Abstract
Preclinical trials confirmed the potential of mesenchymal stromal cells (MSCs) to improve functional recovery after experimental stroke. Beneficial effects of MSCs are often attributed to their immunosuppressive/immunomodulatory functions. Surprisingly, the influence of MSCs on the immune system after stroke is poorly understood, but requires special consideration because cerebral ischemia is associated with stroke-induced immunodepression that predisposes to bacterial infections with increased mortality. In this study, we intravenously transplanted syngeneic murine bone marrow-derived MSCs (mMSCs) into C57BL/6 mice at 6 hours after transient middle cerebral artery occlusion (MCAo; 60 minutes) to investigate the impact of MSCs on stroke-induced immunodepression. Transplantation of syngeneic splenocytes or phosphate-buffered saline (PBS) served as controls. An immune status was determined by flow cytometry on days 3 and 14 after MCAo, which did not reveal any negative effects of cell transplantation on stroke-induced immunodepression. Although our mMSCs were found to exert immunosuppressive effects in vitro, stroke-mediated immune cell dysfunction was not altered by mMSCs in ex-vivo stimulation assays with lipopolysaccharide or concanavalin A. Moreover, systemic inflammatory cytokine levels (interleukin-6, tumor necrosis factorα, interferonγ, monocyte chemoattractant protein-1) remained unchanged in the sera of mice after cerebral ischemia and cell transplantation. These results reduce safety concerns about MSC administration in ongoing clinical stroke trials.
Keywords: bone marrow, cerebral ischemia, mesenchymal stem cell, stroke-induced immunodepression
Introduction
Mesenchymal stromal cells (MSCs) are a heterogeneous population of stem and progenitor cells with tissue-protective and regenerative capacities. Beneficial effects of MSCs have been demonstrated in rodent models of stroke, where intravenous administration of bone marrow-derived MSCs resulted in smaller infarct volumes and improved functional recovery (Chen et al, 2001; Kurozumi et al, 2005). The therapeutic capacities of MSCs were mainly attributed to their paracrine neuroprotective effects (Munoz et al, 2005; Scheibe et al, 2012). However, MSCs are also able to modulate adaptive and innate immune responses, which has been exploited therapeutically in animal models of immune disorders, such as multiple sclerosis (Constantin et al, 2009; Zappia et al, 2005) or graft-versus-host disease (GvHD) (Le Blanc et al, 2004). Among their immunosuppressive effects, MSCs were found to inhibit the proliferation of T-lymphocytes, B-lymphocytes, and natural killer-cells, dampen the respiratory burst of neutrophils, and reduce the antigen-presenting functions of dendritic cells (Singer and Caplan, 2011; Uccelli et al, 2008). This may be desired in ongoing clinical trials using MSCs for the treatment of autoimmune disorders like multiple sclerosis (Uccelli et al, 2011). However, focal cerebral ischemia (stroke) is associated with severe immunodepression and increased susceptibility to bacterial infections, which is the leading cause of stroke-related mortality (Dirnagl et al, 2007). Thus, additional immunosuppressive effects of MSCs may be detrimental. In fact, stroke-induced immunodepression is characterized by autonomic dysfunction and a transient depletion of T-, B-, and natural killer-cell populations, as well as suppression of their effector functions (Prass et al, 2003). Since none of the preclinical or clinical MSC stroke trials has addressed the effects of MSC treatment on stroke-induced immunodepression (Bang et al, 2005; Chen et al, 2001; Honmou et al, 2011; Kurozumi et al, 2005; Lee et al, 2010), we studied the impact of intravenous MSC transplantation on the peripheral immune responses to transient middle cerebral artery occlusion (MCAo) in adult mice.
Materials and methods
Animals and Experimental Model of Stroke (Middle Cerebral Artery Occlusion)
All animal experiments were performed in accordance with ARRIVE guidelines, national and institutional guidelines and were approved by the LAGeSo. Male C57BL/6 mice aged 8 to 14 weeks (Charles River, Sulzfeld, Germany) were exposed to a 60-minute filamentous MCAo as described (Katchanov et al, 2001). In sham-operated mice, an identical surgical procedure was performed, but the filament was directly withdrawn to allow immediate reperfusion.
Six hours after MCAo, mice were transplanted by tail vein injection with either 1 × 106 murine MSCs (mMSCs), 1 × 106 splenocytes from C57BL/6 mice, or with the vehicle PBS in a volume of 400 μL. Cell suspensions and PBS were supplemented with 25 IE/mL heparin (Ratiopharm, Ulm, Germany) to prevent pulmonary embolisms in mice after cell transplantation. Neurological sensory-motor deficits were assessed using the Bederson score (0=no observable deficit, 1=forelimb flexion, 2=decreased resistance to lateral push (and forelimb flexion), without circling, 3=same behavior as grade 2, with circling) (Bederson et al, 1986).
Infarct Volumetry
Histological determination of infarct lesion volume was performed at 24 hours or 3 days after MCAo or sham surgery. The brains were cut into 20 μm-thick sections on a cryostat and direct and indirect infarct lesion sizes were determined as previously described (Endres et al, 2003). Direct infarct volumes represent the whole infarcted area of a brain hemisphere including edema. Indirect infarct volumes are corrected for edema. Infarct volumetry of mice that survived for 14 days was performed on day 3 after MCAo using magnetic resonance imaging. The magnetic resonance imaging machine consisted of a 7-T rodent scanner (Pharmascan 70/16, Bruker BioSpin, Rheinstetten, Germany) with a 16-cm horizontal bore magnet and a 9-cm (inner diameter) shielded gradient with H-resonance frequency of 300 MHz and a maximum gradient strength of 300 mT/m. During the imaging, mice were placed on a heated pad to ensure constant body temperature of 37°C. Anesthesia was induced with 3% and maintained with 1.5% to 2.0% isoflurane (Forene, Abbot, Baar, Switzerland) delivered in 0.5 L/min of 100% O2 via a facemask under constant ventilation monitoring (Small Animal Monitoring & Gating System, SA Instruments, NY, NY, USA). A T2-weighted 2D turbo spin-echo sequence was used for imaging of the ischemic area (imaging parameters: repetition time/echo time =4,200/36 milliseconds, rapid acquisition with refocused echoes factor 8.4 averages). Twenty axial slices with a slice thickness of 0.5 mm, a field of view of 2.85 × 2.85 cm2 and a matrix of 256 × 256 were positioned over the brain excluding the olfactory bulb. Data acquisition and image processing were carried out with the Bruker software Paravision 4.0. The ischemic lesion volumes were determined on T2-weighted images using custom written imaging processing tools programmed with MATLAB (The Mathworks, Ismaning, Germany). For each slice, the ischemic area was marked and infarct volume was calculated taking the slice thickness of 0.5 mm into account.
Cell Culture of Murine Mesenchymal Stromal Cells and PKH26 Labeling
Murine MSCs were obtained from the bone marrow of tibias and femurs of C57BL/6 mice aged 8 to 12 weeks (BfR, Berlin, Germany). Murine MSC isolation and culture techniques, their characterization by cell surface epitope expression and differentiation assays into fat, bone, and cartilage were previously described (Scheibe et al, 2011).
For the labeling of mMSCs with PKH26 (Sigma-Aldrich, Schnelldorf, Germany), cells were washed with serum-free alphaMEM (Biochrom, Berlin, Germany), centrifuged and resuspended in a 16 × 10−6 mol/L PKH26 staining solution. After incubation at 25°C for 5 minutes, the staining reaction was blocked by adding 1 mL fetal calf serum (Biochrom) for 1 minute. After three washing steps, the cells were counted and prepared for injection.
Mixed Lymphocyte Reaction and Mitogen-Stimulated Proliferation Assay
Immunosuppressive functions of mMSCs were investigated in vitro by mixed lymphocyte reaction (MLR) and by a mitogen-stimulated proliferation assay. For that purpose, mononuclear splenocytes from C57BL/6 (responder cells) and Balb/c mice (donor cells) were isolated by density gradient centrifugation (Histopaque 1083; Sigma-Aldrich). Donor cells were irradiated with 30 Gy using a cesium source and washed once subsequently. Responder cells were labeled with 2.5 μM 5-,6-carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) (Molecular Probes, Darmstadt, Germany), plated in a 1:1 responder to donor cell ratio in 96-well-plates and were cultured in complete RPMI 1640 supplemented with 10% fetal calf serum, 100 U/mL Penicillin, 100 μg/mL Streptomycin, 2 mM L-Glutamine, 5 mM HEPES, 50 μM β-mercaptoethanole (all Biochrom) at 37°C for up to 5 days. Mixed lymphocyte reactions were cultured either alone or in the presence of mMSCs (at a ratio of 1:10 and 1:50) or with murine fibroblasts as control cells. In all, 5 μg/mL of the mitogen concanavalin A (ConA; Sigma-Aldrich) were added as stimulatory agent. Samples were collected on days 2 and 5, stained with a rat anti-mouse CD4 (GK1.5) antibody (BD Pharmingen, Heidelberg, Germany) for further FACS analysis with gating on the CD4+/CFDA-SE+ lymphocyte population. FACS measurements were performed on a FACSCalibur and analyzed with CellQuest sofware (both BD Bioscience, Heidelberg, Germany).
Blood Samples and Preparation of Cell Suspensions
Mice were killed and blood specimens were collected into heparinized or citrate-filled syringes by puncture of the inferior vena cava. Whole blood samples were diluted 1:1 with PBS and peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation with Histopaque 1083 at 400 g for 25 minutes at room temperature.
Single-cell suspensions from spleen and lymph nodes (mediastinal, axillary, brachial, pancreatic, renal, mesenteric, lumbar, inguinal, and iliac) were prepared by forcing a donor spleen or pooled lymph nodes from one animal diluted in 5 mL PBS through a 100-μm cell strainer and thereafter through a 40-μm cell strainer. Mononuclear splenocytes were obtained after density gradient centrifugation with Histopaque 1083.
For the isolation of lung leukocytes, the right ventricles of mice were punctured and the lung circulation was perfused with PBS to remove blood cells. Thereafter, the lung tissue was digested with DNase I (Sigma-Aldrich) and collagenase D (Roche, Mannheim, Germany) following the protocol ‘Preparation of single-cell suspensions from mouse lung' provided by Miltenyi Biotech (Bergisch Gladbach, Germany). Thereafter, pulmonary leukocytes were purified by MACS (magnetic-activated cell sorting) from single-cell suspensions of the lung after positive selection with mouse CD45-microbeads (Miltenyi Biotech) in accordance to the manufacturer's instructions. CD45-positive pulmonary leukocytes were washed with RPMI 1640 and counted using trypan blue. Magnetic-activated cell sorting separations were controlled by surface staining and FACS analysis revealed purity between 98.1% and 99.4% of CD45-positive selections.
Flow Cytometry
Immune status was obtained from PBMCs, mononuclear splenocytes, and lymph node cells by flow cytometric analysis of different leukocyte subsets. Cell suspensions were stained with the following antibodies: rat anti-mouse CD3 (17A2), rat anti-mouse CD4 (GK1.5), rat anti-mouse CD8a (53-6.7), rat anti-mouse CD11b (M1/70), rat anti-mouse CD19 (6D5), rat anti-mouse CD25 (3C7), rat anti-mouse CD45 (30-F11), rat anti-mouse CD86 (GL-1) and rat anti-mouse I-A/I-E (M5/114.15.2) (all BioLegend, Fell, Germany). Flow cytometric measurements were acquired on a MACSQuant Analyzer (Miltenyi Biotec) and analyzed using the FlowJo software (Tree Star, Ashland, OR, USA).
Since immunodepression after stroke correlates with infarct size, only MCAo animals with a direct infarct volume >35 mm3 were included in the immune status data analysis (excluded animals MCAo 3 days: PBS n=2, splenocyte n=1, mMSC n=0; MCAo 14 days: PBS n=6, splenocyte n=4, mMSC n=5). However, the immune status was also determined in all of the excluded animals.
Cytokine Secretion
Cytokine secretion was assessed in cell culture supernatants of in-vitro MLR and in the sera of animals at 24 hours post-MCAo using the BD Cytometric Bead Array Mouse Inflammation Kit (BD Biosciences) for the analysis of interleukin (IL)-6, IL-10, MCP-1 (monocyte chemoattractant protein-1), interferon (IFN)γ, and tumor necrosis factor (TNF)α. For this procedure, frozen supernatants and sera were thawed and samples were prepared according to the manufacturer's instructions. Measurements were performed by flow cytometry using a FACSCanto II (BD Biosciences) and analyzed using the FCAP Array software (BD Biosciences).
Ex-Vivo Stimulation Assays
Heparinized whole blood samples from animals killed 24 hours after MCAo were diluted 1:5 in RPMI 1640 (Biochrom) and were stimulated with 100 ng/mL lipopolysaccharide (LPS; endotoxin) from Escherichia coli 0127:B8 (Sigma-Aldrich) in endotoxin-free 1.5 mL tubes at 37°C and 5% CO2 for 4 hours. Splenocytes, lymph node cells, and CD45-positive lung cells from the same mice were seeded with 2 × 106 cells/mL and stimulated with 100 ng/mL LPS for 24 hours before obtained supernatants were quantified for TNFα-secretion. For the analysis of IFNγ-production, either 2 × 106/mL splenocytes or 2 × 106/mL CD45+ leukocytes from lung tissue were stimulated with 5 μg/mL ConA for 72 hours. Collected supernatants were analyzed for cytokine secretion by commercially available ELISA kits for TNFα and IFNγ (both from eBioscience, Frankfurt/Main, Germany) following the manufacturer's instructions.
Statistical Analysis
Statistical analysis was performed using the software GraphPad Prism 5.01 (GraphPad Software, La Jolla, CA, USA). Different statistical tests were applied: unpaired t-test for in-vitro proliferation studies, one-way ANOVA (analysis of variance) with Tukey's post-hoc test for infarct volume analysis, one-way ANOVA with Bonferroni's post-test for body weight, Mann–Whitney rank sum test for Bederson score, two-way ANOVA for cytometric bead array and ex-vivo stimulation assays, one-way ANOVA with Bonferroni's post-hoc test or Kruskal–Wallis analysis with Dunn's method as post-hoc test for the analysis of the immune status (these tests were chosen because the aim of the statistical analysis consisted in subgroup comparisons of mMSCs versus splenocyte versus PBS effects; assessment of direct group comparisons (control versus sham versus MCAo) was disregarded because this has already been published by Prass et al, 2003). Data are presented as mean values+s.d. Differences between groups were considered significant at *P<0.05; **P<0.01 and ***P<0.001.
Results
Murine Mesenchymal Stromal Cells Show Immunosuppressive Effects In Vitro
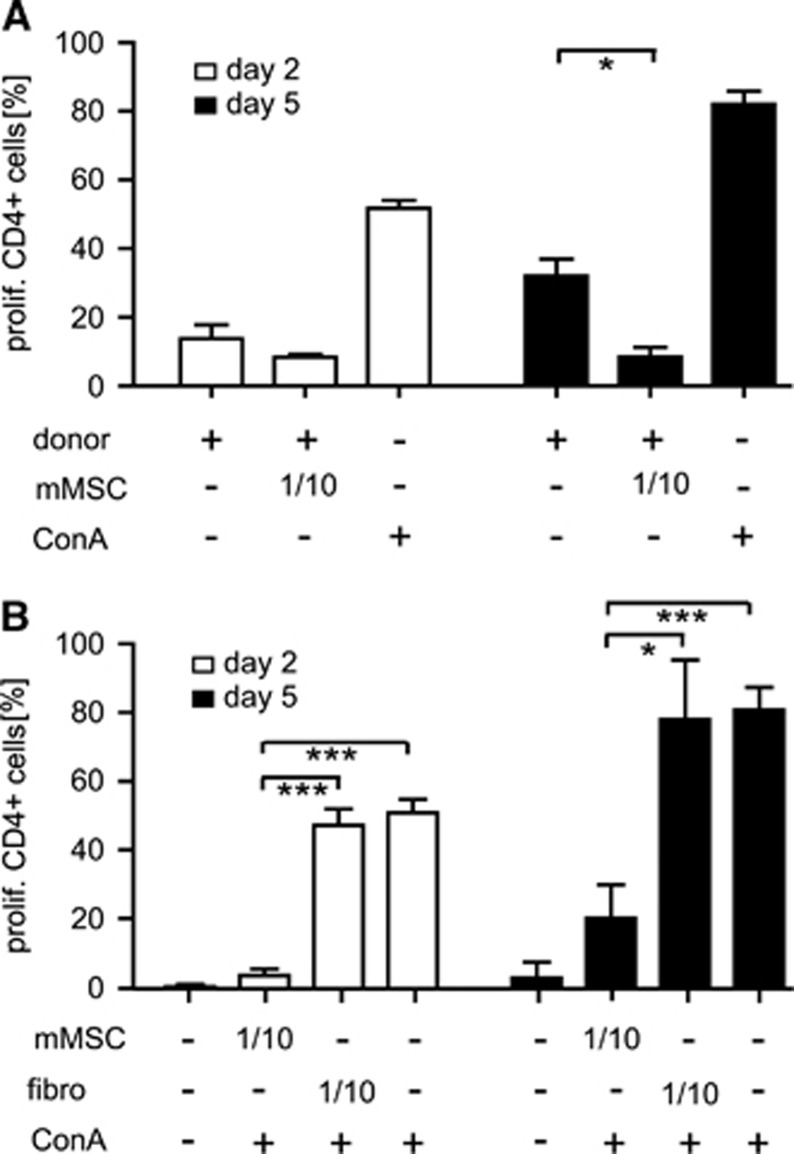
Immunosuppressive functions of mMSCs were assessed in vitro by MLR and mitogen-stimulated proliferation assay of mononuclear splenocytes in the presence of syngeneic mMSCs at a ratio of 1:10. Significantly reduced proliferation rates of CD3+CD4+ T-lymphocytes were demonstrated for cocultures with mMSCs, but not for cocultures with murine fibroblasts, indicating specificity of the immunosuppressive effects of mMSCs in MLR and ConA-stimulated proliferation assays (Figures 1A and 1B). Similar findings were obtained for CD3+CD8+ T-lymphocytes (data not shown). The inhibitory effect of mMSCs on T-lymphocyte proliferation was dose-dependent, since addition of syngeneic mMSCs at a ratio of 1:50 failed to reduce T-lymphocyte proliferation in MLR and mitogen-stimulated proliferation assays (data not shown). The levels of the proinflammatory cytokines TNFα and IFNγ were decreased in supernatants from ConA-stimulated responder lymphocytes cultured in the presence of mMSCs compared with control cultures without mMSCs or cocultures with fibroblasts (data not shown).
Figure 1.
Murine mesenchymal stromal cells (mMSCs) suppress allogeneic T-cell proliferation in vitro. Proliferation of allogeneic CD4+ responder T cells (C57BL/6) was assessed on days 2 and 5 by CFDA-SE-based proliferation assay in mixed lymphocyte cocultures with irradiated donor splenocytes (Balb/C) (A), and by mitogen-induced proliferation studies with ConA (5 μg/mL) (B) in the absence or presence (ratio 1/10) of syngeneic mMSCs, or murine fibroblasts (fibro) as controls. The proportion of proliferated CFDA-SE+CD3+CD4+ T cells was determined by flow cytometry. ConA (5 μg/mL) was used as positive control in mixed lymphocyte reactions. Data are presented as mean values+s.d. (n=3). *P<0.05; ***P<0.001; (unpaired t-test).
Transplantation of Murine Mesenchymal Stromal Cells After Focal Cerebral Ischemia in Mice
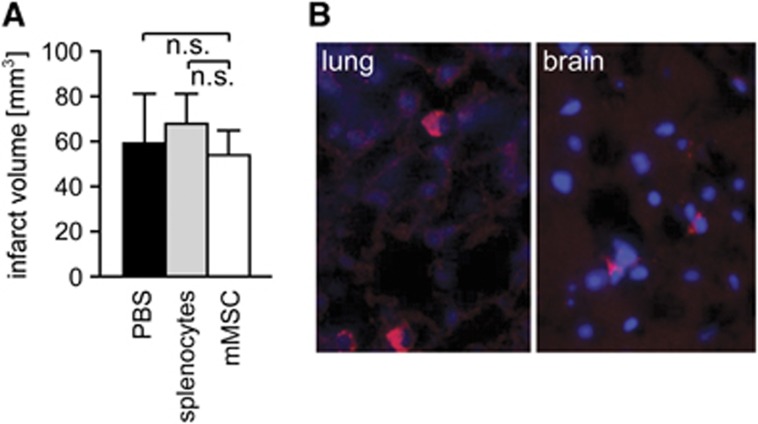
Adult C57BL/6 mice were subjected to MCAo for 60 minutes. At 6 hours after reperfusion, three groups of animals received either 1 × 106 mMSCs, 1 × 106 splenocytes (as cellular control), or PBS (as vehicle control) by tail vein injection. All MCAo animals of mMSC, splenocyte or PBS groups showed no significant differences in indirect infarct volumes at 3 days after MCAo (Figure 2A). The same was true for direct infarct volumes (mMSC group: 114.4±42.1 mm3, splenocyte group: 118.9±32.3 mm3, PBS group: 113.6±22.0 mm3). A preplanned (a priori) sample size calculations for direct infarct volumes is presented in Supplementary Figure S1. We also did not observe differences in infarct volumes between mMSC-, splenocyte-, and PBS-treated animals, which were killed at 24 hours or 14 days post-MCAo (data not shown). This is an important prerequisite for the analysis of MSC effects on stroke-induced immunodepression, which correlates with infarct size. No significant differences in body weight or Bederson scores were observed between mMSC, splenocyte, and PBS groups up to 14 days post-MCAo (Supplementary Figures S2A and S2B). In order to track MSCs and follow their fate in recipient mice after MCAo, we labeled the donor cells with the red fluorescent cell linker compound PKH26. PKH26-marked mMSCs were primarily detected in the lungs at 3 days after MCAo (Figure 2B, left panel). Only very few PKH26-labeled mMSCs were found in the ischemic hemisphere of the brain (Figure 2B, right panel) and in lymphoid tissues (data not shown).
Figure 2.
Murine mesenchymal stromal cells (mMSCs) engraft in organs, but have no impact on infarct volumes after stroke. (A) Histological determination of infarct volumes revealed no differences for indirect infarct volumes between mMSC (n=9), splenocyte (n=10), and phosphate-buffered saline (PBS) (n=10) groups at day 3 after middle cerebral artery occlusion (MCAo). (B) Engraftment of PKH26-labeled mMSCs at 3 days after intravenous administration in MCAo mice. PKH26-labeled mMSCs (red) were observed in the lung (left panel) and very rarely in the ischemic brain hemisphere (right panel). Nuclei were counterstained with DAPI (blue). Data are presented as mean values+s.d. n.s., not significant (one-way analysis of variance (ANOVA) with Tukey's post-hoc test). DAPI, 4′,6-diamidino-2-phenylindole. The color reproduction of this figure is available on the Journal of Cerebral Blood Flow and Metabolism journal online.
Murine Mesenchymal Stromal Cells Do Not Affect Stroke-Induced Immunodepression in Mice
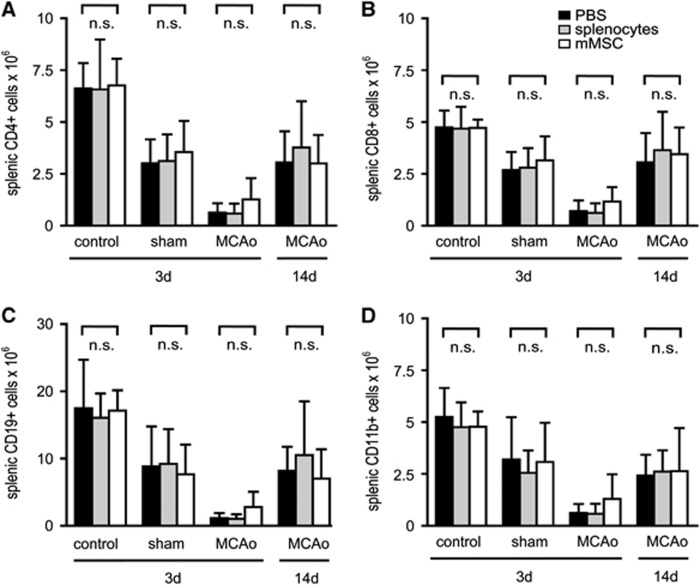
An immune status was obtained from PBMCs, lymph nodes, and spleens of C57BL/6 mice after MCAo and intravenous injection of mMSCs, splenocytes, or PBS. Sham-operated mice and naive mice served as controls. Absolute cell counts of different leukocyte subpopulations were quantified by flow cytometry. At 3 days after MCAo, a striking twofold to threefold reduction of T-cells (CD4+ and CD8+), B-cells (CD19+), and monocytes (CD11b+) was observed in peripheral blood (Figures 3A–3D). Moreover, we found a dramatic 5- to 15-fold loss of T- and B-cells, but also of CD11b+ macrophages in lymph nodes (Figures 4A–4D) and in spleens (Figures 5A–5D) after MCAo. Mild lymphopenia and reduction of monocytes/macrophages were also detected in sham-operated animals, but to a much lesser extent, suggesting that the observed findings were more related to ischemia rather than to surgical stress. Most importantly, mMSC administration did not induce any changes of complete cell counts and also not of defined leukocyte subsets in MCAo, sham, or PBS control groups in peripheral blood (Figure 3), lymph nodes (Figure 4), and spleens (Figure 5). In order to analyze whether mMSC effects on the immune system might occur later in the course of stroke-induced immunodepression, we also analyzed the immune status in blood, lymph nodes, and spleens at 14 days after stroke. The absolute cell counts of different leukocyte populations remained decreased in blood (Figures 3A–3D), but started to increase in lymph nodes (Figures 4A–4D) and have nearly recovered in spleens (Figures 5A–5D). Again, no effect of mMSCs on immune cell recovery after stroke was observed. Administration of splenocytes also remained without effect on poststroke immunodepression in mice (Figures 3, 4 and 5).
Figure 3.
Murine mesenchymal stromal cell (mMSC) administration has no impact on stroke-induced immunodepression in peripheral blood mononuclear cells (PBMCs). Absolute cell counts of PBMC leukocyte subsets in control animals, sham-operated animals, and mice receiving 60 minutes middle cerebral artery occlusion (MCAo) were determined by flow cytometry. In sham-operated animals, reperfusion was allowed for 3 days. Mice with MCAo were analyzed after 3 days (MCAo 3d) or 14 days (MCAo 14d). All mice were injected intravenously with either phosphate-buffered saline (PBS) (black bars), splenocytes (gray bars), or mMSCs (white bars) after 6 hours. Absolute cell numbers of CD4+ T-lymphocytes (A), CD8+ T-lymphocytes (B), CD19+ B-lymphocytes (C), and CD11b+ monocytes/macrophages (D) in PBMCs were analyzed. Data are presented as mean values+s.d. (control n=3, sham n=5 to 7, MCAo 3 days n=5 to 7, MCAo 14 days n=6 to 8). n.s., not significant (subgroup analysis by one-way analysis of variance (ANOVA) with Bonferroni's post-test and Kruskal–Wallis test with Dunns post-test, respectively).
Figure 4.
Murine mesenchymal stromal cell (mMSC) administration does not affect poststroke immunodepression in lymph nodes. Absolute cell counts of leukocyte subsets in lymph nodes (LN) of control animals, sham-operated animals, and mice receiving 60 minutes middle cerebral artery occlusion (MCAo) were analyzed by flow cytometry. After ischemia, reperfusion was allowed for 3 days (sham and MCAo 3 days) or 14 days (MCAo 14 days). All mice were injected intravenously with either phosphate-buffered saline (PBS) (black bars), splenocytes (gray bars), or mMSCs (white bars) after 6 hours. Absolute cell numbers of CD4+ T-lymphocytes (A), CD8+ T-lymphocytes (B), CD19+ B-lymphocytes (C), and CD11b+ monocytes/macrophages (D) in lymph nodes were determined. Data are presented as mean values+s.d. (control n=3 to 5, sham n=5 to 7, MCAo 3 days n=5 to 7, MCAo 14 days n=6 to 8). n.s., not significant (subgroup analysis by one-way analysis of variance (ANOVA) with Bonferroni's post-test and Kruskal–Wallis test with Dunns post-test, respectively).
Figure 5.
Murine mesenchymal stromal cell (mMSC) administration has no effect on stroke-induced immunodepression in mononuclear splenocytes. Absolute cell counts of mononuclear leukocyte subsets in spleens of control animals, sham-operated animals, and mice receiving 60 minutes middle cerebral artery occlusion (MCAo) were determined by flow cytometry. In sham-operated animals, reperfusion was allowed for 3 days. Mice with MCAo were analyzed after 3 days (MCAo 3 days) or 14 days (MCAo 14 days). All mice were injected intravenously with either phosphate-buffered saline (PBS) (black bars), splenocytes (gray bars), or mMSCs (white bars) after 6 hours. Absolute cell numbers of CD4+ T-lymphocytes (A), CD8+ T-lymphocytes (B), CD19+ B-lymphocytes (C), and CD11b+ monocytes/macrophages (D) in mononuclear splenocytes were assessed. Data are presented as mean values+s.d. (control n=3 to 5, sham n=5 to 7, MCAo 3 days n=5 to 7, MCAo 14 days n=6 to 8). n.s., not significant (subgroup analysis by one-way analysis of variance (ANOVA) with Bonferroni's post-test and Kruskal–Wallis test with Dunns post-test, respectively).
In the MCAo experiments, we excluded mice with a direct infarct lesion size <35 mm3 to maintain comparability (see Materials and methods section). The excluded animals were also fully evaluated. The results are shown in Supplementary Figures S3A–S3C for the 14 days after MCAo groups. Interestingly, animals with smaller infarct volumes (<35 mm3) showed less CD8+ lymph node cells than animals with larger infarct volumes (>35 mm3) in all three experimental groups (PBS, splenocytes, MSCs).
Moreover, we quantified the following absolute cell counts of immune cell populations from PBMCs, lymph nodes, and spleens: whole leukocyte count (CD45), activated T-cell count (CD45/CD4/CD25), major histocompatibility complex class II expression on leukocytes, CD11b+ monocytes/macrophages and B-cells, and also CD86 expression on monocyte/macrophages and B-cells. Data were obtained from control, sham, and MCAo mice at 3 and 14 days after cerebral ischemia. Absolute cell counts confirmed the significant reduction of leukocyte numbers as a result of stroke-induced immunodepression in peripheral blood, lymph nodes, and spleens. No difference between the mMSC, splenocyte, and PBS groups were observed at any time point (Supplementary Figures S4–S6).
Murine Mesenchymal Stromal Cells Do Not Restore Immune Cell Function After Stroke
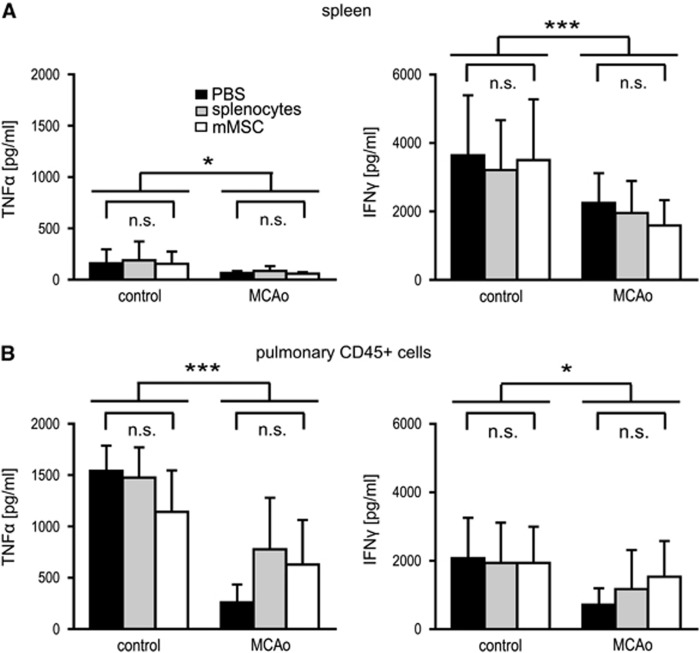
Middle cerebral artery occlusion is not only associated with dramatic apoptotic cell death of all immune cell fractions, but also with severe impairment of immune cell functions. This phenomenon is worst at 1 to 2 days after stroke and usually normalizes a couple of days later (Prass et al, 2003). Since mMSCs did not ameliorate ischemia-related cell death of immune cells, we speculated that mMSCs might have at least an impact on cellular immune functions after stroke. To test this hypothesis, blood samples, lymph node cells, splenocytes, and also CD45-positive leukocytes from lung tissue were ex vivo stimulated with LPS (endotoxin) to assess monocyte function. Endotoxin-induced TNFα-secretion of splenocytes, pulmonary leukocytes (Figures 6A and 6B), and blood (Supplementary Figure S7A) were significantly reduced in the MCAo group compared with control. Lymph node cells showed slight, but nonsignificant reduction of TNFα-production (Supplementary Figure S7B). Ex-vivo stimulation of splenocytes and CD45-positive pulmonary leukocytes with ConA for 72 hours resulted in a significant decline of IFNγ-secretion in MCAo animals compared with controls (Figures 6A and 6B). Interestingly, we found a trend toward better restoration of immune cell function of CD45-positive pulmonary leukocytes after MCAo as determined by TNFα- and IFNγ-production after LPS and ConA stimulation when mMSCs or splenocytes were transplanted compared with PBS (Figure 6B).
Figure 6.
Cytokine release following ex-vivo stimulation of immune cells after middle cerebral artery occlusion (MCAo) is not altered by murine mesenchymal stromal cell (mMSC) injection. Splenocytes (A) and pulmonary CD45+ leukocytes (B) were collected from control mice and from mice with MCAo after 24 hours of reperfusion. All animals received an intravenous injection of either phosphate-buffered saline (PBS) (black bars), splenocytes (gray bars), or mMSCs (white bars) after 6 hours. Cytokine release was analyzed by ELISA in cell culture supernatants after ex-vivo stimulation with either 100 ng/mL lipopolysaccharide for 24 hours (tumor necrosis factor (TNF)α-production) or 5 μg/mL ConA for 72 hours (interferon (IFN)γ-production). Data are presented as mean values+s.d. (control n=5 to 7, MCAo n=7 to 11). *P<0.05; ***P<0.001 (two-way analysis of variance (ANOVA) with Bonferroni's post-test); n.s., not significant (subgroup analysis by one-way ANOVA with Bonferroni's post-test).
Murine Mesenchymal Stromal Cells Do Not Alter the Levels of Proinflammatory Cytokines/Chemokines in the Sera of Mice After Middle Cerebral Artery Occlusion
Cytometric bead arrays were performed with the sera of control and MCAo mice at 24 hours and 3 days after stroke to assess potential changes of proinflammatory cytokine/chemokine profiles (IL-6, IL-10, TNFα, IFNγ, and MCP-1). Interleukin-6 and MCP-1 were significantly increased after MCAo, whereas IFNγ and TNFα were not (Figure 7). In fact, IFNγ was barely detectable (Figure 7A) and IL-10 was undetectable (data not shown). At 3 days after MCAo, the levels of the respective cytokines/chemokines were already greatly reduced compared with day 1 (data not shown). Neither mMSC nor splenocyte administration had any impact on the systemic release of proinflammatory cytokines/chemokines.
Figure 7.
Murine mesenchymal stromal cell (mMSC) administration does not change the secretion of proinflammatory cytokines/chemokines after middle cerebral artery occlusion (MCAo). Cytokine expression levels of interferon (IFN)γ (A), tumor necrosis factor (TNF)α (B), IL-6 (C), and monocyte chemoattractant protein (MCP)-1 (D) were determined by cytometric bead array and compared between the sera of control mice and the sera of mice at 24 hours post-MCAo. All animals were injected intravenously with either phosphate-buffered saline (PBS) (•), splenocytes (Δ), or mMSCs (□) after 6 hours. Data are presented as mean values (n=7 to 9). **P<0.01; ***P<0.001 (two-way analysis of variance (ANOVA) with Bonferroni's post-test); n.s., not significant (subgroup analysis by one-way ANOVA with Bonferroni's post-test).
Discussion
In spite of their known immunosuppressive functions, we did not observe any effects of mMSC administration in experimental stroke. Several immunological parameters, namely the immune status and the extent of immune cell death in blood and peripheral lymphoid tissues, the immune cell effector functions, and also the levels of systemic proinflammatory cytokines/chemokines remained unaltered after intravenous mMSC administration post-MCAo.
Moreover, there were no significant differences between the infarct volumes of mice that received mMSCs, splenocytes, or PBS after MCAo. Since infarct lesion sizes are highly correlated with the severity of stroke-induced immunodepression (Liesz et al, 2009), similar infarct sizes between the respective treatment groups are a prerequisite for adequate measurement of immunoregulatory mMSC functions. Our data are in line with the previous reports where the systemic transplantation of mMSCs after cerebral ischemia in mice only showed neuroprotective capacity when the cells were genetically modified and targeted to the brain by a DETA-NONOate upregulation of SDF-1/CXCR4 (Cui et al, 2007) or the cells were directly injected into brain parenchyma (Ohtaki et al, 2008). In contrast, studies using the rat MCAo model reported reduced infarct volumes after administration of 1 to 10 × 106 naïve MSCs (Nomura et al, 2005; Onda et al, 2008). The discrepancies may be related to different experimental protocols, such as application route or cell dosage. Moreover, neuroanatomical or physiological species differences (e.g., a higher intrinsic regenerative capacity of mice after MCAo) may account for the discrepancies and conceal potential tissue-protective MSC effects.
In general, the regenerative capacities of MSCs are mainly attributed to their paracrine and immunological functions (English et al, 2010). Data from autoimmune-mediated disorders like experimental autoimmune encephalomyelitis suggest that MSC administration before disease onset results in reduced disease severity with decreased inflammatory infiltrates, less demyelination, and less axonal loss in affected mice (Zappia et al, 2005). Moreover, MSCs appear to enhance regeneration by the release of prooligodendrogenic and neuroprotective factors, as well as by the induction of peripheral T-cell tolerance to the immunizing antigen (Uccelli et al, 2011) or a shift of Th2-type cytokines in T-cells (Constantin et al, 2009). Interestingly, even in a model of multiple system atrophy, intravenous MSC administration improved the survival of tyrosine hydroxylase-expressing neurons by modulation of T-cell responses and downregulation of cytokine levels, for example, IL-2, IL-17, and TNFα, in the brain stem (Stemberger et al, 2011).
Similar immunosuppressive capacities of MSCs have been shown in other disease models, such as GvHD (Le Blanc et al, 2004), collagen-induced arthritis (Schurgers et al, 2010), or after organ transplantation (Crop et al, 2009). The absence of immunomodulatory effects of MSCs in stroke-induced immunodepression may result from several factors:
First, MSCs require activation by certain cytokines, for example, IFNγ, TNFα, IL-1β, and IL-1α, in order to develop immunosuppressive functions (Ren et al, 2008; Salem and Thiemermann, 2010). This has been demonstrated in an in-vivo model of GvHD, where MSCs were only immunosuppressive after IFNγ-induced synthesis of nitric oxide (Ren et al, 2008). Inflammatory cytokines like IFNγ, TNFα, and IL-1 induce the expression of adhesion molecules such as intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 on the surface of MSCs, which appears to be mandatory for the induction of cell contact-dependent immunosuppressive functions (Ren et al, 2010). However, our cytometric bead array data suggest that systemic TNFα- and IFNγ-levels are extremely low after MCAo, which may prevent immunosuppressive activities of the transplanted mMSCs. In addition to the persistently low levels of TNFα and IFNγ, the stroke-related reduced capacity of ex-vivo cultured blood, spleen, lymph node, and CD45-positive lung cells to produce IFNγ and TNFα after ConA or LPS stimulation underscores the lack of sufficient activation of MSCs in the MCAo model of stroke. The complexity of stroke-induced immunodepression with systemic and local immune cell loss and dysfunction may keep MSCs in an immunologically quiescent state. At least in part, lung tissue might be an exception because LPS and ConA stimulation of pulmonary leukocytes resulted in a trend towards enhanced TNFα- and IFNγ-secretion in the MSC and splenocyte groups. This may be due to the fact that the vast majority of donor cells entrap in the lungs as a first pass effect and may engage in cell contacts with resident immune cells, whereas significantly less cells reach the lymph nodes or spleen (Lee et al, 2009).
Second, recent reports suggest that immune functions of MSCs are highly dependent on the disease model in which MSCs are applied (Ren et al, 2008). Moreover, the effects of MSCs may also vary within a specific disease model. In collagen-induced arthritis, MSCs were found to reduce disease severity by a switch from Th1/Th17 to Th2 immune responses and by IL-6-dependent secretion of PGE2 (Bouffi et al, 2010). However, other studies failed to reproduce these encouraging in vivo results (Schurgers et al, 2010). Moreover, Flk-1+ MSCs even aggravated the course of collagen-induced arthritis in vivo (Chen et al, 2010). Controversial data also exist for GvHD (Le Blanc et al, 2008). The reasons for the contradictory findings might be related to different sources of the cells (different tissue origin, different in-vitro culture conditions, different species), the time window of cell administration or the dose of applied cells. Thus, we cannot exclude that MSCs from different species or tissues, or administration of MSCs at higher doses or later time points after MCAo may result in immunomodulatory effects. However, we found that intravenous transplantation of higher concentrations of MSCs resulted in pulmonary embolisms, which required pretreatment of the mice with heparin, a potential new confounder. Moreover, transplantation of 1 × 106 mMSCs or less was sufficient to induce beneficial immune changes in immune-mediated disorders, such as multiple sclerosis (Gerdoni et al, 2007) or GvHD (Polchert et al, 2008).
Nonetheless, the encouraging preclinical data of MSC transplantation in rat stroke models has already led to the initiation of multiple clinical stroke trials with human MSCs (http://www.clinicaltrials.gov). In this context, our results provide urgently needed experimental evidence that MSC administration after cerebral ischemia may not further deteriorate the natural course of stroke-induced immunodepression, nor raise immunological concerns on safety aspects of MSC transplantation after stroke.
Acknowledgments
The authors thank Peggy Mex, Monika Dopatka, and Susanne Müller for their excellent technical assistance and the BCRT FACS-core facility for providing instruments and technical assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www. nature.com/jcbfm)
This study was supported by a grant of the German Ministry for Education and Research (BMBF) and the Berlin-Brandenburg Center for Regenerative Therapies.
Supplementary Material
References
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Bouffi C, Bony C, Courties G, Jorgensen C, Noel D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 2010;5:e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Hu J, Liao L, Sun Z, Han Q, Song Z, Zhao RC. Flk-1+ mesenchymal stem cells aggravate collagen-induced arthritis by up-regulating interleukin-6. Clin Exp Immunol. 2010;159:292–302. doi: 10.1111/j.1365-2249.2009.04069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, Galie M, Turano E, Budui S, Sbarbati A, Krampera M, Bonetti B. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27:2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Alwayn IP, Weimar W, Hoogduijn MJ. Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation. 2009;87:896–906. doi: 10.1097/TP.0b013e31819b3d72. [DOI] [PubMed] [Google Scholar]
- Cui X, Chen J, Zacharek A, Li Y, Roberts C, Kapke A, Savant-Bhonsale S, Chopp M. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007;25:2777–2785. doi: 10.1634/stemcells.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, Prass K, Meisel A. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- Endres M, Gertz K, Lindauer U, Katchanov J, Schultze J, Schrock H, Nickenig G, Kuschinsky W, Dirnagl U, Laufs U. Mechanisms of stroke protection by physical activity. Ann Neurol. 2003;54:582–590. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation. Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, Uccelli A. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61:219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katchanov J, Harms C, Gertz K, Hauck L, Waeber C, Hirt L, Priller J, von Harsdorf R, Bruck W, Hortnagl H, Dirnagl U, Bhide PG, Endres M. Mild cerebral ischemia induces loss of cyclin-dependent kinase inhibitors and activation of cell cycle machinery before delayed neuronal cell death. J Neurosci. 2001;21:5045–5053. doi: 10.1523/JNEUROSCI.21-14-05045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Hagmann S, Zschoche C, Adamek J, Zhou W, Sun L, Hug A, Zorn M, Dalpke A, Nawroth P, Veltkamp R. The spectrum of systemic immune alterations after murine focal ischemia: immunodepression versus immunomodulation. Stroke. 2009;40:2849–2858. doi: 10.1161/STROKEAHA.109.549618. [DOI] [PubMed] [Google Scholar]
- Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci USA. 2005;102:18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136:161–169. doi: 10.1016/j.neuroscience.2005.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:329–340. doi: 10.1038/sj.jcbfm.9600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe F, Gladow N, Mergenthaler P, Tucker AH, Meisel A, Prockop DJ, Priller J.2011Nonviral gene delivery of erythropoietin by mesenchymal stromal cells Gene Theradvance online publication, 29 September 2011; doi: 10.1038/gt.2011.139(e-pub ahead of print) [DOI] [PubMed]
- Scheibe F, Klein O, Klose J, Priller J.2012Mesenchymal stromal cells rescue cortical neurons from apoptotic cell death in an in vitro model of cerebral ischemia Cell Mol Neurobioladvance online publication, 1 February 2012; doi: 10.1007/s10571-012-9798-2(e-pub ahead of print) [DOI] [PMC free article] [PubMed]
- Schurgers E, Kelchtermans H, Mitera T, Geboes L, Matthys P. Discrepancy between the in vitro and in vivo effects of murine mesenchymal stem cells on T-cell proliferation and collagen-induced arthritis. Arthritis Res Ther. 2010;12:R31. doi: 10.1186/ar2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- Stemberger S, Jamnig A, Stefanova N, Lepperdinger G, Reindl M, Wenning GK. Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection. PLoS One. 2011;6:e19808. doi: 10.1371/journal.pone.0019808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10:649–656. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.