Abstract
We present here an initial characterization of ATP binding cassette (ABC) transporter function and regulation at the blood–spinal cord barrier. We isolated capillaries from rat spinal cords and studied transport function using a confocal microscopy-based assay and protein expression using western blots. These capillaries exhibited transport function and protein expression of P-glycoprotein (Abcb1), multidrug resistance protein 2 (Mrp2, Abcc2), and breast cancer-related protein (Bcrp, Abcg2). Exposing isolated capillaries to dioxin (activates aryl hydrocarbon receptor) increased transport mediated by all three transporters. Brain and spinal cord capillaries from dioxin-dosed rats exhibited increased P-glycoprotein-mediated transport and increased protein expression for all three ABC transporters. These findings indicate similar ABC transporter expression, function, and regulation at the blood–spinal cord and blood–brain barriers.
Keywords: capillaries, confocal imaging, Dioxin, membrane transport, P-glycoprotein
Introduction
Multiple barriers separate the central nervous system from the periphery. Of these, the blood–brain barrier, which resides within the capillary endothelium, is the most studied and thus the most fully characterized (Abbott et al, 2010). This barrier is characterized by low paracellular permeability and the expression of specialized plasma membrane transport proteins and receptors, which together limit and regulate the exchange of solutes between the brain and the periphery. Certain members of the ATP binding cassette (ABC) family of transporters, e.g., P-glycoprotein (Abcb1), breast cancer-related protein (Bcrp, Abcg2), and multidrug resistance-associated protein (Mrp2, Abcc2), are substantial contributors to barrier selectivity. These plasma membrane proteins are ATP-driven efflux pumps that face the vascular space and limit brain entry of foreign chemicals (xenobiotics), including neurotoxicants and therapeutic drugs (Miller, 2010).
A morphologically similar, capillary-based barrier, the blood–spinal cord barrier, separates the spinal cord from the periphery (Bartanusz et al, 2011). As in brain capillaries, spinal cord capillary endothelial cells express multiple tight junctional proteins and adherens junction-associated proteins (Ge and Pachter, 2006). Low tight junctional permeability of the blood–spinal cord barrier presents a substantial physical barrier to macromolecules (Bartanusz et al, 2011). In contrast, little is known about ABC transporter function and regulation at the blood–spinal cord barrier. As in brain capillaries, P-glycoprotein is localized to the luminal plasma membrane of the spinal cord capillary endothelial cells (Sugawara et al, 1990); inhibiting P-glycoprotein in vivo increases spinal cord uptake of certain drugs that are known substrates (Lotsch et al, 2002; Marier et al, 2005).
Here, we present an initial characterization of ABC transporter function and regulation at the rat blood–spinal cord barrier. In isolated capillaries, we show transport activity and protein expression of P-glycoprotein, Bcrp, and Mrp2. We show that activity of these transporters increased when capillaries were exposed to subnanomolar concentrations of 3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a persistent environmental toxicant and high affinity ligand for the arylhydrocarbon receptor (AhR). P-glycoprotein activity and P-glycoprotein, Bcrp and Mrp2 protein expression also increased in TCDD-dosed rats. These findings define one mechanism by which expression of three ABC transporters is regulated at the blood–spinal cord barrier.
Materials and methods
Materials
[N-ɛ(4-nitrobenzofurazan-7-yl)-D-Lys8]-cyclosporine A (NBD-CSA) was custom synthesized as described previously (Schramm et al, 1995). PSC833 was kindly provided by Novartis (Basel, Switzerland). MK571 was obtained from Cayman Chemical (Ann Arbor, MI, USA). BODIPY-prazosin was obtained from Invitrogen (Carlsbad, CA, USA). The TCDD stock solution was prepared by RTI International (Research Triangle Park, NC, USA). Texas red (TR) (sulforhodamine 101 free acid), Ficoll and all other chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA). Mouse polyclonal P-glycoprotein antibody (C219) was from Covance, and antibodies against Mrp2 and Bcrp were from Alexis Biochemicals (San Diego, CA, USA).
Animals
All experiments were performed in compliance with NIH animal care and use guidelines (Guide for the Care and Use of Laboratory Animals, National Research Council) and approved by the NIEHS Animal Care and Use Committee (ARRIVE Guidelines). Male, 6 to 9 months old, retired breeder Sprague-Dawley rats and 12-week-old male, wild-type (FVB) and P-glycoprotein-null (FVB.129P2-Abcb1atm1BorAbcb1btm1Bor N12) mice were obtained from Taconic, (Germantown, NY). Animals were housed in temperature-controlled rooms under a 12-hour light/12-hour dark cycle and given ad libitum access to food and water. For brain and spinal cord capillary collection, animals were euthanized by CO2 inhalation.
For in-vivo dosing of rats, TCDD dissolved in corn oil (Sigma, St Louis, MO, USA) was administered as a single 5 μg/kg dose by intraperitoneal injection (10 rats). Controls received an equal volume of corn oil intraperitoneally (10 rats). After 48 hours, rats were euthanized by CO2 inhalation and brain and spinal cord capillaries isolated as described above for transport experiments and western blots.
Capillary Isolation and Transport Assay
A detailed procedure for brain capillary isolation was described previously (Hartz et al, 2004). Briefly, for each preparation, white matter, meninges, midbrain, choroid plexus, blood vessels and olfactory lobes were removed from the 5 to 10 brains under a dissecting microscope and brain tissue homogenized. Tissue was kept in cold phosphate-buffered saline (PBS) (2.7 mmol/L KCl, 1.5 mmol/L KH2PO4, 136.9 mmol/L NaCl, 8.1 mmol/L Na2HPO4, 1 mmol/L CaCl2, 0.5 mmol/L MgCl2, 5 mmol/L D-glucose, and 1 mmol/L sodium pyruvate) throughout the isolation procedure. An aliquot of 30% Ficoll was added to an equal volume of brain homogenate and capillaries were separated from the parenchyma by centrifuging at 5,800 g for 20 minutes. Capillary pellets were washed with 1% bovine serum albumin in PBS and passed through a syringe column filled with glass beads. Capillaries bound to the glass beads were released by gentle agitation, then washed with PBS and used immediately. The procedure for spinal cord capillary isolation (5 to 10 animals per preparation) was identical except that the final Ficoll concentration was 20%.
Confocal microscopy-based transport assays with isolated brain capillaries have been described previously (Hartz et al, 2004); an identical procedure was used for spinal cord capillaries. All experiments were performed at room temperature in coverslip-bottomed imaging chambers filled with PBS. In general, capillaries were exposed for 3 hours to nuclear receptor ligands without or with additional inhibitors. Fluorescent substrates, NBD-CSA for P-glycoprotein, TR for Mrp2, and BODIPY-prazosin for Bcrp, were added to each chamber and luminal substrate accumulation was assessed 1 hour later. In some experiments, specific transport inhibitors were included in the incubation medium. To acquire images, the chamber containing the capillaries was mounted on the stage of a Zeiss Model 510 inverted confocal laser scanning microscope (Thornwood, NY, USA) and imaged through a × 40 water-immersion objective (numeric aperture=1.2) using a 488-nm laser line for NBD-CSA and BODIPY-prazosin or a 543-nm laser line for TR. Images were saved to disk and luminal fluorescence was subsequently quantitated by Image J software (NIH, Bethesda, MD, USA). Data are presented as arbitrary fluorescence units from 8-bit images.
Western Blots
Membranes were isolated from control and ligand-exposed capillaries as described previously (Hartz et al, 2004). An aliquot of the membrane protein was mixed with NuPAGE 4 × sample buffer (Invitrogen), loaded onto 4% to 12% Bis-Tris NuPAGE gel, electrophoresed, and then transferred onto an Immobilon-FL membrane (Millipore, Bedford, MA, USA). The membrane was blocked with Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln, NE, USA) at room temperature for 1 hour, and then immunoblotted with antibodies against P-glycoprotein, Mrp2, or Bcrp. The membrane was stained with corresponding goat anti-rabbit or goat anti-mouse fluorescence dyes IRDye 680 (or IRDye 800) in PBS with 0.1% Tween-20 at room temperature for 45 minutes and then imaged using an Li-Cor Biosciences Odyssey Infrared Imaging System. β-Actin (42 kDa) (1:2,000) was used as a loading control; the corresponding secondary antibody was IRDye goat anti-mouse antibody (1:15,000).
Electrophoretic Mobility Shift Assay
Nuclear protein extract was isolated from control and TCDD-induced spinal cord capillaries using an NE-PER kit (Pierce, Rockford, IL, USA), following manufacturer's instructions. Complementary DNA oligonucleotides 5′-GAT CCG GCT CTT CTC ACG CAA CTC CGA GCT CA-3′ and 5′-TGA GCT CGG AGT TGC GTG AGA AGA GCC GGA TC-3′ (dioxin response element recognition sequence highlighted) were end labeled with IRDye700 fluorescent dyes by IDT (Coralville, IA, USA) and annealed at 100°C for 5 minutes. The EMSA (Electrophoretic Mobility Shift Assay) samples were prepared using Odyssey Infrared EMSA Buffer Kit (Li-Cor Biosciences), with 5 μg of nuclear protein extract used in each AhR/dioxin response element binding reaction. The binding reaction was incubated at the room temperature for 20 minutes and the DNA-protein complexes were resolved on a precast, 6% native polyacrylamide gel in 0.5 × TBE buffer. The gel was removed from the electrophoresis unit and directly imaged on the Odyssey Infrared Imaging System.
Statistical Analyses
Data are expressed as mean±standard error of the mean. Differences between treatment means were considered significant when P<0.05 using one-way analysis of variance (Newman–Keuls multiple comparison test; Prism 4.0 software, La Jolla, CA, USA).
Results
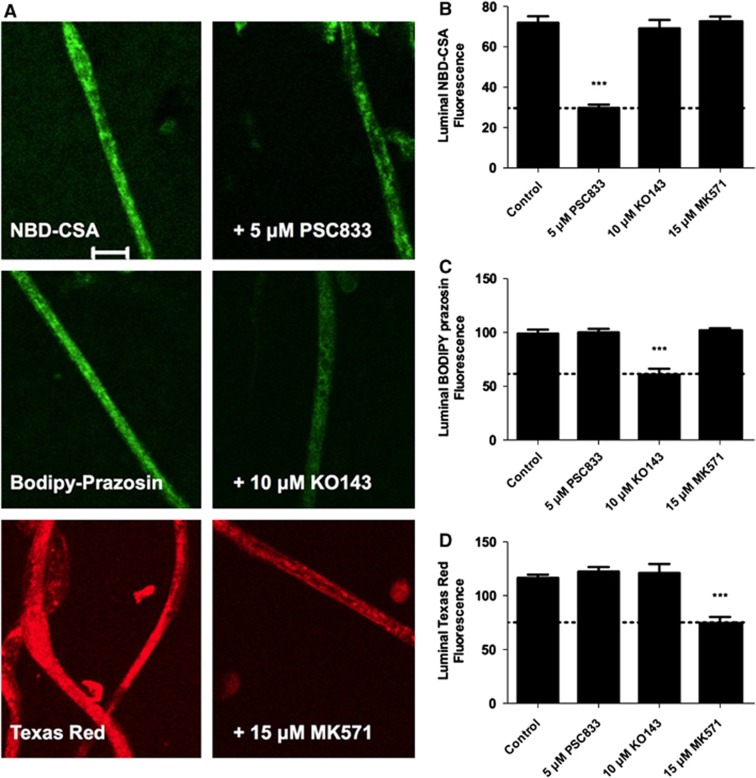
We established previously an assay to measure efflux transporter activity in living, isolated brain capillaries (Hartz et al, 2004). It is based on the use of confocal microscopy and digital image analysis to measure the specific accumulation of fluorescent substrates (NBD-CSA for P-glycoprotein, BODIPY-prazosin for Bcrp, and TR for Mrp2) within capillary lumens (Bauer et al, 2008; Hartz et al, 2004; Wang et al, 2010). In those studies, we also validated selective inhibitors for each transporter: PSC833 for P-glycoprotein, Ko143 for Bcrp, and MK571 for Mrp2. In initial experiments with spinal cord capillaries, we found rapid luminal accumulation of all three fluorescent substrates, with steady state established within 45 minutes (not shown). Figure 1A shows representative confocal images of rat spinal cord capillaries after 60 minutes incubation in medium containing 2 μmol/L NBD-CSA, BODIPY-prazosin, and TR. In these control capillaries, all three fluorescent compounds accumulate within the luminal space. For each compound, luminal fluorescence was substantially reduced when the appropriate inhibitor was added to the medium. Measurements of average pixel intensity within capillary lumens indicated reduced fluorescence only when capillaries were exposed to the appropriate inhibitor: PSC833 for NBD-CSA as a P-glycoprotein substrate, Ko143 for BODIPY-prazosin as a Bcrp substrate, and MK571 for TR as an Mrp2 substrate (Figures 1B to 1D).
Figure 1.
ATP binding cassette (ABC) transporter function at the rat blood–spinal cord barrier. (A) Representative confocal images showing luminal accumulation of the fluorescent substrates for P-glycoprotein (NBD-CSA), Bcrp (BODIPY-prazosin), and Mrp2 (Texas red). Right column shows capillaries incubated with substrate alone; left column shows capillaries incubated with substrate plus specific inhibitor of transport (see text). The scale bar indicates 10 μm. (B to D) Quantification of luminal steady-state (60 minutes) fluorescence in spinal cord capillaries incubated with NBD-CSA (B), BODIPY-prazosin (C), or Texas red (D). Data are shown for total luminal accumulation; the values above the dotted lines indicate the mediated component of transport. Each bar represents the mean value for 7 to 11 capillaries from a single preparation (pooled tissue from 10 rats); variability is shown as standard error bars. Statistical comparisons: ***significantly different from control, P<0.001. NBD-CSA, [N-ɛ(4-nitrobenzofurazan-7-yl)-D-Lys8]-cyclosporine A; Mrp2, multidrug resistance protein 2; Bcrp, breast cancer-related protein.
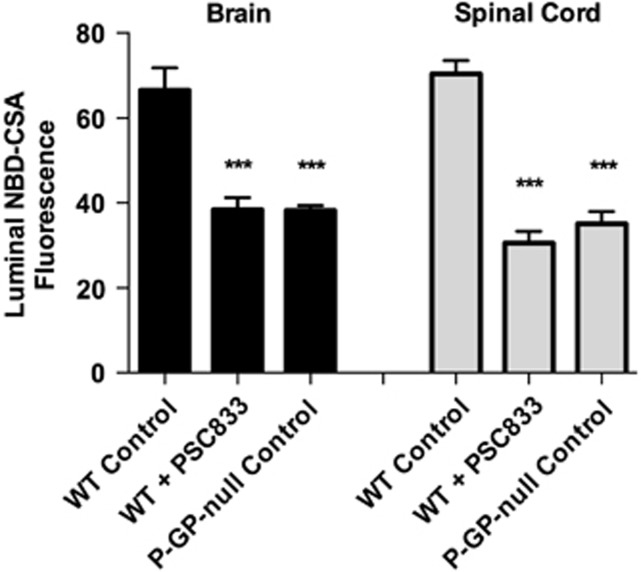
To further validate the assay, we measured luminal accumulation of NBD-CSA in brain and spinal cord capillaries from wild-type and P-glycoprotein-null mice. For both tissues, luminal NBD-CSA accumulation in capillaries from wild-type mice was over twice that in capillaries from P-glycoprotein-null mice (Figure 2). Moreover, in both tissues, luminal NBD-CSA accumulation in capillaries from P-glycoprotein-null mice was same as in capillaries from wild-type mice that had been exposed to PSC833 (Figure 2). Note that previous studies indicated that luminal fluorescence remaining after PSC833 inhibition of transport represents nonspecific accumulation, likely from diffusive entry plus binding to cellular elements (Hartz et al, 2004). Together, the experiments in Figures 1 and 2 define assays that measure transport activity of three ABC transporters in rat spinal cord capillaries. For the following experiments concerned with transporter regulation, we use steady-state, specific (inhibitable) luminal accumulation of the fluorescent substrates as a measure of transporter activity in the isolated capillaries.
Figure 2.
NBD-CSA transport in brain and spinal cord capillaries from wild-type (WT) and P-glycoprotein (P-GP)-null mice. Capillaries were incubated for 60 minutes in medium with 2 μmol/L NBD-CSA without (control) or with 5 μmol/L PSC833 and then imaged to measure luminal fluorescence. Each bar represents the mean value for 8 to 10 capillaries from a single preparation (pooled tissue from 10 rats or mice); variability is shown as standard error bars. Statistical comparisons: ***significantly different from control, P<0.001. NBD-CSA, [N-ɛ(4-nitrobenzofurazan-7-yl)-D-Lys8]-cyclosporine A.
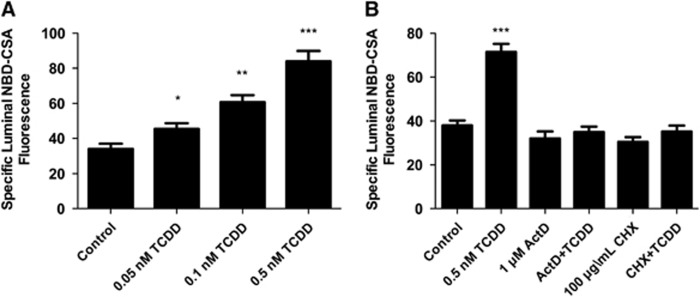
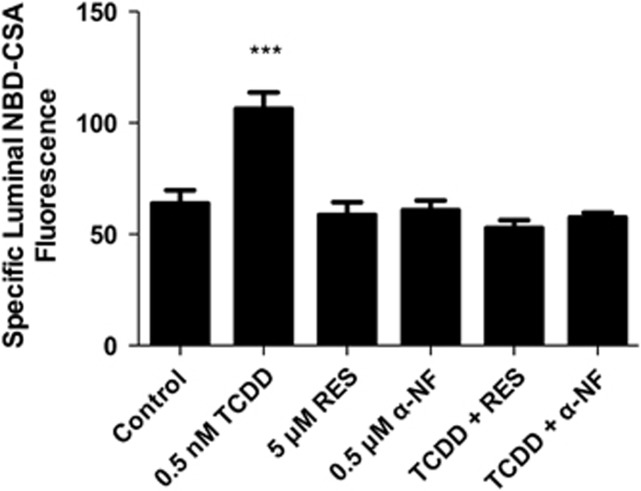
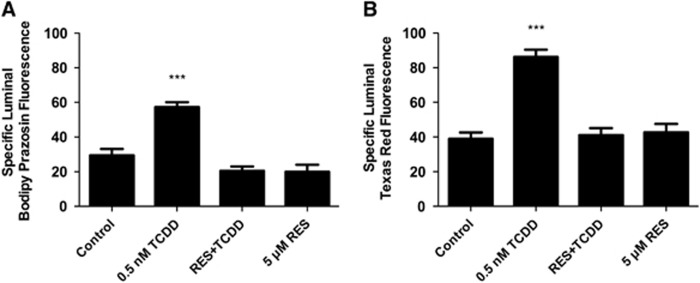
We recently reported that transport activity and protein expression of ABC transporters at the blood–brain barrier are upregulated by ligands that activate the AhR, e.g., the dioxin, TCDD (Wang et al, 2011). Exposing spinal cord capillaries to 0.05 to 0.5 nmol/L TCDD increased specific P-glycoprotein transport activity in a concentration-dependent manner (Figure 3A). Pretreating the capillaries with actinomycin D or cycloheximide abolished the increase in transport activity caused by TCDD exposure (Figure 3B). Exposing the capillaries to AhR antagonists, resveratrol or α-naphthoflavone, also abolished the increase in transport activity (Figure 4). Figures 5A and 5B show a similar pattern of effects for Bcrp and Mrp2. For both, transport activity was roughly doubled after exposure to 0.5 nmol/L TCDD and the AhR antagonist, resveratrol, abolished the increase. Thus, at subnanomolar concentrations, TCDD increased transport activity of P-glycoprotein, Bcrp, and Mrp2. This increase was dependent on transcription and translation and mediated by AhR.
Figure 3.
TCDD exposure increases P-glycoprotein-mediated transport. (A) TCDD dose response. Capillaries were exposed to TCDD for 3 hours. NBD-CSA was added to the medium and capillaries were imaged after an additional hour. (B) TCDD effects are blocked by 1 μmol/L actinomycin D (ActD) and 100 μg/ml cyclohexamide (CHX). Capillaries were preincubated for 30 minutes without or with ActD or CHX. TCDD (0.5 nmol/L) was added to the medium. After 3 hours, NBD-CSA was added to the medium; capillaries were imaged after an additional hour. Each bar represents the mean value for 7 to 14 capillaries from a single preparation (pooled tissue from 10 rats); variability is shown as standard error bars. Statistical comparisons: *significantly different from control, P<0.05; **significantly different from control, P<0.01; ***significantly different from control, P<0.001. TCDD, 3,7,8-tetrachlorodibenzo-p-dioxin; NBD-CSA, [N-ɛ(4-nitrobenzofurazan-7-yl)-D-Lys8]-cyclosporine A.
Figure 4.
TCDD effects on P-glycoprotein-mediated transport are blocked by arylhydrocarbon receptor (AhR) antagonists, 0.5 μmol/L α-naphthoflavone (α-NF) and 5 μmol/L resveratrol (RES). Capillaries were preincubated for 30 minutes without or with α-NF or RES. TCDD (0.5 nmol/L) was added to the medium. After 3 hours, NBD-CSA was added to the medium; capillaries were imaged after an additional hour. Each bar represents the mean value for 8 to 14 capillaries from a single preparation (pooled tissue from 10 rats); variability is shown as standard error bars. Statistical comparisons: ***significantly different from control, P<0.001. TCDD, 3,7,8-tetrachlorodibenzo-p-dioxin; NBD-CSA, [N-ɛ(4-nitrobenzofurazan-7-yl)-D-Lys8]-cyclosporine A.
Figure 5.
TCDD exposure increases Bcrp- (A) and Mrp2-mediated transport (B). Capillaries were preincubated for 30 minutes without or with 5 μmol/L resveratrol (RES). TCDD was added to the medium. After 3 hours, BODIPY-prazosin or Texas red was added to the medium; capillaries were imaged after an additional hour. Each bar represents the mean value for 8 to 11 capillaries from a single preparation (pooled tissue from 10 rats); variability is shown as standard error bars. Statistical comparisons: ***significantly different from control, P<0.001. TCDD, 3,7,8-tetrachlorodibenzo-p-dioxin; Mrp2, multidrug resistance protein 2; Brcp, breast cancer-related protein.
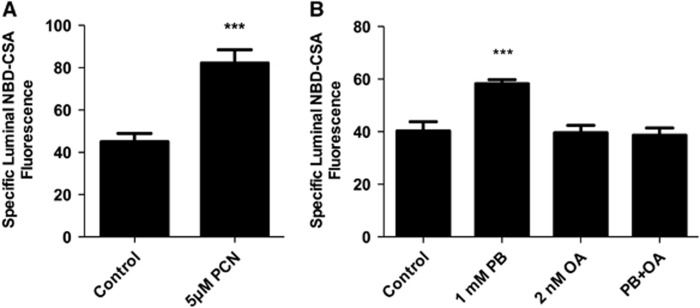
In rats and mice, activation of the pregnane-X-receptor (PXR) and the constitutive adrostane receptor (CAR) increases ABC transporter expression at the blood–brain barrier (Miller, 2010). In initial experiments we exposed spinal cord capillaries to pregnenolone 16α-carbonitrile (PXR ligand) or phenobarbital (PB; CAR ligand) and found increased P-glycoprotein transport activity (Figure 6A, PXR; Figure 6B, CAR). The CAR activation by PB involves the recruitment of cytoplasmic CAR retention protein and dephosphorylation by PP2A (Timsit and Negishi, 2007; Yoshinari et al, 2003). Consistent with a role for PP2A in CAR activation, 10 nmol/L okadaic acid inhibited PB upregulation of cytochrome P450 2B genes in rodent liver (Kawamoto et al, 1999). Figure 6B shows that inhibiting PP2A with 10 nmol/L okadaic acid abolished the PB-induced increase in transport activity. Thus, as in brain capillaries, the three xenobiotic activated nuclear receptors, AhR, PXR, and CAR, upregulate ABC transporter activity in spinal cord capillaries.
Figure 6.
Exposing rat spinal cord capillaries to 5 μmol/L pregnenolone 16α-carbonitrile (PCN; pregnane-X-receptor ligand; A) or 1 mmol/L phenobarbital (PB; constitutive adrostane receptor ligand; B) increases P-glycoprotein-mediated transport. Capillaries were preincubated for 30 minutes without or with 2 nmol/L okadaic acid (OA; only in panel B). TCDD (0.5 nmol/L) was then added to the medium. After 3 hours, NBD-CSA was added to the medium; capillaries were imaged after an additional hour. Each bar represents the mean value for 7 to 11 capillaries from a single preparation (pooled tissue from 10 rats); variability is shown as standard error bars. Statistical comparisons: ***significantly different from control, P<0.001. TCDD, 3,7,8-tetrachlorodibenzo-p-dioxin; NBD-CSA, [N-ɛ(4-nitrobenzofurazan-7-yl)-D-Lys8]-cyclosporine A.
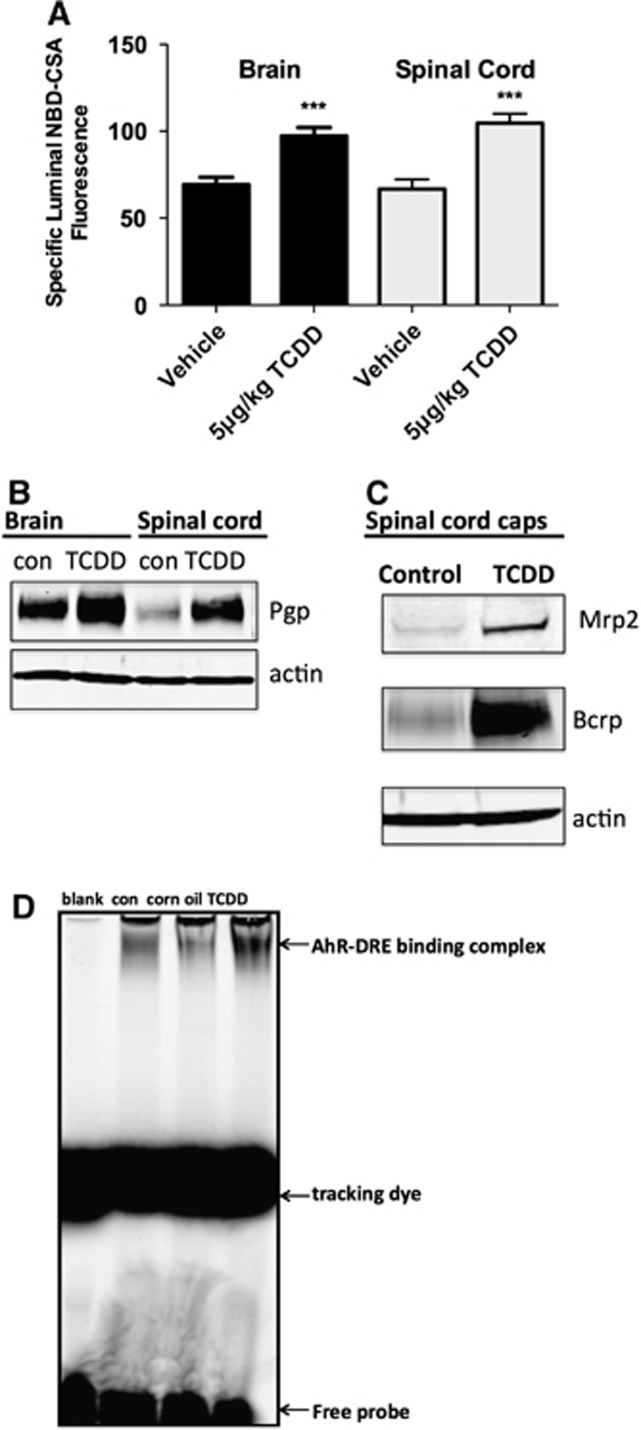
To determine the effects of TCDD exposure in vivo, we dosed 10 rats intraperitoneally with 5 μg/kg TCDD in corn oil and 48 hours later collected brain and spinal cord capillaries from TCDD-dosed rats and 10 control rats that received corn oil alone. As shown previously (Wang et al, 2011), TCDD dosing increased P-glycoprotein transport activity and protein expression in isolated brain capillaries (Figures 7A and 7B). Importantly, in isolated spinal cord capillaries from TCDD-dosed rats, we found increased P-glycoprotein transport activity (Figure 7A) and increased protein expression for P-glycoprotein (Figure 7B), Bcrp, and Mrp2 (Figure 7C). We previously used EMSA with a fluorescent probe containing a dioxin response element consensus sequence to show nuclear translocation of AhR in rat brain capillaries exposed to TCDD in vitro and in vivo (Wang et al, 2011). In spinal cord capillaries from TCDD-dosed rats, the probe band in the gel was shifted to higher mass and the intensity of the shifted band had increased (Figure 7D). This shift was not visible when 200-fold excess unlabeled probe was added to the assay (not shown).
Figure 7.
ATP binding cassette (ABC) transporter function and expression in spinal cord and brain capillaries from control and TCDD-dosed rats (5 μg/kg intraperitoneally in corn oil; capillaries isolated 48 hours after dosing). (A) P-glycoprotein transport activity. (B) P-glycoprotein (P-GP) expression. (C) Mrp2 and Bcrp expression. (D) Electrophoretic mobility shift assay (EMSA) shows nuclear translocation of arylhydrocarbon receptor (AhR) in spinal cord capillaries from TCDD-dosed rats. Each bar represents the mean value for nine capillaries from a single preparation (pooled tissue from 10 rats); variability is shown as standard error bars. Statistical comparisons: ***significantly different from control, P<0.001. TCDD, 3,7,8-tetrachlorodibenzo-p-dioxin; DRE, dioxin response element; Mrp2, multidrug resistance protein 2; Brcp, breast cancer-related protein.
Discussion
Our understanding of the molecular basis of blood–spinal cord barrier function and dysfunction lags substantially behind that of the blood–brain barrier. This is especially true for the ABC transporters that provide an important, selective element of the barrier. In the present study, we show that specific ABC transporter activity can be measured in isolated spinal cord capillaries using the same confocal microscopy-based assays developed previously for brain capillaries (Miller et al, 2000). We confirm the presence of P-glycoprotein on capillary membranes and show its transport function in the isolated capillaries from rats and mice. As shown previously in mouse (Ge and Pachter, 2006), at the protein level, P-glycoprotein expression appears to be decrease in rat spinal cord capillaries than in rat brain capillaries. Importantly, we show for the first time at the blood–spinal cord barrier transport function and protein expression for Bcrp and Mrp2. Although we have not yet immunostained spinal cord capillaries for Mrp2 and Bcrp, the present transport data are entirely consistent with luminal localization of both transporters. Our unpublished experiments with spinal cord capillaries isolated from multiple mouse strains show similar results for ABC transporter function.
Certain ligand-activated nuclear receptors upregulate genes that code for proteins involved in phase 1 and phase 2 xenobiotic metabolism and xenobiotic excretion. Although most work in this area has been focused on peripheral barrier and excretory tissues, e.g., liver, recent studies show similar responses at the blood–brain barrier for PXR, CAR, and AhR (Miller, 2010; Wang et al, 2011). Consider AhR, which is activated by environmental toxicants, e.g., TCDD, and drugs. In-vivo exposure of rats to TCDD increases blood–brain barrier expression of phase 2 drug enzymes, Cyp1a1 and Cyp1b1, and three ABC transporters, P-glycoprotein, Bcrp, and Mrp2 (Wang et al, 2011). One consequence of TCDD exposure is reduced brain accumulation of the P-glycoprotein substrate, verapamil. The present results and our preliminary experiments indicate a parallel picture for the three ABC transporters at the blood–spinal cord barrier. That is, transporter activity increased in spinal cord capillaries exposed to TCDD in-vitro and transporter protein expression and transport activity had increased in capillaries from TCDD-dosed rats. These findings imply reduced ability of therapeutic drugs to enter the spinal cord in patients exposed to xenobiotics that are ligands for PXR, CAR, and AhR. Such chemicals include a large number of commonly prescribed drugs and persistent environmental pollutants.
Finally, blood–brain barrier, P-glycoprotein and Bcrp, substantially determine the extent to which many small molecule drugs enter the brain (Hartz et al, 2010). Of these transporters, P-glycoprotein handles a wider range of drugs, and is thus considered as a major obstacle to drug delivery to the brain. Available data indicate that blood–brain barrier P-glycoprotein expression is altered in central nervous system disease, e.g., increased in epilepsy and stroke and reduced in Alzheimer's disease (Zlokovic, 2008, 2011).
In this regard, altered blood–spinal cord barrier function is increasingly recognized as one consequence of traumatic injury, e.g., spinal cord and peripheral nerve injury, as well as neurodegenerative disease, e.g., amyotrophic lateral sclerosis. Both tight junction permeability and transporter expression/function are implicated. On the one hand, altered barrier function can disturb homeostatic mechanisms that depend on the composition of spinal cord interstitial fluid. On the other hand, altered expression of drug efflux pumps can affect pharmacotherapy. Consider amyotrophic lateral sclerosis, recent studies show altered blood–spinal cord barrier function before symptoms become apparent both in an animal model of the disease (transgenic SOD1 mouse) and in patients with sporadic and familial amyotrophic lateral sclerosis (Zlokovic, 2011). Moreover, the SOD1 mouse exhibits increased P-glycoprotein expression at the blood–spinal cord barrier (Boston-Howes et al, 2008; Milane et al, 2010), suggesting reduced ability to deliver certain drugs to the spinal cord. How this disease leads to increased transporter expression is not known. We posit that understanding the molecular basis for drug transport at the blood–spinal cord barrier and its regulation will provide strategies to improve delivery of therapeutic drugs.
The authors declare no conflict of interest.
Footnotes
This work was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M. The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol. 2011;70:194–206. doi: 10.1002/ana.22421. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Lucking JR, Yang X, Pollack GM, Miller DS. Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab. 2008;28:1222–1234. doi: 10.1038/jcbfm.2008.16. [DOI] [PubMed] [Google Scholar]
- Boston-Howes W, Williams EO, Bogush A, Scolere M, Pasinelli P, Trotti D. Nordihydroguaiaretic acid increases glutamate uptake in vitro and in vivo: therapeutic implications for amyotrophic lateral sclerosis. Exp Neurol. 2008;213:229–237. doi: 10.1016/j.expneurol.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pachter JS. Isolation and culture of microvascular endothelial cells from murine spinal cord. J Neuroimmunol. 2006;177:209–214. doi: 10.1016/j.jneuroim.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol. 2004;66:387–394. doi: 10.1124/mol.104.001503. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Mahringer A, Miller DS, Bauer B. 17-beta-Estradiol: a powerful modulator of blood-brain barrier BCRP activity. J Cereb Blood Flow Metab. 2010;30:1742–1755. doi: 10.1038/jcbfm.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotsch J, Schmidt R, Vetter G, Schmidt H, Niederberger E, Geisslinger G, Tegeder I. Increased CNS uptake and enhanced antinociception of morphine-6-glucuronide in rats after inhibition of P-glycoprotein. J Neurochem. 2002;83:241–248. doi: 10.1046/j.1471-4159.2002.01177.x. [DOI] [PubMed] [Google Scholar]
- Marier JF, Deschenes JL, Hage A, Seliniotakis E, Gritsas A, Flarakos T, Beaudry F, Vachon P. Enhancing the uptake of dextromethorphan in the CNS of rats by concomitant administration of the P-gp inhibitor verapamil. Life Sci. 2005;77:2911–2926. doi: 10.1016/j.lfs.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Milane A, Fernandez C, Dupuis L, Buyse M, Loeffler JP, Farinotti R, Meininger V, Bensimon G. P-glycoprotein expression and function are increased in an animal model of amyotrophic lateral sclerosis. Neurosci Lett. 2010;472:166–170. doi: 10.1016/j.neulet.2010.01.078. [DOI] [PubMed] [Google Scholar]
- Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31:246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol. 2000;58:1357–1367. doi: 10.1124/mol.58.6.1357. [DOI] [PubMed] [Google Scholar]
- Schramm U, Fricker G, Wenger R, Miller DS. P-glycoprotein-mediated secretion of a fluorescent cyclosporin analogue by teleost renal proximal tubules. Am J Physiol. 1995;268:F46–F52. doi: 10.1152/ajprenal.1995.268.1.F46. [DOI] [PubMed] [Google Scholar]
- Sugawara I, Hamada H, Tsuruo T, Mori S. Specialized localization of P-glycoprotein recognized by MRK 16 monoclonal antibody in endothelial cells of the brain and the spinal cord. Jpn J Cancer Res. 1990;81:727–730. doi: 10.1111/j.1349-7006.1990.tb02636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timsit YE, Negishi M. CAR and PXR: the xenobiotic-sensing receptors. Steroids. 2007;72:231–246. doi: 10.1016/j.steroids.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hawkins BT, Miller DS. Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. FASEB J. 2011;25:644–652. doi: 10.1096/fj.10-169227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sykes DB, Miller DS. Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. Mol Pharmacol. 2010;78:376–383. doi: 10.1124/mol.110.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinari K, Kobayashi K, Moore R, Kawamoto T, Negishi M. Identification of the nuclear receptor CAR:HSP90 complex in mouse liver and recruitment of protein phosphatase 2A in response to phenobarbital. FEBS Lett. 2003;548:17–20. doi: 10.1016/s0014-5793(03)00720-8. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]