Abstract
Inflammation is an essential component for glial scar formation. However, the upstream mediator(s) that triggers the process has not been identified. Previously, we showed that the expression of CD36, an inflammatory mediator, occurs in a subset of astcotyes in the peri-infarct area where the glial scar forms. This study investigates a role for CD36 in astrocyte activation and glial scar formation in stroke. We observed that the expression of CD36 and glial fibrillary acidic protein (GFAP) coincided in control and injured astrocytes and in the brain. Furthermore, GFAP expression was attenuated in CD36 small interfering RNA transfected astrocytes or in the brain of CD36 knockout (KO) mice, suggesting its involvement in GFAP expression. Using an in-vitro model of wound healing, we found that CD36 deficiency attenuated the proliferation of astrocytes and delayed closure of the wound gap. Furthermore, stroke-induced GFAP expression and scar formation were significantly attenuated in the CD36 KO mice compared with wild type. These findings identify CD36 as a novel mediator for injury-induced astrogliosis and scar formation. Targeting CD36 may serve as a potential strategy to reduce glial scar formation in stroke.
Keywords: astrocytes, cerebral ischemia, GFAP, glial scar, stroke
Introduction
In response to CNS (central nervous system) injury, astrocytes change their morphology, proliferate, and migrate to the injury sites to form a scar. These reactive astrocytes display hypertrophic morphology and express high levels of glial fibrillary acidic protein (GFAP), a major intermediate filament protein (Pekny and Nilsson, 2005). The formed glial scar isolates and protects the noninjured tissue from injury, inhibits the spread of inflammation, and regulates the extracellular milieu (Bush et al, 1999; Faulkner et al, 2004). However, the scar creates a physical barrier for neurite outgrowth and also produces redox reactants and inflammatory mediators that cause further tissue damage (Askalan et al, 2006; Bush et al, 1999; Silver and Miller, 2004; Sofroniew, 2005). Accordingly, strategies aimed at reducing scar formation have been suggested to overcome the physical barrier to promote axonal growth (Desclaux et al, 2009).
Sterile inflammation, an inflammatory response in the absence of infection, occurs in postischemic tissues (Bamboat et al, 2010a, 2010b). It is a rapid and coordinated process involving an array of cellular and molecular events that leads to glial scar formation. Literature suggests that inflammation is an essential component for the formation of glial scar. Several inflammatory factors including TGF-β (transforming growth factor β), IFN-γ (interferon-γ), interleukins (ILs), fibroblast growth factor 2, monocyte chemoattractant protein 1 (MCP-1) as well as blood components are involved in the processes of astrogliosis (Fitch and Silver, 1997; Giulian et al, 1988; Hughes et al, 2002; Levison et al, 2000; Mocchetti et al, 1996; Schachtrup et al, 2010; Yong et al, 1991).
CD36, a class B scavenger receptor, has been implicated in pathological conditions associated with inflammation including stroke, atherosclerosis, and Alzheimer's disease (Kim et al, 2008; Febbraio et al, 2000; El Khoury et al, 2003). The receptor is expressed in many different cell types such as microglia, microvascular endothelial cells, monocytes/macrophages, and platelets (Febbraio et al, 2001; Febbraio and Silverstein, 2007). Substantial evidence suggests a role for CD36 in stroke pathology associated with inflammation (Cho et al, 2005; Cho and Kim, 2009). Ischemic stroke produces several CD36 ligands including oxidized/modified low-density lipoprotein and thrombospondins (Kim et al, 2008; Lin et al, 2003; Qin et al, 2011). We previously reported that CD36 contributes to free radical generation in the postischemic brain and that its deficiency ameliorates stroke-induced inflammation and injury (Cho et al, 2005; Cho and Kim, 2009; Kim et al, 2008). In addition to CD36 function in stroke-induced inflammation, its expression also occurred in a subset of GFAP+ astrocytes in the peri-infarct area where the glial scar forms (Cho et al, 2005). The purpose of this study is to address a potential role for CD36 in astrocyte proliferation and glial scar formation in stroke. Using an astrocytic cell line and a mouse model of transient focal cerebral ischemia in wild-type (WT) and CD36 knockout (KO) mice, here we report that CD36 is a novel mediator for astrogliosis and scar formation in stroke.
Materials and methods
Cell Culture
Mouse astrocytic cell line C8-D1A was obtained from ATCC (CRL-2541, American Type Culture Collection, Manassas, VA, USA) and cultured in DMEM (Dulbecco's Modified Eagle's Medium; high glucose, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Mediatech, Manassas, VA, USA), 100 I.U. Penicillin and 100 μg/mL Streptomycin (Invitrogen) at 37 °C in a humidified 5% CO2 incubator.
Animals
The use of animals and performance of procedures were approved by the Institutional Animal Care and Use Committee of Weill Medical College of Cornell University. C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Breeding pairs of each WT and CD36 KO strain (seven times backcrossed with C57BL/6, 99.7% C57BL/6 background) were derived at the time of the heterozygote cross. From these breeding pairs, only male F1 generation were used for the study without further sister–brother mating. Breeding pairs were replaced every 6 to 9 months by another heterozygote cross. This strategy prevented the genetic drifting associated with the propagation of homozygote lines. The procedures for genotyping have been described previously (Febbraio et al, 1999, 2000).
Transient Middle Cerebral Artery Occlusion
The procedure for middle cerebral artery occlusion (MCAO) has been described previously (Cho et al, 2005; Kim et al, 2008; Qin et al, 2011). Briefly, 8- to 10-week-old male mice were anesthetized with a mixture of isoflurane/oxygen/nitrogen and a 6-0 Teflon-coated black monofilament surgical suture (Doccol, Redland, CA, USA) was inserted into the exposed external carotid artery, advanced into the internal carotid artery, and wedged into the circle of Willis to obstruct the origin of the middle cerebral artery. The filament was left in place for either 30 or 45 minutes and then withdrawn. The cerebral blood flow in the center of the ischemic territory was monitored by Laser-Doppler flowmetry (Periflux System 5010; Perimed, Jarfalla, Sweden). Animals exhibiting reduced cerebral blood flow >80% during MCAO and restored cerebral blood flow 80% of baseline by 10 minutes following reperfusion were included in the study. The criteria resulted in reproducible infarcts involving both the cerebral cortex and the striatum.
Tissue Preparation for Infarct Volume and Biochemical Measurement
The brains were excised, frozen, and sectioned using a cryostat. Temporal CD36 and GFAP mRNA changes in the postischemic brain were initially performed to select time points to compare stroke-induced GFAP levels. For this temporal study, we collected an entire hemisphere from C57BL/6 mice. The rest of the samples were serially collected by an unbiased stereological sampling strategy. Infarct typically spans about 6 mm rostrocaudal, roughly from +2.8 to −3.8 mm bregma. To collect tissue to closely reflect the infarct area, the infarct region was cryosectioned for infarct volume measurement and slide immunohistochemistry (20 μm thickness) or for gene and protein expression (four sections at 50 μm thickness) and collected serially at 600-μm intervals. To assess infarct volume, mice were killed 3 days postischemia. The serial sections (total 12 to 13 sections for each mouse) were stained with Cresyl Violet or subjected to phase contrast to outline infarct and to determine infarct volume using Axiovision software (Carl Zeiss Microimaging, Thornwood, NY, USA). The contribution due to swelling was corrected using a method described previously (Lin et al, 1993). For gene and protein expression, the sections were cut in half and collected for each hemisphere.
RNA Interference
C8-D1A cells were transfected with mouse CD36 Stealth RNAi small interfering RNA (siRNA) (Invitrogen) using lipofectamine RNAiMAX (Invitrogen) for 12 hours according to the manufacturer's instructions. In all, 20 nmol/L of siRNA (Oligo ID: MSS202775, MSS202776, and MSS202777, abbreviated to 75/76/77) were added either alone or in combination. For controls, an equivalent amount of nonspecific control siRNA (Stealth RNAi siRNA Negative Control Low GC, Invitrogen) was used. Since siRNA 75 was the most effective, this siRNA (sense, 5′-UAG CUU GGC CAA UAG GAC AAA UUC C-3′ antisense, 5′-GGA AUU UGU CCU AUU GGC CAA GCU A-3′) was used for the further experiments.
Real-Time Reverse Transcription PCR
The levels of mRNA were quantified with real-time quantitative reverse transcription PCR using fluorescent TaqMan technology. Total RNA was reverse transcribed using a QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA, USA). PCR primers specific for CD36, GFAP, MCP-1, IL-6, and internal controls 18s rRNA (for the RNA interference experiments) and β-actin (for the rest of experiments) were obtained from Applied Biosystems (Foster City, CA, USA). The PCR reaction was performed using TaqMan Universal PCR Master mix and 7500 Fast Real-Time PCR system (Applied Biosystems), according to the instructions of the manufacturer. Reactions were performed in 20 μl total volumes and incubated at 95 °C for 10 minutes, followed by 45 cycles of 15 seconds at 95 °C and 1 minute at 60 °C. The results were analyzed by 7500 Fast Real-Time PCR System software.
Western Blot Analysis
Protein was extracted from cultured astrocytes and brain tissues, and Western blot analysis was performed as described previously (Cho et al, 2005; Kim et al, 2008). Briefly, samples were homogenized in radioimmunoprecipitation assay buffer (for cells, Sigma-Aldrich, St Louis, MO, USA) or CelLytic MT Mammalian Tissue Lysis/Extraction Reagent (for tissues, Sigma-Aldrich) with freshly added protease inhibitor (Roche Diagnostics, Indianapolis, IN, USA). After incubating for 15 minutes on ice, the homogenate was centrifuged at 10,000 r.p.m. for 10 minutes at 4 °C. In all, 20 μg of protein was loaded on a gel, electrophoresed and transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA, USA) using an electroblotting apparatus. After incubation with blocking buffer (LI-COR, Lincoln, NE, USA) for 1 hour, the membrane was incubated with one of the following antibodies: anti-CD36 polyclonal antibody (1:1,000, AF2519, R&D Systems, Minneapolis, MN, USA), anti-GFAP monoclonal antibody for cell line (1:1,000, G3893, Sigma), anti-GFAP polyclonal antibody for mouse brain (1:1,000, AB5541, Chemicon, Millipore, Billerica, MA, USA), or anti-actin antibody (1:1,000, sc-8432, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and then incubated with the appropriate secondary antibody: Alexa Fluor 680 rabbit anti-goat IgG (1: 10,000 for CD36, Invitrogen), IRDye 680 Donkey anti-Chicken IgG (1:10,000 for GFAP, LI-COR) or IRDye 800 Donkey anti-mouse IgG (1:10,000 for actin or GFAP, LI-COR). The membrane was washed and proteins visualized using the Odyssey Imaging System (LI-COR).
Cytotoxicity Assay
Cell viability was assessed by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Invitrogen). Briefly, 48 hours after scratch, cells treated with control or CD36 Stealth RNAi siRNA were incubated in the cell culture medium containing MTT for 4 hours at 37 °C. After washing with phosphate-buffered saline, the cells were dissolved in the sodium dodecyl sulfate HCl solution for 16 hours at 37 °C. Absorbance was measured at 570 nm.
Degree of cell death was determined by TdT-mediated dUTP nick end labeling (TUNEL) staining (DeadEnd Colorimetric TUNEL system, Promega, Madison, WI, USA). Briefly, 24 and 48 hours after scratch, cells treated with control or CD36 Stealth RNAi siRNA, were fixed with 4% paraformaldehyde, treated with ethanol/acetic acid and triton, and incubated in an equilibration buffer as described in the kit. The TdT enzyme and nucleotide mix were then added at proportions specified in the kit. Nuclei were counterstained by methyl green.
Assessment of Cellular Proliferation
Immunohistochemistry of Ki-67 was performed 12 and 48 hours after scratch to investigate the effect of CD36 Stealth RNAi siRNA on cellular proliferation. For each culture, six areas along the scratched edge (about 250 μm away the edge) were randomly selected. The number of proliferating C8-D1A cells was identified by nuclear staining of 4′,6-diamidino-2-phenylindole (DAPI) colocalized with Ki-67-immunoreactive cells. The densities of proliferating C8-D1A cells were calculated by dividing DAPI+ cells from the number of Ki-67+ cells and averaged in triplicates.
Wound Healing Assay
To gauge the degree of wound healing, nonclosed gap area was measured at 0, 24, and 48 hours by using CytoSelect 24-Well Wound Healing Assay Kit (Cell Biolabs, San Diego, CA, USA) and Axiovision software (Zeiss). Briefly, 600 μL from a 5 × 106 cells/mL suspension in DMEM (Invitrogen) was plated in the Control siRNA or CD36 Stealth RNAi siRNA (Invitrogen). After 12 hours incubation at 37 °C and 5% CO2, the insert was removed. Migration rate of the cells into the defined wound area was determined by measuring the lengths of cell migration over 48 hours after scratch (μm/h). The experiments were performed in triplicate.
Immunohistochemistry
Following MCAO, the brains were excised, frozen, and sectioned at a thickness of 20 μm for GFAP (1:1,000, Chemicon) immunohistochemistry according to previously published methods (Cho et al, 2005). The specificity of the immunolabel was tested by omitting the primary antibodies. Images were obtained using a fluorescent microscope and laser scanning confocal microscopy (Carl Zeiss Microimaging, Thornwood, NY, USA).
Data Analysis
Gene and protein levels in cultures were normalized by β-actin or 18s RNA. Three independent experiments with triplicates within each experiment were performed. For culture studies, values (mean±s.e.m.) were presented as a fold increase relative to control (0 hours) cultures. For in-vivo studies, after mRNA levels in the brain were normalized by an internal control, values (mean±s.e.m.) were presented as fold increase relative to control conditions. Due to confounding effect of variability associated protein level detection by Western blots in vivo, GFAP protein levels in the brain (Figure 8) were expressed as a ratio of ipsilateral to contralateral levels. Two groups were compared using Student's t-test. Multiple comparisons were made using analysis of variance followed by a post hoc Newman–Keuls test. Differences were considered significant at P<0.05.
Results
CD36 and Glial Fibrillary Acidic Protein Expression in Astrocytes Occurs in a Coordinated Manner
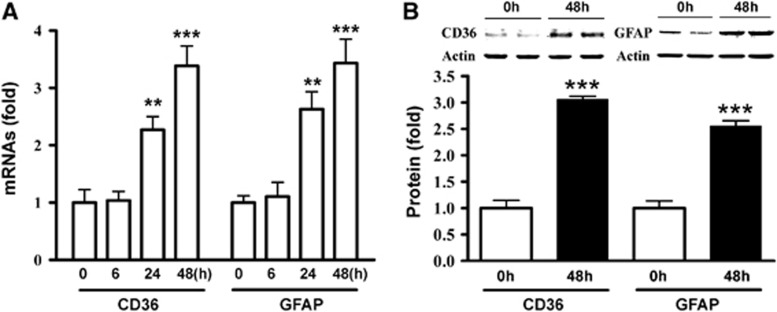
CD36 expression in numerous GFAP-positive (GFAP+) cells in peri-infarct area (Cho et al, 2005) led us to investigate a role for astrocytic CD36 in injury-induced scar formation. We first determined CD36 and GFAP expression in astrocyte cultures following scratch-induced injury. CD36 and GFAP mRNA levels were increased similarly in a time-dependent manner in C8-D1A astrocyte cells (Figure 1A). Similarly, the protein levels were significantly increased in C8-D1A cells 48 hours postscratch, reflecting the gene changes (Figure 1B). The results demonstrate that CD36 and GFAP expression occur in a coordinated manner in injured astrocytes.
Figure 1.
Coordinated expression of CD36 and glial fibrillary acidic protein (GFAP) expression in astrocytes. (A) Fold changes in CD36 and GFAP mRNA levels in C8-D1A astrocytes. 0 hours, control. (B) CD36 and GFAP protein levels in nonscratched control (0 hours) and 48 hours after scratch in C8-D1A cells. Values were expressed as mean±s.e.m. Three independent experiments were performed in triplicate. **P<0.01 and ***P<0.001 compared to respective 0 hours.
CD36 Absence Reduces Glial Fibrillary Acidic Protein Expression, Delays Wound Healing, and Reduces Inflammatory Factor Expression in Astrocytes
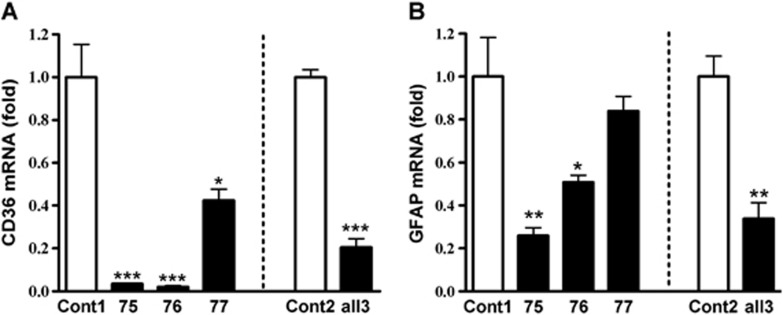
To investigate the requirement of CD36 for GFAP expression, GFAP mRNA levels were determined in CD36-silenced cultures. Transfection of CD36 siRNA 75, 76, and 77, either singly or in combination, significantly attenuated CD36 mRNA levels 48 hours postscratch when compared with their respective control (Figure 2A). There were corresponding reductions in GFAP mRNA levels in CD36 siRNA-treated cultures (Figure 2B), suggesting the necessity of CD36 for GFAP expression.
Figure 2.
Reduced glial fibrillary acidic protein (GFAP) expression in CD36-silenced astrocytes. Effect of CD36 Stealth RNAi small interfering RNA (siRNA) on fold changes in CD36 (A) and GFAP (B) mRNA levels in C8-D1A cells. siRNA 75, 76, or 77 were applied either singly (20 nmol/L) or all together (all 3, 60 nmol/L for 20 nmol/L each) with respective controls (normalized as 1). Cells were collected after treatment of siRNA for 12 hours. Cont1, 20 nmol/L control siRNA, Cont2, 60 nmol/L control siRNA. *P<0.05, **P<0.01, and ***P<0.001 versus respective control (Cont1 or Cont2), n=3 to 6/group.
To address functional significance of CD36 expression, effect of CD36 knockdown on cell viability and also on the extent of wound healing using an in-vitro model (Yu et al, 1993; Wang et al, 2011). MTT assay and TUNEL staining showed no difference in cellular viability between control- and CD36 siRNA-treated C8-D1A cultures, assessed at 48 hours postscratch (Figures 3A and 3B). The cellular proliferation was further examined by Ki-67, a proliferating marker. The density of proliferating cells was significantly decreased along the scratch edge in the cultures treated with CD36 siRNA 75 at both 12 and 48 hours after scratch (Figure 3C) without affecting the density in the area away from the scratched edge (data not shown). Larger gap areas at a given postscratch time point was observed in the CD36 siRNA-treated cultures, suggesting an involvement of CD36 in promoting wound healing (Figure 3D). The delayed gap closure in the CD36 siRNA-treated cultures was associated with a significant reduction in cellular migration rate (Control- versus CD36 siRNA 7.0±0.25 versus 5.4±0.13 μm/h, P<0.05). The expression and function studies suggest the potential involvement of CD36 in astrogliosis and scar formation.
Figure 3.
Delayed wound healing and inflammation in the absence of CD36. (A) Effect of CD36 small interfering RNA (siRNA) on cellular viability by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Cont, 20 nmol/L control siRNA; siRNA, 20 nmol/L CD36 siRNA. n=5/group. (B) Effect of CD36 siRNA on cell death by TUNEL (terminal deoxynucleotidyl transferase-mediated 2'-deoxyuridine 5'-triphosphate-biotin nick end labeling) staining n=4/group. Black arrowheads indicate TUNEL-positive cells. Scale bar, 50 μm. (C) Effect of CD36 siRNA on cellular proliferation and quantification. Representative images of Ki-67 and DAPI staining along the scratch edge in C8-D1A cultures treated with control siRNA (Cont) or CD36 siRNA 75 (siRNA) at 12 hours after scratch injury. White arrowheads indicate Ki-67+ cells. S indicated the scratch-induced gap area. The densities were calculated by dividing DAPI+ cells from the number of Ki-67+ cells and averaged in triplicates. Scale bar, 50 μm. n=4/group. (D) Effect of CD36 siRNA on wound healing. Representative images showed the effect of CD36 Stealth RNAi siRNA on the rate of wound closure following scratch. C8-D1A cells were transfected with CD36 siRNA 75 (20 nmol/L) or control siRNA (Cont) for 12 hours. Gaps were created by inserts and the area of gaps were quantified at 0, 24, and 48 hours. Scale bar, 500 μm. Quantification of gap area according to the wound field surface area. *P<0.05 versus Cont, n=3 to 4/group. (E) IL-6 and monocyte chemoattractant protein (MCP)-1 mRNA levels were increased in C8-D1A cells following mechanical scratch. β-Actin was used as an internal control. *P<0.05 and **P<0.01 versus 0 hours. (F) Effect of CD36 deficiency on scratch-induced IL-6 and MCP-1 expression in C8-D1A cells. The effect of CD36 Stealth RNAi siRNA 75/76/77 (all 3) on IL-6 and MCP-1 mRNA levels were determined 48 hours after scratch. siRNA and the control were treated for 12 hours before scratch. In all, 18 seconds rRNA was used as an internal control. Cont, 60 nmol/L control siRNA. *P<0.05, **P<0.01, and ***P<0.001 versus Cont.
Inflammation is tightly associated with glial scar formation in multiple brain injury models, and CD36 deficiency significantly reduced the levels of CD36-associated pro-inflammatory molecules such as MCP-1 and IL-6 (Glabinski et al, 1996; Hughes et al, 2002; Kim et al, 2008; Okada et al, 2004; Penkowa et al, 2000; Sawada et al, 1992). Following the mechanical injury, MCP-1 mRNA levels in astrocyte cultures were rapidly increased and sustained until 48 hours postinjury, whereas IL-6 expression increased at 48 hours postinjury (Figure 3E). Silencing CD36 significantly reduced both MCP-1 and IL-6 mRNA levels at 48 hours (Figure 3F). Reduced expression of inflammatory factors in the absence of CD36 suggests a mechanism by which CD36 influences glial scar formation through the expression of the inflammatory factors.
CD36 and Glial Fibrillary Acidic Protein Expression in the Brain Occur in a Coordinated Manner During Development
Glial fibrillary acidic protein expression changes during brain development and peaks around 1 week after birth in mice (Kim et al, 2011). To confirm the coordinated expression of CD36 and GFAP in vivo, we determined their mRNA levels in the mouse brain during development. In spite of higher expression of GFAP mRNA levels (∼100-fold) compared with CD36, their expression profiles over time were similar with a peak at postnatal day 7 (Figure 4A), indicating a coordinated expression of CD36 and GFAP during development. To address whether the absence of CD36 affects basal GFAP expression, GFAP mRNA and protein levels were determined in 1-week and 12-week-old wild-type and CD36 KO mice. Glial fibrillary acidic protein mRNA levels at 1 week were not different between the genotypes but decreased at 12 weeks (Figure 4B). Glial fibrillary acidic protein protein showed small but significant increases in CD36 KO mice at both time points (Figure 4C). The mismatch in the basal GFAP gene and protein expression in naive animals indicates that CD36 may regulate GFAP expression differentially in injured versus basal conditions.
Figure 4.
Coordinated expression of CD36 and glial fibrillary acidic protein (GFAP) expression in normal brain. (A) Temporal expression of CD36 and GFAP expression in the brain during postnatal period. *P<0.05, **P<0.01, and ***P<0.001 versus 1 day. (B) Fold changes in GFAP mRNA levels in wild-type (WT) and CD36 knockout (KO) mice at 1 and 12 weeks. Data were expressed as GFAP/β-actin. β-Actin was used as an internal control. n=7 to 9/group. *P<0.05 versus WT. (C) Fold changes in GFAP protein levels in WT and CD36 KO mice at 1 and 12 weeks. Data were expressed as GFAP/actin. n=3 to 5/group. WT, wild-type mice; KO, CD36 KO mice. *P<0.05 versus WT.
Coordinated Expression of CD36 and Glial Fibrillary Acidic Protein Expression in Postischemic Brain
Previously, we reported that CD36 proteins were elevated in the ipsilateral side of the brain and the expression occurred in GFAP+ astrocytes in the peri-infarct area (Cho et al, 2005). Temporal changes of CD36 mRNA and GFAP mRNA expression showed significant increases in the ipsilateral hemisphere at 3, 7 days and 2 weeks after ischemia (Figures 5A and 5B). The expression was relatively unchanged in the contralateral side. The similar gene expression profiles of CD36 and GFAP following stroke suggest their coordinated expression in response to injury. Stroke-induced GFAP protein expression was significantly higher at 3, 7, days and 2 weeks after ischemia in the WT mice (Figure 5C). CD36 KO mice displayed consistently less stroke-induced GFAP expression at 3, 7, days and 2 weeks, suggesting that the absence of CD36 resulted in sustained GFAP reduction in postischemic brain (Figure 5D).
Figure 5.
Coordinated expression of stroke-induced CD36 and glial fibrillary acidic protein (GFAP) expression in the postischemic brain. Fold changes of CD36 mRNA (A), GFAP mRNA (B) in C57BL/6 mice following 30 minutes middle cerebral artery occlusion (MCAO). *P<0.05, **P<0.01, and ***P<0.001 versus corresponding Contl. Representatives of Western blot images and quantification of GFAP protein levels in C57BL/6 mice (C) and CD36 knockout (KO) mice (D) following 30 minutes MCAO. n=4/group. Note the similar temporal expression profiles between CD36 and GFAP. Contralateral side of sham was normalized as 1. Contl, contralateral side; Ipsl, ipsilateral side. *P<0.05 and **P<0.01 versus corresponding Contl.
CD36 Is Required for Injury-Induced Glial Fibrillary Acidic Protein Expression and Glial Scar Formation
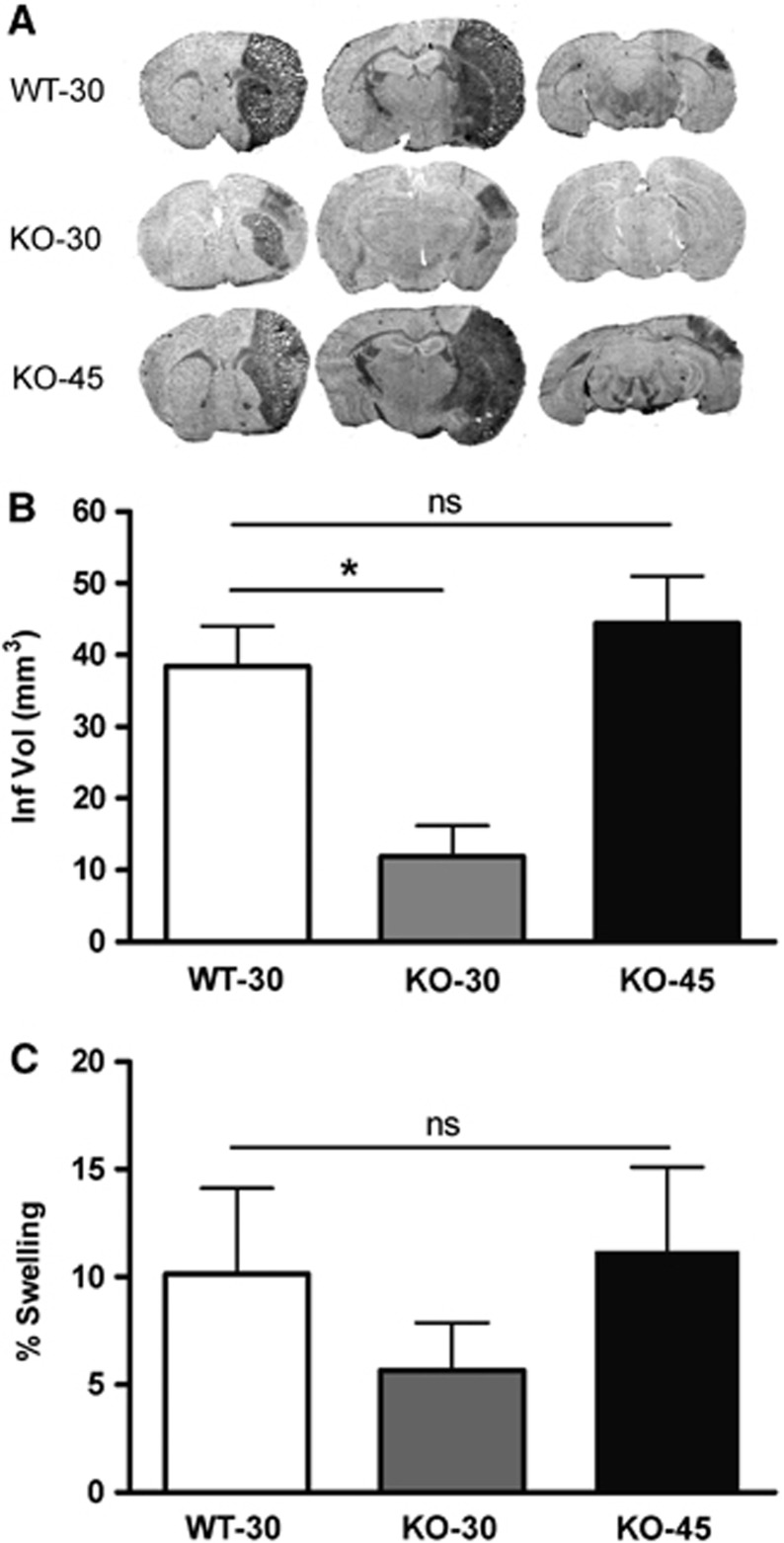
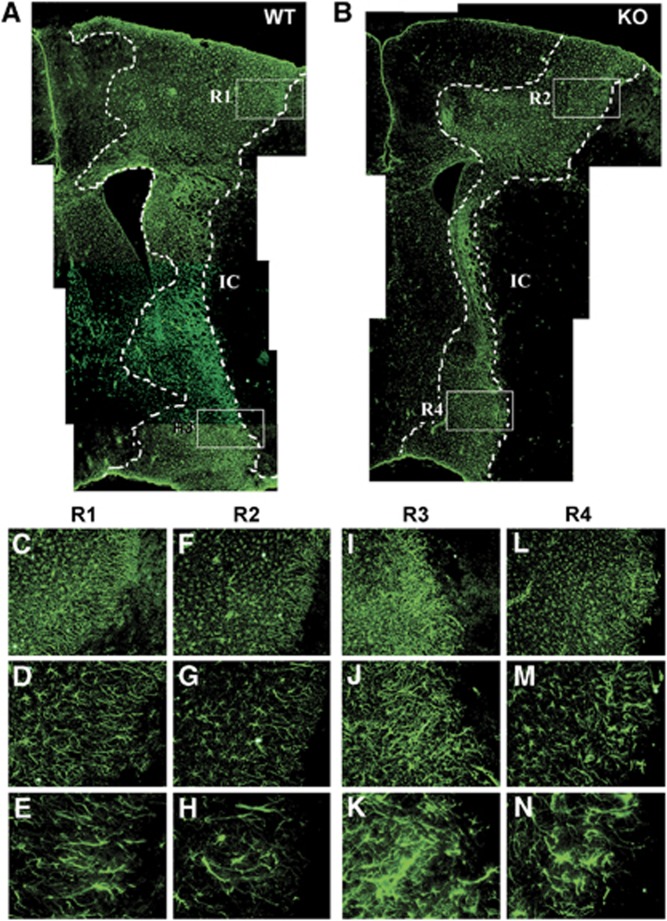
To further define the role of CD36 in scar formation in vivo, GFAP expression was determined in CD36 WT and KO mice after stroke. Since infarct size influences the degree of astrogliosis and scar formation following injury and CD36 KO mice had a significantly smaller infarct size compared with WT mice (Cho et al, 2005), we normalized infarct size between the genotypes by extending ischemic duration in CD36 KO mice. This eliminated the potential confounding effect of injury size on the extent of astrogliosis and glial scar formation. Subjecting CD36 KO mice for 45 minutes (KO-45) MCAO resulted in comparable infarct size and hemispheric swelling in WT mice with 30 minutes (WT-30) MCAO (Figure 6). Glial fibrillary acidic protein immunohistochemistry in the WT mice showed a stronger and wider immunoreactivity in the peri-infarct area, an indication of injury-induced astrocyte proliferation and reactivity (Figure 7A). In spite of a similar infarct size, scar thickness and GFAP staining in the corresponding area in CD36 KO mice with 45 minutes MCAO were attenuated (Figure 7B). Close examination of the morphology of GFAP+ cells revealed that WT mice displayed dense and intricate intercalating fine processes (Figures 7C–7E and 7I–7K) whereas CD36 KO mice exhibited shorter and sparse processes (Figures 7F–7H and 7L–7N), indicating that the degree of hypertrophy in the reactive astrocytes is reduced in CD36 KO mice.
Figure 6.
Effect of CD36 on stroke outcome. (A) Representative phase contrast images in wild-type (WT) and CD36 knockout (KO) mice. Assessment of infarct volume (B) and percent hemispheric swelling (C) 3 days after 30 minutes middle cerebral artery occlusion (MCAO) in WT and 30 or 45 minutes MCAO in CD36 KO mice. Note that extending ischemic duration to 45 minutes in CD36 KO mice resulted in an infarct size comparable to that in WT mice subjected to 30 minutes MCAO. WT-30, WT mice with a 30-minute MCAO (n=11); KO-30, CD36 KO mice with a 30-minute MCAO (n=5); KO-45, CD36 KO mice with a 45-minute MCAO (n=8). *P<0.05 versus WT-30.
Figure 7.
Stroke-induced glial scar formation is attenuated in CD36 knockout (KO) mice. Composite of low magnification of glial fibrillary acidic protein (GFAP) immunohistochemistry micrographs ( × 4) from wild-type (WT) (A) and CD36 KO (B) brains 7 days after ischemia. The mice exhibit similar infarct size (WT, 32.1 mm3, CD36 KO 34.0 mm3). Note that there is an overall reduction in GFAP immunoreactivity in the CD36 KO brain. The dotted lines indicate the boundary of glial scar. IC, Infarct core. (C–N) Higher magnification micrographs of GFAP immunoreactivity; × 10 (C, F, I, L), × 20 (D, G, J, M), and × 40 (E, H, K, N) indicated with white boxes indicate four different regions of interest (R) in panels A, B, and obtained from WT (C–E for R1 and I–K for R3) and CD36 KO mice for the corresponding regions (F–H for R2 and L–N for R4). n=2/group.
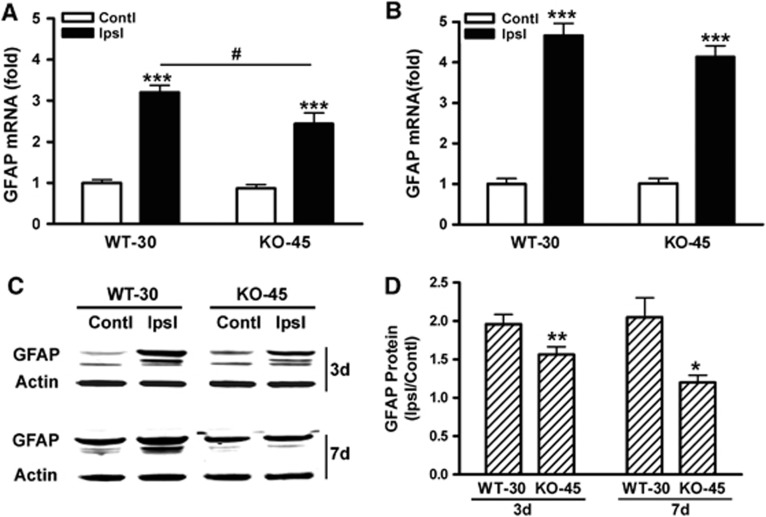
Glial fibrillary acidic protein mRNA levels in CD36 KO mice revealed a moderate but significant attenuation at day 3, but not day 7, postischemia (Figures 8A and 8B). Glial fibrillary acidic protein mRNA levels in the contralateral side were not different between genotypes, suggesting that ischemia–reperfusion and longer MCA occlusion in CD36 KO mice did not have an impact on GFAP gene expression in the contralateral hemisphere. We also found that GFAP protein levels in the stroked side (expressed as ratio of ipsilateral to contralateral) were significantly attenuated in CD36 KO mice at both 3 and 7 days postischemia (Figures 8C and 8D). Further correlation analyses between GFAP mRNA and protein levels showed a significant correlation in the ipsilateral side of the brain (P<0.01, r2=0.75, n=8), but not in the contralateral side (ns, r2=0.1382, n=8). Taken together, the quantitative changes in GFAP expression in the postischemic brain showed that CD36 is involved in stroke-induced GFAP expression and scar formation.
Figure 8.
CD36 deficiency reduces glial fibrillary acidic protein (GFAP) expression in the postischemic brain. Fold changes in GFAP mRNA levels in the brains of wild-type (WT) and CD36 knockout (KO) mice at 3 (A) and 7 days (B) after stroke. Data were expressed as GFAP/β-actin. Contl, contralateral side; Ipsl, ipsilateral side, ***P<0.001 versus Contl, #P<0.05 versus WT-30 Ipsl. n=5 to 8/group. (C, D) GFAP protein levels in the postischemic brain. Values were expressed as a ratio of ipsilateral/contralateral levels. *P<0.05 and **P<0.01 versus WT-30. n=4 to 8/group.
Discussion
Despite an initial protective role, glial scar formed in the CNS hinders outgrowth of regenerating axons. Several studies have aimed at degrading or suppressing the production of inhibitory components from the scar tissue to overcome this nonpermissive milieu (Desclaux et al, 2009; Snow et al, 1990). Our study identifies CD36 as a novel mediator of glial scar formation in stroke as CD36 is coordinately expressed with GFAP, and genetic or molecular suppression of CD36 reduces the expression of the intermediate filaments after injury.
Functionally, the absence of CD36 reduces cellular proliferation, delays the wound closure in an in-vitro model, and attenuates scar formation in the postischemic brain, supporting the view that CD36 is necessary for GFAP expression in injury-induced astrocyte activation and scar formation. In addition, the temporal expression of CD36 coincides with that of GFAP following stroke with profound inductions of both genes at 3 to 7 days after stroke. We observed that CD36 and GFAP gene/protein expression is increased and persisted until 2 weeks after stroke in the presence of CD36. This late expression indicates ongoing repair and remodeling processes following stroke, consistent with a recent report that shows prolonged scar-associated TGF-β signaling in astrocytes in the postischemic brain (Doyle et al, 2010).
Multiple signaling pathways are involved in scar formation. The literature suggests several links by which CD36 may regulate these pathways. Pro-inflammatory factors, such as IL-1, IL-6, MCP-1, and IFN-γ, which have been shown to be involved in GFAP expression and astrogliosis (Giulian et al, 1988; Hughes et al, 2002; Okada et al, 2004; Levison et al, 2000; Yong et al, 1991), are elevated in the postischemic brain. In the absence of CD36, the stroke-induced upregulation of these pro-inflammatory molecules is substantially reduced (Kim et al, 2008). Consistent with the report, we observed reduced expression of MCP-1 and IL-6, pro-inflammatory factors in the CD36-silenced cultures. Alternatively, the process of astrocyte reactivity and scar formation may be triggered by blood components (Preston et al, 2001). A recent study identified fibrinogen in the blood as a mediator that triggers scar formation via activation of latent TGF-β and downstream signaling pathways (Schachtrup et al, 2010; Wang et al, 2007). The activation of latent TGF-β was shown to require interaction with CD36 and its ligand thrombospondin-1 (Yehualaeshet et al, 1999). Thus, CD36-mediated inflammation in the postischemic brain may initiate the activation of astrocytes and scar formation. It is also possible that the presence of excess CD36 ligands including thrombospondins, oxidized lipids, and apoptotic bodies, which are upregulated in the ischemic territory, is likely to elevate CD36 expression, as CD36 expression occurs in a feed-forward manner (Tontonoz et al, 1998). While it is recognized that the ligands produced in the cultures would be substantially different from those in the ischemic brain in vivo, lipid membrane debris, modified low-density lipoproteins, and CD36-associated pro-inflammatory factors resulting from mechanical injury would serve as CD36 ligands and likely result in overlapping inflammatory responses, which may in turn upregulate GFAP expression. Our finding that CD36 and GFAP expression occur in a coordinated manner in injured C8-D1A astrocytes indicates the presence of intrinsic properties that cause GFAP upregulation in astrocytes upon stimulation. This finding, however, does not preclude the role of other CD36 expressing cells as contributors to the GFAP expression in astrocytes in vivo. Infiltrating peripheral immune cells, resident microglia, and endothelial cells that results in postischemic inflammation, may be a potential mechanism for increased GFAP expression.
Ischemic injury size depends in part on the severity of blood flow reduction and the duration of occlusion (Pedrono et al, 2010; Zhu and Auer, 1995). Compared with WT mice, CD36 KO mice subjected to 30 minutes MCAO had a significantly smaller injury in this study, consistent with a previous report (Cho et al, 2005). Since a larger infarct results in a greater glial response, the current study uniquely addressed the specific contribution of CD36 on the degree of astrogliosis and glial scar formation by normalizing infarct size between the genotypes. Reduced GFAP expression and less glial scar formation in CD36 KO mice with similar infarct size confirmed the necessary and specific role of CD36 in astrocyte activation and glial scar formation.
The formation of glial scar following injury can be damaging as well as beneficial. In the absence of CD36, we observed reduction in the injury-induced GFAP expression, cellular proliferation, and wound healing rate. The astrocytes also exhibited less intricate and sparse processes in the CD36 KO postischemic brain. Because physical hindrance and a nonpermissive milieu are associated with scar tissue, these morphological changes in the absence of CD36 may be viewed as beneficial. Caveats to this view are reports showing increased CNS inflammation and injury when scar formation is inhibited. In STAT3 KO mice whose scar formation was impaired, inflammation and injury following spinal cord injury were increased (Herrmann et al, 2008). Similarly, the ablation of intermediate filaments in GFAP and vimentin double KO mice increased stroke- or stab wound-induced injury size (Li et al, 2008). Unlike these mice, CD36 KO mice, however, display reduced infarct size with better neurological outcome (Cho et al, 2005) while the glial scar formation was attenuated even infarct size was normalized. Since CD36 KO mice exhibited a shift to a less inflammatory state in the postischemic brain (Kim et al, 2008), reduced inflammation at the level of the receptor may dampen intracellular signalings that lead glial responses. Although the mechanistic distinction to reduce scar formation between CD36 KO versus other means such as STAT3 KO or GFAP/vimentin double KO mice warrants further investigations, the inflammatory property of CD36 and its involvement in GFAP expression suggest that CD36 may serve as an ideal target to suppress intrinsic inflammation and subsequently reduce astrogliosis/scar formation following stroke.
Acknowledgments
The authors thank Jimmy Payappilly for technical assistance on astrocyte cultures and Nancy Geibel and Eric Shipp for editing the manuscript.
The authors declare no conflict of interest.
Footnotes
This work was supported by NIH Grants HL82511, HL82511-04S1, UL1RR024996, and the Burke Foundation.
References
- Askalan R, Deveber G, Ho M, Ma J, Hawkins C. Astrocytic-inducible nitric oxide synthase in the ischemic developing human brain. Pediatr Res. 2006;60:687–692. doi: 10.1203/01.pdr.0000246226.89215.a6. [DOI] [PubMed] [Google Scholar]
- Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010a;51:621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010b;120:559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Cho S, Kim E. CD36: a multi-modal target for acute stroke therapy. J Neurochem. 2009;109 (Suppl 1:126–132. doi: 10.1111/j.1471-4159.2009.05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Park EM, Febbraio M, Anrather J, Park L, Racchumi G, Silverstein RL, Iadecola C. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci. 2005;25:2504–2512. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclaux M, Teigell M, Amar L, Vogel R, Gimenez YRM, Privat A, Mallet J. A novel and efficient gene transfer strategy reduces glial reactivity and improves neuronal survival and axonal growth in vitro. PLoS One. 2009;4:e6227. doi: 10.1371/journal.pone.0006227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS. TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation. 2010;7:62. doi: 10.1186/1742-2094-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol. 2007;39:2012–30. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. Activated macrophages and the blood-brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp Neurol. 1997;148:587–603. doi: 10.1006/exnr.1997.6701. [DOI] [PubMed] [Google Scholar]
- Giulian D, Woodward J, Young DG, Krebs JF, Lachman LB. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988;8:2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM, Yong VW, Ransohoff RM. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol. 1996;156:4363–4368. [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002;22:308–317. doi: 10.1097/00004647-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Kim E, Tolhurst AT, Qin LY, Chen XY, Febbraio M, Cho S. CD36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci. 2008;28:4661–4670. doi: 10.1523/JNEUROSCI.0982-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Kim J, Kim Y, Yang M, Jang H, Kang S, Kim JC, Kim SH, Shin T, Moon C. Differential patterns of nestin and glial fibrillary acidic protein expression in mouse hippocampus during postnatal development. J Vet Sci. 2011;12:1–6. doi: 10.4142/jvs.2011.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, Jiang FJ, Stoltzfus OK, Ducceschi MH. IL-6-type cytokines enhance epidermal growth factor-stimulated astrocyte proliferation. Glia. 2000;32:328–337. doi: 10.1002/1098-1136(200012)32:3<328::aid-glia110>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34:177–186. doi: 10.1161/01.str.0000047100.84604.ba. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Rabin SJ, Colangelo AM, Whittemore SR, Wrathall JR. Increased basic fibroblast growth factor expression following contusive spinal cord injury. Exp Neurol. 1996;141:154–164. doi: 10.1006/exnr.1996.0149. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Mikami Y, Shimazaki T, Mihara M, Ohsugi Y, Iwamoto Y, Yoshizaki K, Kishimoto T, Toyama Y, Okano H. Blockade of interleukin-6 receptor suppresses reactive astrogliosis and ameliorates functional recovery in experimental spinal cord injury. J Neurosci Res. 2004;76:265–276. doi: 10.1002/jnr.20044. [DOI] [PubMed] [Google Scholar]
- Pedrono E, Durukan A, Strbian D, Marinkovic I, Shekhar S, Pitkonen M, Abo-Ramadan U, Tatlisumak T. An optimized mouse model for transient ischemic attack. J Neuropathol Exp Neurol. 2010;69:188–195. doi: 10.1097/NEN.0b013e3181cd331c. [DOI] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Giralt M, Carrasco J, Hadberg H, Hidalgo J. Impaired inflammatory response and increased oxidative stress and neurodegeneration after brain injury in interleukin-6-deficient mice. Glia. 2000;32:271–285. doi: 10.1002/1098-1136(200012)32:3<271::aid-glia70>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Preston E, Webster J, Small D. Characteristics of sustained blood-brain barrier opening and tissue injury in a model for focal trauma in the rat. J Neurotrauma. 2001;18:83–92. doi: 10.1089/089771501750055794. [DOI] [PubMed] [Google Scholar]
- Qin L, Kim E, Ratan R, Lee FS, Cho S. Genetic variant of BDNF (Val66Met) polymorphism attenuates stroke-induced angiogenic responses by enhancing anti-angiogenic mediator CD36 expression. J Neurosci. 2011;31:775–783. doi: 10.1523/JNEUROSCI.4547-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Suzumura A, Marunouchi T. TNF alpha induces IL-6 production by astrocytes but not by microglia. Brain Res. 1992;583:296–299. doi: 10.1016/s0006-8993(10)80037-x. [DOI] [PubMed] [Google Scholar]
- Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30:5843–5854. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- Wang JN, Shi N, Xie WB, Guo X, Chen SY. Response gene to complement 32 promotes vascular lesion formation through stimulation of smooth muscle cell proliferation and migration. Arterioscler Thromb Vasc Biol. 2011;31:e19–e26. doi: 10.1161/ATVBAHA.111.230706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Moges H, Bharucha Y, Symes A. Smad3 null mice display more rapid wound closure and reduced scar formation after a stab wound to the cerebral cortex. Exp Neurol. 2007;203:168–184. doi: 10.1016/j.expneurol.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Yehualaeshet T, O'Connor R, Green-Johnson J, Mai S, Silverstein R, Murphy-Ullrich JE, Khalil N. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am J Pathol. 1999;155:841–851. doi: 10.1016/s0002-9440(10)65183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW, Moumdjian R, Yong FP, Ruijs TC, Freedman MS, Cashman N, Antel JP. Gamma-interferon promotes proliferation of adult human astrocytes in vitro and reactive gliosis in the adult mouse brain in vivo. Proc Natl Acad Sci USA. 1991;88:7016–7020. doi: 10.1073/pnas.88.16.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AC, Lee YL, Eng LF. Astrogliosis in culture: I. The model and the effect of antisense oligonucleotides on glial fibrillary acidic protein synthesis. J Neurosci Res. 1993;34:295–303. doi: 10.1002/jnr.490340306. [DOI] [PubMed] [Google Scholar]
- Zhu CZ, Auer RN. Graded hypotension and MCA occlusion duration: effect in transient focal ischemia. J Cereb Blood Flow Metab. 1995;15:980–988. doi: 10.1038/jcbfm.1995.124. [DOI] [PubMed] [Google Scholar]