measure 5-HT1A receptor density across the regions of the brain. Further simplifications could be made to the M-I protocol if the desired outcome metric is limited to only Bmax assay, which may be of value for investigating diseases or mechanisms targeting 5-HT1A receptor density.
Acknowledgments
The authors would like to thank the following for their contribution to this research: Dr Jonathan Engle and Professor R Jerry Nickles for assistance with isotope production; Julie Larson and the staff at the Harlow Center for Biological Psychology at the University of Wisconsin for nonhuman primate handling; Dr Alex Converse and Professor Jim Holden for technical discussions; Dr Suresh Pandey and Neil Saigal for providing the trans-tosyl mefway precursor.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by NIH grants AA017706, MH086014, AG030524, AA12277, T32CA009206. Additional support was provided by NIH grants S10RR015801, P30HD003352, and S10RR023033.
Supplementary Material
References
- Bacopoulos NG, Redmond DE, Roth RH. Serotonin and dopamine metabolites in brain regions and cerebrospinal fluid of primate species: effects of ketamine and fluphenazine. J Neurochem. 1979;32:1215–1218. doi: 10.1111/j.1471-4159.1979.tb11048.x. [DOI] [PubMed] [Google Scholar]
- Burnet PWJ, Eastwood SL, Harrison PJ. [3H]WAY-100635 for 5-HT1A receptor autoradiography in human brain: a comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem Int. 1997;30:565–574. doi: 10.1016/s0197-0186(96)00124-6. [DOI] [PubMed] [Google Scholar]
- Carson RE, Lang L, Watabe H, Der MG, Adams HR, Jagoda E. PET evaluation of [18F]FCWAY, an analog of the 5-HT1A receptor antagonist, WAY-100635. Nucl Med Biol. 2000;27:493–497. doi: 10.1016/s0969-8051(00)00118-9. [DOI] [PubMed] [Google Scholar]
- Christian BT, Narayanan T, Shi B, Morris ED, Mantil J, Mukherjee J. Measuring the in vivo binding parameters of [18F]-fallypride in monkeys using a PET multiple-injection protocol. J Cereb Blood Flow Metab. 2004;24:309–322. doi: 10.1097/01.WCB.0000105020.93708.DD. [DOI] [PubMed] [Google Scholar]
- Christian BT, Vandehey NT, Floberg JM, Mistretta CA. Dynamic PET denoising with HYPR processing. J Nucl Med. 2010;51:1147–1154. doi: 10.2967/jnumed.109.073999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes N, Merlet I, Zimmer L, Lavenne F, Cinotti L, Delforge J, Luxen A, Pujol J-F, Le Bars D. Modeling [18F]MPPF positron emission tomography kinetics for the determination of 5-hydroxytryptamine(1A) receptor concentration with multiinjection. J Cereb Blood Flow Metab. 2002;22:753–765. doi: 10.1097/00004647-200206000-00014. [DOI] [PubMed] [Google Scholar]
- Delforge J, Bottlaender M, Loc'h C, Guenther I, Fuseau C, Bendriem B, Syrota A, Mazière B. Quantitation of extrastriatal D2 receptors using a very high-affinity ligand (FLB 457) and the multi-injection approach. J Cereb Blood Flow Metab. 1999;19:533–546. doi: 10.1097/00004647-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Delforge J, Bottlaender M, Pappata S, Loc'h C, Syrota A. Absolute quantification by positron emission tomography of the endogenous ligand. J Cereb Blood Flow Metab. 2001;21:613–630. doi: 10.1097/00004647-200105000-00016. [DOI] [PubMed] [Google Scholar]
- Delforge J, Spelle L, Bendriem B, Samson Y, Bottlaender M, Papageorgiou S, Syrota A. Quantitation of benzodiazepine receptors in human brain using the partial saturation method. J Nucl Med. 1996;37:5–11. [PubMed] [Google Scholar]
- Delforge J, Syrota A, Mazoyer BM. Identifiability analysis and parameter identification of an in vivo ligand-receptor model from PET data. IEEE Trans Biomed Eng. 1990;37:653–661. doi: 10.1109/10.55673. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Kuo A, Tajuddin N. Effects of in utero ethanol exposure on the developing serotonergic system. Alcohol Clin Exp Res. 1991;15:678–684. doi: 10.1111/j.1530-0277.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Farde L, Ginovart N, Ito H, Lundkvist C, Pike VW, McCarron JA, Halldin C. PET-characterization of [carbonyl-11C]WAY-100635 binding to 5-HT1A receptors in the primate brain. Psychopharmacology. 1997;133:196–202. doi: 10.1007/s002130050391. [DOI] [PubMed] [Google Scholar]
- Gallezot J-D, Bottlaender MA, Delforge J, Valette H, Saba W, Dollé F, Coulon CM, Ottaviani MP, Hinnen F, Syrota A, Grégoire M-C. Quantification of cerebral nicotinic acetylcholine receptors by PET using 2-[18F]fluoro-A-85380 and the multiinjection approach. J Cereb Blood Flow Metab. 2008;28:172–189. doi: 10.1038/sj.jcbfm.9600505. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Sargent PA, Bench CJ, Rabiner EA, Osman S, Pike VW, Hume SP, Grasby PM, Lammertsma AA. Tracer kinetic modeling of the 5-HT1A receptor ligand [carbonyl-11C]WAY-100635 for PET. Neuroimage. 1998;8:426–440. doi: 10.1006/nimg.1998.0379. [DOI] [PubMed] [Google Scholar]
- Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, Fletcher A, Cliffe IA, Barf T, Wikström H, Sedvall G. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]WAY-100635. Brain Res. 1997;745:96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- Holden JE, Doudet DJ.2004Positron emission tomography receptor assay with multiple ligand concentrations: an equilibrium approach Methods in enzymology(Abelson JN, Simon MI, eds),vol. 385New York: Academic Press; 169–184. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang S-C, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Khawaja X, Evans N, Reilly Y, Ennis C, Minchin MCW. Characterization of the binding of [3H]WAY-100635, a novel 5-hydroxytryptamine1A receptor antagonist, to rat brain. J Neurochem. 1995;64:2716–2726. doi: 10.1046/j.1471-4159.1995.64062716.x. [DOI] [PubMed] [Google Scholar]
- Köhler C, Radesäter A-C, Lang W, Chan-Palay V. Distribution of serotonin-1A receptors in the monkey and the postmortem human hippocampal region. A quantitative autoradiographic study using the selective agonist [3H]8-OH-DPAT. Neurosci Lett. 1986;72:43–48. doi: 10.1016/0304-3940(86)90615-4. [DOI] [PubMed] [Google Scholar]
- Lang L, Jagoda E, Schmall B, Sassaman M, Magata Y, Eckelman WC. Comparison of F-18 labeled cis and trans 4-fluorocyclohexane derivatives of WAY 100635. J Nucl Med. 1999;40:37P–38P. [Google Scholar]
- Le Bars D, Lemaire C, Ginovart N, Plenevaux A, Aerts J, Brihaye C, Hassoun W, Leviel V, Mekhsian P, Weissmann D, Pujol JF, Luxen A, Comar D. High-yield radiosynthesis and preliminary in vivo evaluation of p-[18F]MPPF, a fluoro analog of WAY-100635. Nucl Med Biol. 1998;25:343–350. doi: 10.1016/s0969-8051(97)00229-1. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Ding YS, Wang G-J, Alexoff DL. A strategy for removing the bias in the graphical analysis method. J Cereb Blood Flow Metab. 2001;21:307–320. doi: 10.1097/00004647-200103000-00014. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Logan J, Volkow ND, Fowler JS, Wang GJ, Dewey SL, MacGregor R, Schlyer D, Gatley SJ, Pappas N, King P, Hitzemann R, Vitkun S. Effects of blood flow on [nC]raclopride binding in the brain: model simulations and kinetic analysis of PET data. J Cereb Blood Flow Metab. 1994;14:995–1010. doi: 10.1038/jcbfm.1994.132. [DOI] [PubMed] [Google Scholar]
- Logan J, Volkow ND, Fowler JS, Wang G-J, Fischman MW, Foltin RW, Abumrad NN, Vitkun S, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Concentration and occupancy of dopamine transporters in cocaine abusers with [11C]cocaine and PET. Synapse. 1997;27:347–356. doi: 10.1002/(SICI)1098-2396(199712)27:4<347::AID-SYN8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Maeda J, Suhara T, Ogawa M, Okauchi T, Kawabe K, Zhang MR, Semba J, Suzuki K. In vivo binding properties of [carbonyl-11C]WAY-100635: effect of endogenous serotonin. Synapse. 2001;40:122–129. doi: 10.1002/syn.1033. [DOI] [PubMed] [Google Scholar]
- Mauger G, Saba W, Hantraye P, Dolle F, Coulon C, Bramoulle Y, Chalon S, Grégoire M-C. Multiinjection approach for D2 receptor binding quantification in living rats using [11C]raclopride and the b-microprobe: crossvalidation with in vitro binding data. J Cereb Blood Flow Metab. 2005;25:1517–1527. doi: 10.1038/sj.jcbfm.9600141. [DOI] [PubMed] [Google Scholar]
- Morris ED, Babich JW, Alpert NM, Bonab AA, Livni E, Weise S, Hsu H, Christian BT, Madras BK, Fischman AJ. Quantification of dopamine transporter density in monkeys by dynamic PET imaging of multiple injections of [11C]-CFT. Synapse. 1996;24:262–272. doi: 10.1002/(SICI)1098-2396(199611)24:3<262::AID-SYN9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Morris ED, Bonab AA, Alpert NM, Fischman AJ, Madras BK, Christian BT. Concentration of dopamine transporters: to Bmax or not to Bmax. Synapse. 1999;32:136–140. doi: 10.1002/(SICI)1098-2396(199905)32:2<136::AID-SYN7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Morris ED, Christian BT, Yoder KK, Muzic RF.2004Estimation of local receptor density, B'max, and other parameters via multiple-injection positron emission tomography experiments Methods in enzymology(Abelson JN, Simon MI, eds),vol. 385New York: Academic Press; 184–213. [DOI] [PubMed] [Google Scholar]
- Muzic RF, Christian BT. Evaluation of objective functions for estimation of kinetic parameters. Med Phys. 2006;33:342–353. doi: 10.1118/1.2135907. [DOI] [PubMed] [Google Scholar]
- Muzic RF, Cornelius S. COMKAT: compartment model kinetic analysis tool. J Nucl Med. 2001;42:636–645. [PubMed] [Google Scholar]
- Muzic RF, Saidel GM, Zhu N, Nelson AD, Zheng L, Berridge MS. Iterative optimal design of PET experiments for estimating β-adrenergic receptor concentration. Med Biol Eng. 2000;38:593–602. doi: 10.1007/BF02344863. [DOI] [PubMed] [Google Scholar]
- Olsson H, Halldin C, Farde L. Differentiation of extrastriatal dopamine D2 receptor density and affinity in the human brain using PET. Neuroimage. 2004;22:794–803. doi: 10.1016/j.neuroimage.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Pike VW, McCarron JA, Lammertsma AA, Osman AA, Hume SP, Sargent PA, Bench CJ, Cliffe IA, Fletcher A, Grasby PM. Exquisite delineation of 5-HT1A receptors in human brain with PET and [carbonyl-11C]WAY-100635. Eur J Pharmacol. 1996;301:R5–R7. doi: 10.1016/0014-2999(96)00079-9. [DOI] [PubMed] [Google Scholar]
- Poyot T, Condé F, Grégoire M-C, Frouin V, Coulon C, Fuseau C, Hinnen F, Dollé F, Hantraye P, Bottlaender M. Anatomic and biochemical correlates of the dopamine transporter ligand [11C]-PE2I in normal and parkinsonian primates: comparison with 6-[18F]fluoro-L-DOPA. J Cereb Blood Flow Metab. 2001;21:782–792. doi: 10.1097/00004647-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Rice OV, Gatley SJ, Shen J, Huemmer CL, Rogoz R, DeJesus OT, Volkow ND, Gifford AN. Effects of endogenous neurotransmitters on the in vivo binding of dopamine and 5-HT radiotracers in mice. Neuropsychopharmacology. 2001;25:679–689. doi: 10.1016/S0893-133X(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Saigal N, Pichika R, Easwaramoorthy B, Collins D, Christian BT, Shi B, Narayanan TK, Potkin SG, Mukherjee J. Synthesis and biologic evaluation of a novel serotonin 5-HT1A receptor radioligand, [18F]-labeled mefway, in rodents and imaging by PET in a nonhuman primate. J Nucl Med. 2006;47:1697–1706. [PubMed] [Google Scholar]
- Salinas C, Muzic RF, Ernsberger P, Saidel GM. Robust experiment design for estimating myocardial beta adrenergic receptor concentration using PET. Med Phys. 2007;34:151–165. doi: 10.1118/1.2402585. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Carson RE, Jagoda E, Lang L, Heilig M, Barr CS, Suomi SJ, Higley JD, Stein EA. Effects of early-life stress on serotonin1A receptors in juvenile rhesus monkeys measured by positron emission tomography. Biol Psychiatry. 2010;67:1146–1153. doi: 10.1016/j.biopsych.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YC, Chatziioanno A, Siegel S, Young J, Newport D, Goble RN, Nutt RE, Cherry SR. Performance evaluation of the microPET P4: a PET system dedicated to animal imaging. Phys Med Biol. 2001;46:1845–1862. doi: 10.1088/0031-9155/46/7/308. [DOI] [PubMed] [Google Scholar]
- Tokugawa J, Ravasi L, Nakayama T, Lang L, Schmidt KC, Seidel J, Green MV, Sokoloff L, Eckelman WC. Distribution of the 5-HT1A receptor antagonist [18F]-FPWAY in blood and brain of the rat with and without isoflurane anesthesia. Eur J Nucl Med Mol Imaging. 2007;34:259–266. doi: 10.1007/s00259-006-0228-x. [DOI] [PubMed] [Google Scholar]
- Vandehey NT, Moirano JM, Converse AK, Holden JE, Mukherjee J, Murali D, Nickles RJ, Davidson RJ, Schneider ML, Christian BT. High-affinity dopamine D2/D3 PET radioligands [18F]-fallypride and [11C]-FLB457: a comparison of kinetics in extrastriatal regions using a multiple-injection protocol. J Cereb Blood Flow Metab. 2010;30:994–1007. doi: 10.1038/jcbfm.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AA, Garcia A, Li J, Dasilva JN, Houle S. Analogues of WAY-100635 as radiotracers for in vivo imaging of 5-HT1A receptors. J Labelled Compd Rad. 1999;42:611–620. [Google Scholar]
- Wooten DW, Hillmer AT, Murali D, Barnhart TE, Schneider ML, Mukherjee J, Christian BT. An in vivo comparison of cis- and trans-[18F]mefway in the nonhuman primate. Nucl Med Biol. 2011b;38:925–932. doi: 10.1016/j.nucmedbio.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten DW, Moraino JM, Hillmer AT, Engle JW, DeJesus OT, Murali D, Barnhart TE, Nickles RJ, Davidson R, Schneider ML, Mukherjee J, Christian BT.2011aIn vivo kinetics of [18F]MEFWAY: a comparison with [11C]WAY-100635 and [18F]MPPF in the nonhuman primate. Synapse 65592–600. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.