Abstract
Parkinson's disease (PD) is a multisystem neurodegenerative disorder. Heterogeneous clinical features may reflect heterogeneous changes in different brain regions. In contrast to the pronounced nigrostriatal denervation characteristic of PD, cholinergic changes are less marked. We investigated cholinergic innervation activity in PD subjects relative to normal subjects. Nondemented PD subjects (n=101, age 65.3±7.2 years) and normal subjects (n=29, age 66.8±10.9 years) underwent clinical assessment and [11C]methyl-4-piperidinyl propionate acetylcholinesterase and [11C]dihydrotetrabenazine monoaminergic positron emission tomography (PET) imaging. Cholinergic projection changes were heterogeneous for 65 out of 101 PD subjects who had neocortical and thalamic acetylcholinesterase activity within the normal range. The remainder had combined neocortical and thalamic (13/101), isolated neocortical (18/101), or isolated thalamic (5/101) acetylcholinesterase activity below the normal range. The low neocortical acetylcholinesterase activity subgroup had significantly lower global cognitive performance compared with the normal range subgroup (F=7.64, P=0.0069) with an independent effect for nigrostriatal denervation (F=7.60, P=0.0074). The low thalamic acetylcholinesterase activity subgroup did not differ from the normal thalamic acetylcholinesterase activity subgroup in cognitive performance or motor impairments except for a history of falls (P=0.0023). Cholinergic denervation is heterogeneous with reduced neocortical and/or thalamic acetylcholinesterase activity in 36% of nondemented PD subjects with corresponding clinical phenotypic variation. Results also show independent cognitive effects for both cholinergic and dopaminergic system changes in nondemented PD subjects.
Keywords: acetylcholinesterase, cognitive impairment, dopamine, motor, Parkinson's disease, PET
Introduction
Parkinson's disease (PD) is viewed traditionally as a motor syndrome arising from nigrostriatal dopaminergic denervation. Abundant data, however, indicate that PD is a multisystem neurodegenerative disorder. PD is clinically heterogeneous with differences in the severity of clinical features among individual PD patients. In the early stages, PD exhibits severe nigrostriatal degeneration of relatively uniform magnitude and distribution. Variation in clinical features may result from deficiency of other neurotransmitter systems and pathologies (Cumming and Borghammer, 2012). In addition to the well-known reductions in dopaminergic pathways, there is also evidence for alterations in cholinergic pathways in PD (Arendt et al, 1983). Previous studies found cognitive correlates of cortical cholinergic denervation (Bohnen et al, 2006b; Shimada et al, 2009), whereas subcortical (pontine tegmentum projection system) denervation is associated with a history of falls and gait problems in PD (Bohnen et al, 2009; Gilman et al, 2010).
In contrast to severe striatal dopaminergic terminal losses, exceeding 50% to 80% in PD subjects with clinically manifest motor symptoms (Frey et al, 1996; Guttman et al, 1997), in vivo acetylcholinesterase studies show cholinergic projection losses in the magnitude of 5% to 25% in PD subjects without or with dementia (Bohnen et al, 2003; Hilker et al, 2005; Shimada et al, 2009; Shinotoh et al, 1999). The more limited and variable cholinergic losses suggest the presence of overlap in cholinergic projection system integrity between PD and control groups. Effectiveness of cholinergic augmentation therapy as a strategy to manage cognitive or mobility problems in PD is expected to be greater in the subset of patients with significant cholinergic denervation compared with patients with relative integrity of cholinergic projections.
We set out to examine the heterogeneity of cholinergic denervation in patients with PD relative to a normal comparison group using acetylcholinesterase positron emission tomography (PET). There are two major brain cholinergic projection systems. The first arises in the basal forebrain complex, providing the principal cholinergic input of the cortical mantle and is known to degenerate in PD. The second arises in the pedunculopontine nucleus, a brainstem locomotor center, and provides cholinergic inputs to the thalamus, cerebellum, basal ganglia, other brainstem nuclei, and the spinal cord (Heckers et al, 1992a). Acetylcholinesterase PET imaging assesses cholinergic terminal integrity with cortical uptake reflecting largely basal forebrain and thalamic uptake principally reflecting pedunculopontine nucleus integrity. We explored differences in clinical features in the subsets of PD patients with below normal versus normal range cholinergic projection integrity. Significant clinical phenotypic variations between the cholinergic subgroups were further assessed while controlling for the degree of nigrostriatal denervation.
Subjects and methods
Subjects and Clinical Test Battery
This cross-sectional study involved 101 subjects with PD (76 men and 25 women), mean age 65.3.±7.2 (range 50 to 84) years, and 29 control non-PD and cognitively normal subjects (16 men and 13 women), mean age 66.8±10.9 (range 50 to 84) years. PD subjects met the UK Parkinson's Disease Society Brain Bank clinical diagnostic criteria (Hughes et al, 1992). The diagnosis of PD was confirmed by the presence of nigrostriatal dopaminergic denervation as revealed by (+)-[11C]dihydrotetrabenazine vesicular monoamine type 2 (VMAT2) PET. In all, 34 PD subjects were taking a combination of dopamine agonist and carbidopa-levodopa medications, 50 were using carbidopa-levodopa alone, 9 were taking dopamine agonists alone, and 8 were not receiving dopaminergic drugs. No subjects were being treated with anticholinergic or cholinesterase inhibitor drugs. Most subjects had moderate severity of disease: 4 patients in stage 1, 6 in stage 1.5, 24 in stage 2, 49 in stage 2.5, 16 in stage 3, and 2 in stage 4 of the modified Hoehn and Yahr classification (Hoehn and Yahr, 1967). Mean duration of disease was 5.9±3.9 (range 0.5 to 19) years.
The Movement Disorder Society-revised Unified Parkinson's Disease Rating Scale (UPDRS) was performed in the practically defined ‘off' state (Goetz et al, 2007). A history of falls was also documented. Subjects receiving dopaminergic medications were examined and imaged first with the (+)-[11C]dihydrotetrabenazine VMAT2 ligand in the morning after dopaminergic medications had been withheld overnight. All patients completed the VMAT2 and acetylcholinesterase PET scan on the same day except for four patients because of scheduling or radiosynthesis reasons. Patients on dopaminergic drugs were allowed to resume their medications during a rest break between the VMAT2 and acetylcholinesterase PET scan. Magnetic resonance imaging (MRI) scans for most subjects were also performed on the same day after a rest break in the afternoon except in 13 patients because of scheduling reasons. For these subjects, MR imaging was performed within several days to few weeks.
Each patient underwent a neuropsychological examination as follows: neuropsychological tests evaluating multiple cognitive domains were used for analysis following the approach previously reported for cognitive impairment in PD (Aarsland et al, 2009). These tests included measures of verbal and nonverbal memory: California Verbal Learning Test (Delis et al, 2000) and Benton Visual Retention Test (Benton, 1974); executive/reasoning functions: WAIS III Picture Arrangement test (Wechsler, 1997), and Stroop Color Word Interference test together with a switching version of the Stroop 3 test in which subjects name the ink, unless the word is surrounded by a box, in which case they read the word itself (Stroop 4). Performance of this task makes an additional demand on cognitive flexibility (Bohnen et al, 1992). Stroop Color Word Interference Test scores were calculated as the time difference for completion of the interference measures minus the noninterference tasks; attention/psychomotor speed as absolute times on the Stroop 1 and 2 subtests; and visuospatial function: Benton Judgment of Line Orientation test (Benton et al, 1975). Composite z-scores were calculated for these different cognitive domains (memory, executive, attention, and visuospatial functions) based on normative data. A global composite z-score was calculated as the average of the four domain z-scores. Patients with evidence of dementia as defined by a global composite z-score of less than −2 and significant impairments of instrumental activities of daily living were not eligible for the study.
The study was approved by and study procedures were followed in accordance with the ethical standards of the Institutional Review Boards of the University of Michigan and Veterans Affairs Ann Arbor Health System for studies involving human subjects. Written informed consent was obtained from all subjects.
Imaging Techniques
All subjects underwent brain MRI and [11C]methyl-4-piperidinyl propionate (acetylcholinesterase) and (+)-[11C]dihydrotetrabenazine VMAT2 PET. MRI was performed on a 3 Tesla Philips Achieva system (Philips, Best, The Netherlands) using an 8-channel head coil and the ‘ISOVOX' exam card protocol primarily designed to yield isotropic spatial resolution. A standard T1-weighted series of a three-dimensional inversion recovery-prepared turbo-field-echo was performed in the sagittal plane using TR/TE/TI=9.8/4.6/1,041 ms; turbo factor=200; single average; field of view=240 × 200 × 160 mm; acquired matrix=240 × 200. A total of 160 slices were reconstructed to 1 mm isotropic resolution. This sequence maximizes contrast among gray matter, white matter, and cerebrospinal fluid and provides high-resolution delineation of cortical and subcortical structures.
The [11C]methyl-4-piperidinyl propionate and (+)-[11C]dihydrotetrabenazine PET imaging were performed in three-dimensional imaging mode using an ECAT HR+ tomograph (Siemens Molecular Imaging, Knoxville, TN), which acquires 63 transaxial slices (slice thickness: 2.4 mm; intrinsic in-plane resolution: 4.1 mm full-width at half maximum over a 15.2 cm axial field of view. A NeuroShield (Scanwell Systems, Montreal, Canada) head-holder/shielding unit was attached to the patient bed to reduce the contribution of detected photon events originating from the body outside the scanner field of view (Thompson et al, 2001). Before [11C]dihydrotetrabenazine and [11C]methyl-4-piperidinyl propionate injections, a 5-minute transmission scan was acquired using rotating 68Ge rods for attenuation correction of emission data using the standard vendor-supplied segmentation and reprojection routines.
The [11C]methyl-4-piperidinyl propionate was prepared in high radiochemical purity (>95%) by N-[11C]methylation of piperidin-4-yl propionate using a previously described method (Snyder et al, 1998). Dynamic PET scanning was performed for 70 minutes as previously reported (Bohnen et al, 2010).
No-carrier-added (+)-[11C]dihydrotetrabenazine (250 to 1,000 Ci/mmol at the time of injection) was prepared as reported previously (Jewett et al, 1997). Dynamic PET scanning was performed for 60 minutes as previously reported (Bohnen et al, 2006a).
Analysis
All image frames were spatially coregistered within subjects with a rigid-body transformation to reduce the effects of subject motion during the imaging session (Minoshima et al, 1993). Interactive Data Language image analysis software (Research Systems, Boulder, CO) was used to manually trace volumes of interest on MRI images to include the thalamus, caudate nucleus, and putamen of each hemisphere. Total neocortical volume of interest were defined using semiautomated threshold delineation of the cortical gray matter signal on the MRI scan.
Acetylcholinesterase [11C]methyl-4-piperidinyl propionate hydrolysis rates (k3) were estimated using the striatal volume of interest (defined by manual tracing on the MRI scan of the putamen and caudate nucleus) as the tissue reference for the integral of the precursor delivery (Nagatsuka et al, 2001). The lower limit of normal is commonly determined using the lower 5th percentile or 95% confidence interval obtained from healthy subjects (White et al, 1988). Therefore, the 5th percentile cutoff of acetylcholinesterase activity in the cognitively normal non-PD elderly was used to define activity levels falling below the normal range.
The (+)-[11C]dihydrotetrabenazine distribution volume ratios were estimated using the Logan plot graphical analysis method with the striatal time activity curves as the input function and the total neocortex as reference tissue, a reference region overall low in VMAT2 binding sites, with the assumption that the nondisplaceable distribution is uniform across the brain at equilibrium (Koeppe et al, 1999).
Standard pooled-variance t or Satterthwaite's method of approximate t-tests (tapprox) were used for group comparisons (SAS version 9.2, SAS Institute, Cary, NC). Analysis of covariance for cognitive variables used rank transformation because of nonnormal distribution of z-scores between cholinergic subgroups. The χ2 testing was performed to compare proportions between groups. Logistic regression analysis was performed to evaluate categorical dependent variables (history of falls) in relationship to thalamic cholinergic subgroups and striatal VMAT2 activity. Bonferroni correction for multiple testing was performed for each main analysis.
Results
Acetylcholinesterase PET Imaging
Analysis of the [11C]methyl-4-piperidinyl propionate acetylcholinesterase PET data showed reduced mean neocortical (−10.3%) and thalamic acetylcholinesterase (−9.5%) activity in the PD group compared with the control subjects (Table 1).
Table 1. Mean (±s.d.) neocortical and thalamic [11C]methyl-4-piperidinyl propionate acetylcholinesterase hydrolysis rates (k3; per minute) and striatal (+)-[11C]dihydrotetrabenazine VMAT2 distribution volume ratio in Parkinson's disease and control subjects.
| Parkinson's disease subjects (n=101) | Normal subjects (n=29) | Statistical significance | |
|---|---|---|---|
| Neocortical acetylcholinesterase k3 | 0.0236±0.0027 | 0.0263±0.0027 | t=4.7; P<0.0001a |
| Thalamic acetylcholinesterase k3 | 0.0542±0.0056 | 0.0599±0.0074 | tapprox=3.9; P=0.0004a |
| Striatal VMAT2 distribution volume ratio | 1.93±0.27 | 3.03±0.31 | t=18.7, P<0.0001a |
VMAT2, vesicular monoamine type 2.
Significant variable after Bonferroni correction.
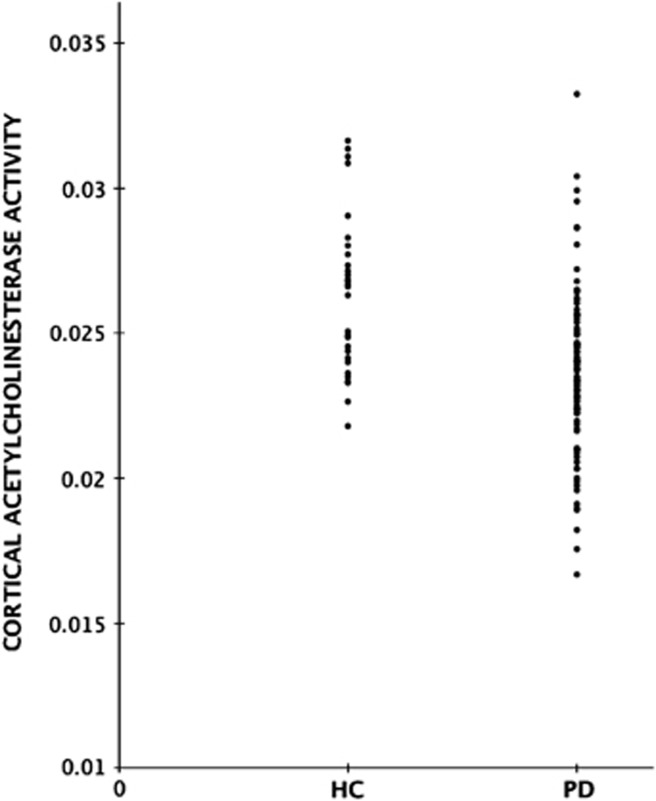
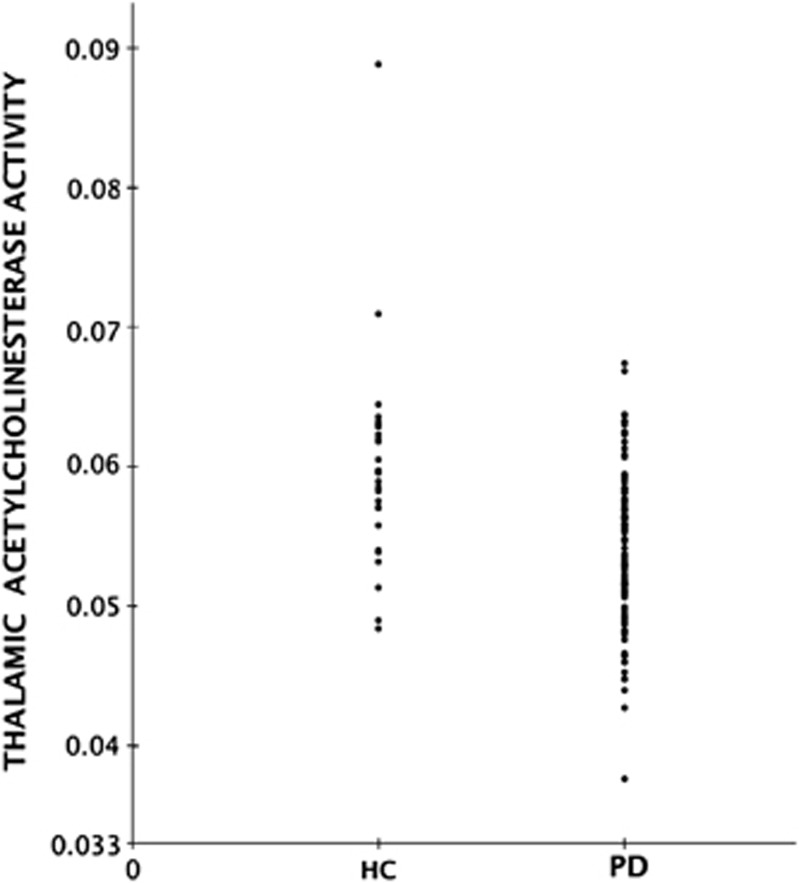
Although average neocortical and thalamic acetylcholinesterase activities were significantly reduced in the PD compared with the normal group, a significant number of subjects with PD had acetylcholinesterase activities in the normal range for both neocortical and thalamic regions (Figures 1 and 2).
Figure 1.
Group scatter plot of distribution of neocortical [11C]methyl-4-piperidinyl propionate acetylcholinesterase activity (k3 hydrolysis rate, per minute) in healthy control (HC) and Parkinson's disease (PD) subjects.
Figure 2.
Group scatter plot of distribution of thalamic [11C]methyl-4-piperidinyl propionate acetylcholinesterase activity (k3 hydrolysis rate, per minute) in healthy control (HC) and Parkinson's disease (PD) subjects.
PD Subjects with Reduced Acetylcholinesterase Activity
Based on the 5th percentile cutoff for normal range acetylcholinesterase activity, there were 31 out of 101 (31%) subjects with below normal range neocortical acetylcholinesterase activity and 18 subjects (18%) with below normal range thalamic acetylcholinesterase activity. Table 2 lists the frequency of combined low versus normal range neocortical and thalamic acetylcholinesterase activity. Sixty-five subjects (65%) had both normal range neocortical and thalamic acetylcholinesterase activity.
Table 2. Frequency of combined low versus normal range neocortical and thalamic [11C]methyl-4-piperidinyl propionate acetylcholinesterase activity in Parkinson's disease without dementia.
| Neocortical acetylcholinesterase | Thalamic acetylcholinesterase | Frequency | Percent | Cumulative frequency |
|---|---|---|---|---|
| Low | Low | 13 | 13% | 13% |
| Low | Normal range | 18 | 18% | 31% |
| Normal range | Low | 5 | 5% | 36% |
| Normal range | Normal range | 65 | 64% | 100% |
Clinical Correlates of Neocortical Acetylcholinesterase Activity
Out of 101 patients, 31 (31%) had below normal range neocortical acetylcholinesterase activity. Patients with below normal range neocortical acetylcholinesterase activity were slightly older (Table 3). There were no significant differences in striatal VMAT2 binding, duration of disease, or the Unified Parkinson's Disease Rating Scale scores between the two groups. Global cognitive performance was decreased in the patients with below normal versus normal range neocortical acetylcholinesterase activity (Table 3).
Table 3. Mean (±s.d.) values of clinical, striatal (+)-[11C]dihydrotetrabenazine VMAT2 distribution volume ratio, and cognitive z-scores in the subgroup of patients with Parkinson's disease below versus normal range neocortical [11C]methyl-4-piperidinyl propionate acetylcholinesterase activity.
| Low neocortical acetylcholinesterase group (n=31) | Normal range neocortical acetylcholinesterase group (n=70) | Statistical significance | |
|---|---|---|---|
| Age | 69.0±7.0 | 63.7±6.8 | t=3.70; P=0.0004a |
| Duration of motor disease | 7.0±4.3 | 5.5±3.7 | t=1.88; P=0.065 |
| Striatal VMAT2 distribution volume ratio | 1.84±0.22 | 1.98±0.28 | t=2.31, P=0.023 |
| Nonmotor experiences of daily living | 6.24±4.25 | 6.63±4.87 | t=0.38, P=0.70 |
| Motor experiences of daily living | 9.86±5.50 | 7.96±6.31 | t=1.45, P=0.15 |
| Motor UPDRS | 36.16±13.62 | 30.30±13.79 | t=1.98, P=0.051 |
| 8.5-m walking time | 9.48±2.78 | 8.02±1.82 | tapprox=2.67, P=0.011 |
| History of falls | 11 out of 31 (36%) | 16 out of 70 (23%) | χ2=1.75, P=0.19 |
| Global composite z-score | −0.80±1.04 | −0.14±0.63 | tapprox=3.16, P=0.0031a |
UPDRS, Unified Parkinson Disease Rating Scale; VMAT2, vesicular monoamine type 2.
Significant variable after Bonferroni correction.
Subjects with a history of falls are presented as proportions.
A post hoc analysis of the cognitive domain z-scores showed significantly lower performance for the verbal learning (t=2.68, P=0.0087), executive functions (tapprox=2.76, P=0.0084), and attention (tapprox=2.96, P=0.0051) domains but not visuospatial domain scores (tapprox=1.91, P=0.064) in the patients with below normal versus normal range neocortical acetylcholinesterase activity.
Analysis of covariance was used to compare cognitive performance scores between the subgroups of normal range versus low cortical acetylcholinesterase activity using striatal (+)-[11C]dihydrotetrabenazine VMAT2 activity as covariate. The overall model was significant (F=9.45, P=0.0002), with significantly lower global cognitive z-scores in the low compared with the normal range neocortical acetylcholinesterase group (F=7.64, P=0.0069) and an independently significant regression for striatal (+)-[11C]dihydrotetrabenazine VMAT2 binding (F=7.50, P=0.0074). Results for the specific cognitive domains showed significant cholinergic subgroup effects for verbal learning, executive functions, and attention z-scores (Table 4). These domains also revealed independent striatal dopaminergic effects. The analysis of covariance model for the visuospatial domain z-score was not significant.
Table 4. Analysis of covariance results for rank-transformed cognitive z-scores for the Parkinson's disease subgroup of low versus normal range neocortical [11C]methyl-4-piperidinyl propionate acetylcholinesterase activity levels using striatal (+)-[11C]dihydrotetrabenazine VMAT2 distribution volume ratio as covariate.
| Low versus normal cortical acetylcholinesterase group effect | Striatal VMAT2 distribution volume ratio covariate effect | Total model | |
|---|---|---|---|
| Global composite z-score | F=7.64, P=0.0069 | F=7.50, P=0.0074 | F=9.45, P=0.0002a |
| Verbal learning z-score | F=4.78, P=0.031 | F=4.22, P=0.043 | F=5.82, P=0.0041a |
| Executive functions z-score | F=6.25, P=0.014 | F=7.26, P=0.0083 | F=8.42, P=0.0004a |
| Visuospatial function z-score | F=1.55, P=0.22 | F=0.75, P=0.39 | F=1.47, P=0.24 |
| Attention z-score | F=6.67, P=0.011 | F=5.33, P=0.023 | F=7.70, P=0.0008a |
VMAT2, vesicular monoamine type 2.
Significant variable after Bonferroni correction.
Clinical Correlates of Thalamic Acetylcholinesterase Activity
Eighteen patients (18%) had below normal range thalamic acetylcholinesterase activity. Other than a history of falls, there were no significant differences in clinical, striatal VMAT2, motor, or cognitive variables between groups after correction for multiple testing (Table 5).
Table 5. Mean (±s.d.) values of clinical, striatal (+)-[11C]dihydrotetrabenazine VMAT2 distribution volume ratio and cognitive z-scores in the subgroup of patients with Parkinson's disease below versus normal range thalamic [11C]methyl-4-piperidinyl propionate acetylcholinesterase activity.
| Low thalamic acetylcholinesterase group (n=18) | Normal range thalamic acetylcholinesterase group (n=83) | Statistical significance | |
|---|---|---|---|
| Age | 67.3±7.6 | 64.9±7.0 | t=1.29; P=0.20 |
| Duration of motor disease | 8.0±3.9 | 5.5±3.8 | t=2.53; P=0.013 |
| Striatal VMAT2 distribution volume ratio | 1.82±0.19 | 1.96±0.28 | t=1.96, P=0.053 |
| Nonmotor experiences of daily living | 6.25±3.74 | 6.56±4.87 | t=0.26, P=0.80 |
| Motor experiences of daily living | 9.47±5.70 | 8.34±6.21 | t=0.71, P=0.48 |
| Motor UPDRS | 33.11±13.55 | 31.88±14.08 | t=0.34, P=0.74 |
| 8.5-m walking time | 9.11±2.48 | 8.36±2.21 | t=1.26, P=0.21 |
| History of falls | 10 out of 18 (56%) | 17 out of 83 (23%) | χ2=9.30, P=0.0023a |
| Global composite z-score | −0.52±0.71 | −0.31±0.86 | t=0.95, P=0.35 |
UPDRS, Unified Parkinson Disease Rating Scale; VMAT2, vesicular monoamine type 2.
Significant variable after Bonferroni correction.
Subjects with a history of falls are presented as proportions.
There were 27 subjects (27%) who reported a history of falls. Falls were more frequent in the subgroup of subjects with low thalamic acetylcholinesterase activity, 56% versus 21% fallers, in the normal range thalamic acetylcholinesterase group (χ2=9.3, P=0.0023). Logistic regression analysis using fall history as an dependent variable and thalamic acetylcholinesterase group and striatal (+)-[11C]dihydrotetrabenazine VMAT2 activity as independent variables revealed a significant overall model effect (likelihood ratio χ2=12.1, P=0.0023) with a significant acetylcholinesterase group effect (Wald χ2=6.3, P=0.012) and a nonsignificant trend for striatal (+)-[11C]dihydrotetrabenazine VMAT2 activity (Wald χ2=3.3, P=0.07).
Discussion
Heterogeneity of Cholinergic Denervation in PD and Clinical Phenotypic Variations
Our data show that the extent of cholinergic denervation is variable in nondemented PD patients, with normal range neocortical and thalamic [11C]methyl-4-piperidinyl propionate acetylcholinesterase activity present in 64% of patients. The subgroup with below normal range neocortical acetylcholinesterase is larger (31%) than the subgroup with below normal range thalamic acetylcholinesterase levels (18%). Patients with below normal range neocortical acetylcholinesterase were slightly older compared with the normal range subgroup.
The major clinical difference between patients with below normal versus normal range neocortical [11C]methyl-4-piperidinyl propionate acetylcholinesterase activity was decreased cognitive performance. Lower performances were seen in all tested cognitive domains except for visuospatial function measures. These findings agree with the current concept that cognitive changes in PD are heterogeneous. A recent multicenter pooled analysis, for example, disclosed evidence of cognitive impairments in memory, visuospatial, attention, and executive function domains in PD without dementia (Aarsland et al, 2010). The lack of visuospatial function findings in our study may be related to differences in cognitive test battery between our and other studies.
Our subgroup definition reflects the tail end or continuum of an apparently normal distribution of the [11C]methyl-4-piperidinyl propionate acetylcholinesterase activity in the PD patient group rather than the existence of two dichotomous groups. We found that neocortical and thalamic acetylcholinesterase activity in the PD group showed substantial overlap with normal range activity, as evidenced by the small absolute mean difference (∼10% decrease) across all PD patients compared with the control group for both neocortical and thalamic [11C]methyl-4-piperidinyl propionate acetylcholinesterase activity. This is strikingly different from the typical complete group separation for nigrostriatal denervation levels (especially at the level of the posterior putamen) between PD and control groups (Bohnen et al, 2006a). Our approach of defining a patient subgroup with below normal range cholinergic activity is intended as a more effective strategy to investigate clinical phenotypic variations because of cholinergic denervation in PD.
There is increasing evidence that cholinergic denervation occurs early in a subset of PD patients and that progressive cholinergic denervation is associated with dementia (Shimada et al, 2009). In vivo imaging studies have also shown that the presence of dementia in PD is associated with more severe and widespread cholinergic denervation compared with PD without dementia (Bohnen et al, 2003; Hilker et al, 2005; Kuhl et al, 1996; Shinotoh et al, 1999). These imaging results are consistent with post-mortem evidence that basal forebrain cholinergic system degeneration appears early in PD and worsens in parallel with the appearance of dementia (Ruberg et al, 1986). The cross-sectional design of our study is a limitation and future longitudinal research is needed to determine whether selective cholinergic denervation predicts development of dementia in PD.
Thalamic cholinergic changes are also described in PD without dementia and are associated with a propensity for falls (Bohnen et al, 2009). Although there were no significant differences in the parkinsonian motor scores between the two subgroups of low versus normal range thalamic acetylcholinesterase activity, the subgroup with lower thalamic acetylcholinesterase activity had a greater proportion of patients with a history of falls compared with the normal range subgroup. Gait impairment, falls, and cognition may be related (Yarnall et al, 2011), as evidenced by findings that fallers performed less well on a test of divided attention than did nonfallers (Allcock et al, 2009). Cholinergic system degeneration may also provide a conceptual framework to explain why patients with higher postural instability and gait disturbances in PD are at an increased risk of developing dementia (Alves et al, 2006; Taylor et al, 2008). However, we did not find a specific association between thalamic cholinergic activity and cognitive performance in our study. A recent post-mortem study showed that patients with PD and falls have greater loss of acetylcholinesterase-containing neurons in the mesencephalon compared with nonfallers (Karachi et al, 2010). This study also showed that bilateral lesioning of the cholinergic part of the pedunculopontine nucleus induced gait and postural deficits in monkeys (Karachi et al, 2010). These findings raise the question as to whether acetylcholinesterase inhibitor therapy may have a role in the treatment of falls in PD as suggested by the results of a recent small placebo-controlled clinical trial (Chung et al, 2010).
Mixed Cholinergic and Dopaminergic Correlates of Cognitive Performance in PD Without Dementia
We found that low cortical acetylcholinesterase is robustly associated with decreased cognitive performance in PD without dementia. We also found that cognitive performance in PD is independently related to both neocortical cholinergic and nigrostriatal dopaminergic denervation in multiple domains except for visuospatial functions. We did not find evidence of either exclusive cholinergic or dopaminergic substrates for specific cognitive domains to identify neurotransmitter-specific cognitive circuits. Although dopamine has long been known to modulate cortical striatal circuits and performance on executive tasks such as working memory (Goldman-Rakic, 1998) and attentional control (Williams-Gray et al, 2008), we found evidence of independent cholinergic and dopaminergic effects underlying this group of functions. PD is now recognized as a multisystem neurodegenerative disorder and multiple neurotransmitter deficits likely contribute to cognitive impairment in PD (Kehagia et al, 2010).
Acetylcholinesterase has been long recognized as a reliable marker for brain cholinergic pathways including in the human brain (Selden et al, 1998; Shute and Lewis, 1966). Acetylcholinesterase is localized predominantly in cholinergic cell bodies and axons. In the cortex, acetylcholinesterase is present in axons innervating it from the basal forebrain (Selden et al, 1998). There is also acetylcholinesterase in intrinsic cortical neurons, and low levels of acetylcholinesterase are probably present in the noncholinergic structures postsynaptic to the nucleus basalis innervation (Heckers et al, 1992b). Our group has shown similar reductions in cortical and pedunculopontine nucleus cholinergic activity levels in PD using the vesicular acetylcholine transporter and the [123I]iodobenzovesamicol (IBVM) radioligand (Kuhl et al, 1996). These data support the validity of [11C]methyl-4-piperidinyl propionate PET as a reliable marker, especially for the forebrain and midbrain cholinergic projections. A limitation of the PMP radioligand is that it does not allow accurate assessment of the high acetylcholinesterase activity levels in the striatum and cerebellum using noninvasive analysis technique. Furthermore, acetylcholinesterase activity may be a less robust marker of cerebellar cholinergic nerve terminals because cerebellar vesicular acetylcholine transporter levels are low in this region. Consequently, motor correlates of cholinergic denervation in these structures cannot be excluded by our [11C]methyl-4-piperidinyl propionate PET study (Bohnen et al, 2009; Gilman et al, 2010).
We conclude that cholinergic denervation in PD without dementia is variable with approximately one third of patients exhibiting decreased cholinergic terminals. The presence of variable cholinergic denervation of basal forebrain and pedunculopontine nuclear projections correlates with important clinical features of PD and is consistent with the general hypothesis that the clinical heterogeneity of PD results from variable involvement of different brain systems. Our findings also imply that in cholinergic augmentation therapy, only symptoms of cognitive impairment or possibly falls should be selectively targeted.
Acknowledgments
The authors thank Christine Minderovic, Virginia Rogers, the PET technologists, cyclotron operators, and chemists for their assistance. This work was supported by the Department of Veterans Affairs, the Michael J. Fox Foundation, and the NIH (grant numbers P01 NS015655 and RO1 NS070856).
Dr Bohnen and Dr Müller have research support from the NIH and the Department of Veteran Affairs. Dr Kotagal has no disclosures. Dr Koeppe serves on the Board of the International Society of Cerebral Blood Flow and Metabolism; receives research support from NIH (NINDS, NIA); and is a consultant for Johnson & Johnson and Merck. Dr Kilbourn has research support from the NIH, Bayer, and General Electric Medical Systems. He is a scientific board member of Avid Radiopharmaceuticals (Eli Lilly subsidiary) and a consultant for Eli-Lilly and Bristol-Myers-Squibb companies. He has royalties from Avid Radiopharmaceuticals and holds stock options in Avid Radiopharmaceuticals. Dr Gilman has research support from the NIH. Dr Albin has received compensation for expert witness testimony in litigation regarding dopamine agonist-induced impulse control disorders. He serves on the editorial boards of Neurology, Experimental Neurology, and Neurobiology of Disease. He receives grant support from the National Institutes of Health and the Department of Veterans Affairs. He served on the Data Safety and Monitoring Boards for the QE3 and HORIZON trials. Dr Frey has research support from the NIH, GE Healthcare, and AVID Radiopharmaceuticals (Eli Lilly subsidiary). He also serves as a consultant to AVID Radiopharmaceuticals, MIMVista, Bayer-Schering, and GE healthcare. He also holds equity (common stock) in GE, Bristol-Myers, Merck, and Novo-Nordisk.
References
- Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72:1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, Burn D, Barone P, Pagonabarraga J, Allcock L, Santangelo G, Foltynie T, Janvin C, Larsen JP, Barker RA, Emre M. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75:1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allcock LM, Rowan EN, Steen IN, Wesnes K, Kenny RA, Burn DJ. Impaired attention predicts falling in Parkinson's disease. Parkinsonism Relat Disord. 2009;15:110–115. doi: 10.1016/j.parkreldis.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord. 2006;21:1123–1130. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- Arendt T, Bigl V, Arendt A, Tennstedt A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer's disease, paralysis agitans and Korsakoff's disease. Acta Neuropathol (Berl) 1983;61:101–108. doi: 10.1007/BF00697388. [DOI] [PubMed] [Google Scholar]
- Benton AL. Revised Visual Retention Test, 4th ed. New York, NY: Psychological Corporation; 1974. [Google Scholar]
- Benton AL, Varney NR, Hamsher K. Judgment of Line Orientation, Form V. Iowa City, IA: University of Iowa Hospitals; 1975. [Google Scholar]
- Bohnen N, Jolles J, Twijnstra A. Modification of the Stroop color word test improves differentiation between patients with mild head injury and matched controls. Clin Neuropsychol. 1992;6:178–184. doi: 10.1080/13854049208401854. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, Frey KA. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006a;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Constantine GM, Mathis Ch A, Davis JG, Moore RY, Dekosky ST. Cognitive correlates of cortical cholinergic denervation in Parkinson's disease and parkinsonian dementia. J Neurol. 2006b;253:242–247. doi: 10.1007/s00415-005-0971-0. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, Mathis CA, Moore RY, DeKosky ST. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003;60:1745–1748. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Muller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, Albin RL. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73:1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Muller MLTM, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, Frey KA. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson disease. Brain. 2010;133:1747–1754. doi: 10.1093/brain/awq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology. 2010;75:1263–1269. doi: 10.1212/WNL.0b013e3181f6128c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming P, Borghammer P.2012Molecular imaging and the neuropathologies of Parkinson's disease Curr Top Behav Neuroscidoi: 10.1007/7854_2011_165(PMID 22034053) [DOI] [PubMed]
- Delis DC, Kramer JH, Kaplan E, Ober BA.2000California Verbal Learning Test Manual, Adult Version2nd edn. San Antonio: The Psychological Corporation [Google Scholar]
- Frey KA, Koeppe RA, Kilbourn MR, Vander Borght TM, Albin RL, Gilman S, Kuhl DE. Presynaptic monoaminergic vesicles in Parkinson's disease and normal aging. Ann Neurol. 1996;40:873–884. doi: 10.1002/ana.410400609. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koeppe RA, Nan B, Wang CN, Wang X, Junck L, Chervin RD, Consens F, Bhaumik A. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology. 2010;74:1416–1423. doi: 10.1212/WNL.0b013e3181dc1a55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, Tilley BC, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, Lewitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, Van Hilten JJ, Lapelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22:41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Guttman M, Burkholder J, Kish SJ, Hussey D, Wilson A, DaSilva J, Houle S. [11C]RTI-32 PET studies of the dopamine transporter in early dopa-naive Parkinson's disease: implications for the symptomatic threshold. Neurology. 1997;48:1578–1583. doi: 10.1212/wnl.48.6.1578. [DOI] [PubMed] [Google Scholar]
- Heckers S, Geula C, Mesulam M. Cholinergic innervation of the human thalamus: dual origin and differential nuclear distribution. J Comp Neurol. 1992a;325:68–82. doi: 10.1002/cne.903250107. [DOI] [PubMed] [Google Scholar]
- Heckers S, Geula C, Mesulam MM. Acetylcholinesterase-rich pyramidal neurons in Alzheimer's disease. Neurobiol Aging. 1992b;13:455–460. doi: 10.1016/0197-4580(92)90072-6. [DOI] [PubMed] [Google Scholar]
- Hilker R, Thomas AV, Klein JC, Weisenbach S, Kalbe E, Burghaus L, Jacobs AH, Herholz K, Heiss WD. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65:1716–1722. doi: 10.1212/01.wnl.0000191154.78131.f6. [DOI] [PubMed] [Google Scholar]
- Hoehn M, Yahr M. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinicopathologic study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett DM, Kilbourn MR, Lee LC. A simple synthesis of [11C]dihydrotetrabenazine (DTBZ) Nucl Med Biol. 1997;24:197–199. doi: 10.1016/s0969-8051(96)00213-2. [DOI] [PubMed] [Google Scholar]
- Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, Belaid H, Bardinet E, Prigent A, Nothacker HP, Hunot S, Hartmann A, Lehericy S, Hirsch EC, Francois C. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest. 2010;120:2745–2754. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9:1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- Koeppe RA, Frey KA, Kuhl DE, Kilbourn MR. Assessment of extrastriatal vesicular monoamine transporter binding site density using stereoisomers of [11C]dihydrotetrabenazine. J Cereb Blood Flow Metab. 1999;19:1376–1384. doi: 10.1097/00004647-199912000-00011. [DOI] [PubMed] [Google Scholar]
- Kuhl D, Minoshima S, Fessler J, Frey K, Foster N, Ficaro E, Wieland D, Koeppe R. In vivo mapping of cholinergic terminals in normal aging, Alzheimer's disease, and Parkinson's disease. Ann Neurol. 1996;40:399–410. doi: 10.1002/ana.410400309. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Fessler JA, Mintun MA, Berger KL, Taylor SF, Kuhl DE. Integrated and automated data analysis method for neuronal activation studying using O15 water PET. Ann Nucl Med. 1993;7 (Suppl:S74–5. [Google Scholar]
- Nagatsuka S, Fukushi K, Shinotoh H, Namba H, Iyo M, Tanaka N, Aotsuka A, Ota T, Tanada S, Irie T. Kinetic analysis of [(11)C]MP4A using a high-radioactivity brain region that represents an integrated input function for measurement of cerebral acetylcholinesterase activity without arterial blood sampling. J Cereb Blood Flow Metab. 2001;21:1354–1366. doi: 10.1097/00004647-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Ruberg M, Rieger F, Villageois A, Bonnet AM, Agid Y. Acetylcholinesterase and butyrylcholinesterase in frontal cortex and cerebrospinal fluid of demented and non-demented patients with Parkinson's disease. Brain Res. 1986;362:83–91. doi: 10.1016/0006-8993(86)91401-0. [DOI] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121:2249–2257. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Shimada H, Hirano S, Shinotoh H, Aotsuka A, Sato K, Tanaka N, Ota T, Asahina M, Fukushi K, Kuwabara S, Hattori T, Suhara T, Irie T. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73:273–278. doi: 10.1212/WNL.0b013e3181ab2b58. [DOI] [PubMed] [Google Scholar]
- Shinotoh H, Namba H, Yamaguchi M, Fukushi K, Nagatsuka S, Iyo M, Asahina M, Hattori T, Tanada S, Irie T. Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson's disease and progressive supranuclear palsy. Ann Neurol. 1999;46:62–69. [PubMed] [Google Scholar]
- Shute CC, Lewis PR. Electron microscopy of cholinergic terminals and acetylcholinesterase-containing neurones in the hippocampal formation of the rat. Z Zellforsch Mikrosk Anat. 1966;69:334–343. doi: 10.1007/BF00406286. [DOI] [PubMed] [Google Scholar]
- Snyder SE, Tluczek L, Jewett DM, Nguyen TB, Kuhl DE, Kilbourn MR. Synthesis of 1-[11C]methylpiperidin-4-yl propionate ([11C]PMP) for in vivo measurements of acetylcholinesterase activity. Nucl Med Biol. 1998;25:751–754. doi: 10.1016/s0969-8051(98)00045-6. [DOI] [PubMed] [Google Scholar]
- Taylor JP, Rowan EN, Lett D, O'Brien JT, McKeith IG, Burn DJ. Poor attentional function predicts cognitive decline in patients with non-demented Parkinson's disease independent of motor phenotype. J Neurol Neurosurg Psychiatry. 2008;79:1318–1323. doi: 10.1136/jnnp.2008.147629. [DOI] [PubMed] [Google Scholar]
- Thompson CJ, Kecani S, Boelen S. Evaluation of a neck-shield for use during neurological studies with a whole-body PET scanner. IEEE Trans Nucl Sci. 2001;48:1512–1517. [Google Scholar]
- Wechsler D. WAIS III Technical Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- White J, Yeats A, Skipworth G. Cheltenham, UK: Stanley Thornes Publishers; 1988. Tables for Statisticians. [Google Scholar]
- Williams-Gray CH, Hampshire A, Barker RA, Owen AM. Attentional control in Parkinson's disease is dependent on COMT val 158 met genotype. Brain. 2008;131:397–408. doi: 10.1093/brain/awm313. [DOI] [PubMed] [Google Scholar]
- Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson's disease. Mov Disord. 2011;26:2496–2503. doi: 10.1002/mds.23932. [DOI] [PubMed] [Google Scholar]