Abstract
The aim of this study was to examine the patterns of disclosure of BRCA1/2 genetic test results to employers by unaffected carriers. In a national prospective cohort study on unaffected BRCA1/2 mutation carriers, disclosure to employers was assessed prospectively, using self-administered questionnaires, up to 2 years after their test results were delivered by cancer geneticists. Kaplan–Meier curves and Cox-regression analysis were used to assess the factors associated with time to disclosure to the employer. Mean age of the 146 women BRCA1/2 carriers who were employed when their test results were delivered was 37.1 years (range: 19–57). At the end of the second year of follow-up, 47 of them (32.2%) had disclosed their results to their employers; median time to disclosure was 6 months. Reasons spontaneously expressed were first to inform the employer that medical surveillance/surgery was necessary for cancer risk management although these carriers did not actually have cancer. After multivariate adjustment on age, women with a lower educational level (HRadj=2.00, P=0.026) and those who had undergone prophylactic surgery during the 2 years of follow-up (HRadj=2.18, P=0.019) had disclosed their BRCA status to their employers earlier and more frequently. One-third of the female carriers not affected by breast/ovarian cancer disclosed their BRCA1/2 genetic test results spontaneously to their employers, mainly to inform them that they were disease-free but required medical surveillance or a surgical intervention to reduce the risk of cancer.
Keywords: BRCA1/2, genetic test, employment, disclosure
Introduction
The political processes used to deal with fears of genetic discrimination differ from one country to another. In France, access to genetic test results is thought to be a sensitive enough issue to require specific legal provisions. To prevent the risk of discrimination, a law (2002-303 Art. L. 1141-1) was promulgated in 2005, which prohibits health and life insurance companies and employers from using genetic information based on the ‘analysis of genetic characteristics' to the detriment of carriers; this law is still in force. In the United States, President George W Bush signed the Genetic Information Non-discrimination Act (known as ‘GINA' http://www.eeoc.gov/laws/types/genetic.cfm).1 This act was designed to prevent health insurers from discriminating against gene defect/mutation carriers. This legislation also bars employers from basing employment-related decisions on individuals' genetic data. ‘In the United Kingdom, following the Government's Discrimination Law Review and the decision not to include specific protections against genetic discrimination in the Equality Bill, the Human Genetic Commission agreed to set up a time-limited working group to carry out a specific investigation into the issue of genetic discrimination, to identify methods of evidence collection and monitoring, to assess the appropriateness and durability of existing safeguards, and to make recommendations'. The group is expected to report in the near future to the Ministers responsible http://www.hgc.gov.uk/Client/Content.asp?ContentId=254. The regulations pertaining in various european and other industrialised countries such as Australia have been described elsewhere in greater detail http://www.eurogentest.org/web/info/public/unit4/ethical_legal_papers.xhtml#legal_3).2, 3, 4
However, although employers cannot oblige their employees to disclose the results of genetic tests, in France as in many other Western countries, medical information belongs to the individuals involved, who can disclose this information to whoever they want.
Women in a national cohort of BRCA1/2 mutation carriers (GENEPSO) were asked prospectively up to 2 years after receiving their test results from their cancer geneticists whether or not they had disclosed their genetic status to their employers. The sociodemographic, psychological and medical factors involved in this disclosure were studied. The hypothesis was that the rate of disclosure to employers was likely to be very low in the context of unaffected carriers, and that the main factor involved was likely to be educational status. This issue has never been investigated so far to our knowledge.
Methods
The women included in this analysis were all those who had completed the 2-year follow-up after receiving their genetic test results and had a job at the time when the results were delivered (which was taken as the baseline). These women were BRCA1/2 carriers who were cancer-free up to the end of the 2-year follow-up period.
Disclosure of genetic test results to employers was assessed prospectively, using self-administered questionnaires mailed 15 days, 6 months, 1 year and 2 years after disclosure of the genetic test results at a consultation with the geneticist. The following questions were asked: ‘Who have you informed about your own BRCA genetic test results (either spontaneously or at their request)?' The following items were proposed: ‘Your employer?' ‘Your health insurance company' and ‘Your life insurance company' and the possible answers were ‘Yes, no, I have no employer/health insurance company/life insurance company'. An open-ended question was added, asking respondents to explain in their own words the circumstances under which they had informed their employer or health/life insurance company, if relevant. Specific items were proposed here as regards family disclosure, such as ‘Your partner', ‘Your sister(s)' and ‘Your brother(s)'.
The women's psychological characteristics were assessed at the same time. Depression was measured using the French version of the Center for Epidemiologic Studies Depression Scale (CES-D)5 and the psychological impact of testing with the 15-item Impact of Events Scale (IES).6
Other characteristics such as sociodemographics (age, educational level, occupational status, living with a partner, number of children) were also recorded at baseline and updated with time.
The SPSS (Statistical Package for Social Science) 17.0 software program (SPSS Inc., Chicago, IL, USA) was used for all the statistical analyses.
Time to disclosure to the employer was calculated from the date of receiving the BRCA test results from the medical team to the date of disclosure to the employer. Subjects who did not disclose their BRCA test results were censored.
Kaplan–Meier analysis was used to determine the cumulative rates of disclosure of genetic data to the employer during the 2-year follow-up period. Log-rank tests were performed to test the difference between the Kaplan–Meier estimation curves obtained on each variable.
Independent variables associated with disclosure of genetic test results to the employer were analysed using Cox's proportional hazard regression model. The 95% confidence intervals (CIs) were calculated. Variables with a P-value <0.25 in the univariate analyses were taken to be eligible for inclusion in the multivariate model. Only variables still significantly associated with the outcome variable with a P-value <0.05 were kept in the final model.
Results
Description of the sample
Among the female BRCA1/2 carriers who completed the first questionnaire before test result disclosure (N=259), 13 were excluded because they had developed breast/ovarian cancer (N=11) or because they died (N=2) during the 2-year follow-up period. The respondents included for 2 years after test result disclosure (N=221) were all in employment at baseline (N=146). They did not differ from unemployed participants in terms of any of the baseline sociodemographic, psychological or medical characteristics except for age: the working women were younger on average (37.1 years, SD=8.2) than the unemployed women (40.7 years, SD=13.1; P=0.010). In the sample studied, 86 (58.9%) women had reached an educational level equal to or above secondary school leaving certificate level, and 111 (76.0%) had a partner; 8 (5.5%) participants had undergone prophylactic mastectomy and 39 (26.7%), prophylactic oophorectomy by the second year of follow-up. Two weeks after the women had received their genetic test results, the mean IES score was 18.9 (SD: 18.9) and the mean CES-D score was 17.4 (SD: 16.0).
Disclosure to employers
Within 2 years after being informed by the cancer geneticists about their BRCA1/2 carrier status, 47 women (32.2%) had disclosed their results to their employers. Median time to disclosure was 6 months; mean age at disclosure was 37.7 years. In the same sample and the same time frame, 100% of the women had disclosed their results to their partner, 97.4% to their sisters and 86.5% to their brothers; 8.3% had disclosed their results to their health insurance company and 3.6% to their life insurance company.
Factors associated with disclosure to the employer
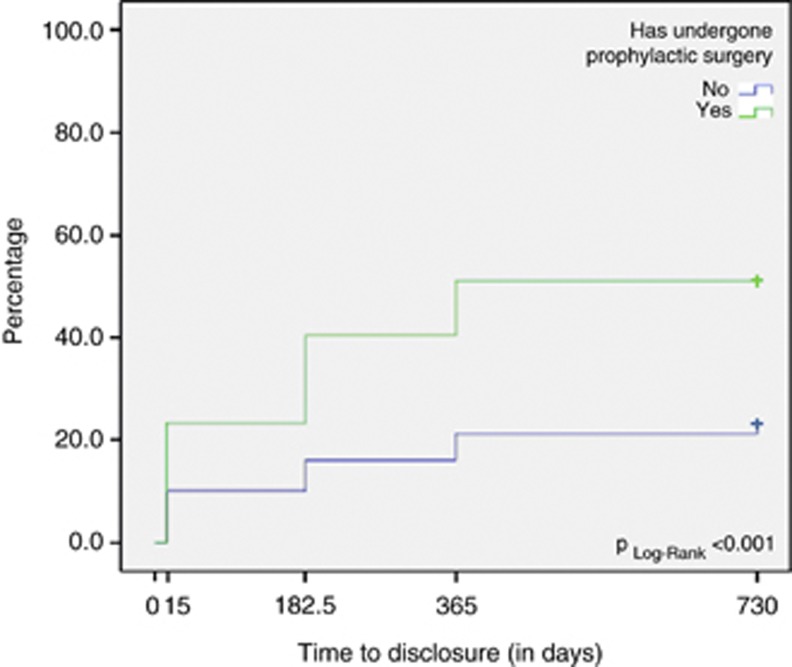
After multivariate adjustment (Table 1), two parameters were found to be significantly associated with higher rates of disclosure: a lower educational level, HR 2.00 (95% CI 1.09–3.69), and having undergone prophylactic surgery, HR 2.18 (95% CI 1.14–4.18). The timing of disclosure to the employer is presented in Figure 1, depending on whether or not the women underwent prophylactic surgery during the 2 years after delivery of the BRCA1/2 results by the cancer geneticist. None of the other independent variables tested, such as sociodemographic, psychological or medical variables, were found to be significant after multivariate adjustment.
Table 1. Cox regression analysis giving the HR on disclosure of genetic test results to the employer during the 2-year follow-up period – GENEPSO cohort, n=146.
| Disclosure to employer BRCA+ (n=146) | |||||
|---|---|---|---|---|---|
| Cox regression, univariate | Cox regression, multivariate | ||||
| Variable | n (row %) | HR (95%CI) | P-value | HRadj (95%CI)a | P-value |
| Level of education | |||||
| Low (n=60) | 28 (53.3) | 2.34 (1.31–4.20) | 0.004 | 2.00 (1.09–3.69) | 0.026 |
| High (n=86) | 19 (22.1) | 1 | 1 | ||
| Prophylactic surgery | |||||
| Yes (n=44) | 22 (50.0) | 2.53 (1.43–4.49) | 0.002 | 2.18 (1.14–4.18) | 0.019 |
| No (n=102) | 102 (24.5) | 1 | 1 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio.
HR adjusted on age.
Figure 1.
Rates of BRCA1/2 disclosure to the employer during the 2-year follow-up period, depending on whether respondents underwent prophylactic surgery – GENEPSO cohort, N=146.
On the basis of the qualitative information obtained in response to the open question, two main reasons for disclosure to the employer were expressed by 26 women out of the 47 who disclosed this information. First (20 of the 28 comments), these women felt the need to explain that the reason for their absence from work was that they had to undergo prophylactic surgical procedures (N=10) or be monitored regularly (by undergoing IRM screening, for example): they were not being treated for cancer but for risk reduction purposes at specialised centres (N=10); and secondly (8 of the 28 comments), they mentioned having friendly or close relationships with their employers.
Discussion
It emerged from this study that one-third of the gene carriers questioned had disclosed their results to their employers (32.2%). This rate of disclosure was unexpectedly high. Here we talk about employer as ‘informal' employer, meaning the higher in order, and not the institution. Two parameters were found to be significantly associated with higher rates of disclosure to employers: first, a lower educational level, which might result in insufficient awareness of the risk of discrimination but may also correspond to having a lower position in the occupational hierarchy; women with lower educational levels may feel compelled to inform their superiors about the reasons for their absence from work.7 The second parameter was having undergone prophylactic surgery. This is consistent with the qualitative information obtained. Women felt compelled to explain to their employers why they had been hospitalised for a surgical intervention, usually in cancer wards or hospitals. French legislation about disclosure of genetic tests prohibits the use of genetic information by health and life insurance companies and employers. The law applies even if this information is provided by employees or the applicants for insurance themselves. This precaution was taken to prevent ‘mutation-free' persons from asking for a discount8 or from using this information at work to elicit positive discrimination because those who refrain from informing their employers might be ‘mutation carriers'. As observed in our survey, spontaneous disclosure of genetic test results to employers by employees who are carriers is not an unusual occurrence. Two main reasons were given by the women questioned here. Although being on friendly terms with their employers does not raise any ethical concerns, and might constitute an expression of guilty feelings or social responsibility, the main reason (which was mentioned by almost 50% of the respondents) is more worrying: the women frequently disclosed this information because they felt compelled to justify their absence from work to undergo medical procedures. This is not actually a genetic issue but a medical and sociological one: it emerges from these findings that people who have to undergo surgery or surveillance at specialised cancer hospitals or cancer screening clinics often have to give their employers the exact reason for their absence from work, and in this case, to specify that they have not actually developed cancer. Future studies could deal with this issue in greater depth: qualitative studies seem to be a more informative means of approaching questions of this kind.
These data were based on a prospective population-based (nation-wide) survey. However, the absolute number of gene carriers participating was low (N=146) because they included only unaffected working women, and this sample may not be representative of all female gene carriers, as inclusion in this survey depended on the willingness to participate of both hereditary cancer clinics and the women carriers.
As no other data are available as far as we know on this topic, these findings cannot be yet either confirmed or challenged. Genetic discrimination has been investigated and described in the context of Huntington's disease or other genetic diseases, and the types of discrimination unaffected carriers are liable to undergo in their lifetime, especially in the framework of insurance and at the workplace.9, 10, 11, 12 Transmission of medical information to employers has been reported to occur in 51% of patients with cystic fibrosis13 and even in 76% of those with arthritis,14 and further knowledge is required about the issues involved in to self-disclosure of conditions of this kind. The existence of a supportive occupational environment and supportive co-workers seems to be a key issue for these patients.
The present data show that legal constraints and regulations might not suffice if genetic information has to be disclosed to prevent unfair discrimination. Regulatory bodies and physicians should be aware of this problem. Although exchanging experience at work with one's co-workers may have positive psychological and supportive effects, this situation also means that the cancer geneticists and physicians involved upstream in the information delivery process should explain in greater detail the risk of potential occupational discrimination and emphasise patients' right not to disclose personal medical details.
It would be extremely interesting to compare results obtained in different settings in order to determine whether the social and cultural background may have an impact on the rates of self-disclosure of genetic test results, especially in the case of BRCA1/2 genetic testing.
Acknowledgments
We thank all the members of the Genepso cohort team of investigators, including Claude Adenis, Pascaline Berthet, Yves-Jean Bignon, Valérie Bonadona, Olivier Caron, Annie Chevrier, Odile Cohen-Haguenauer, Isabelle Coupier, Liliane Demange, Capucine Delnatte, Hélène Dreyfus, Catherine Dugast, Laurence Faivre, Marc Frenay, Jean-Pierre Fricker, Marion Gauthier-Villars, Paul Gesta, Laurence Gladieff, Michel Longy, Elisabeth Luporsi, Emmanuelle Mouret-Fourme, Tan Dat Nguyen, Hagay Sobol, Laurence Venat-Bouvet, Philippe Vennin, Hélène Zattara-Cannoni. Anne-Deborah Bouhnik is acknowledged for her statistical expertise. This work was supported by Institut National du Cancer INCA R08097 AA/RPT08011AAA.
The authors declare no conflict of interest.
References
- Hudson KL, Holohan MK, Collins FS. Keeping pace with the times--the Genetic Information Nondiscrimination Act of 2008. N Engl J Med. 2008;358:2661–2663. doi: 10.1056/NEJMp0803964. [DOI] [PubMed] [Google Scholar]
- Godard B, Raeburn S, Pembrey M, Bobrow M, Farndon P, Ayme S. Genetic information and testing in insurance and employment: technical, social and ethical issues. Eur J Hum Genet. 2003;11 (Suppl 2:S123–S142. doi: 10.1038/sj.ejhg.5201117. [DOI] [PubMed] [Google Scholar]
- Otlowski M. Essentially yours: the protection of human genetic information in Australia. Genewatch. 2006;19:9–12. [PubMed] [Google Scholar]
- Surbone A. Social and ethical implications of BRCA testing. Ann Oncol. 2011;22 (Suppl 1:i60–i66. doi: 10.1093/annonc/mdq668. [DOI] [PubMed] [Google Scholar]
- Fuhrer F. La version française de l'échelle CES-D. Description and translation of the autoevaluation scale (in French) Psychiatrie et Psychobiologie. 1989;4:163–166. [Google Scholar]
- Horowitz M, Wilner N, Alvarez W. Impact of event scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Marmot M, Feeney A, Shipley M, North F, Syme SL. Sickness absence as a measure of health status and functioning: from the UK Whitehall II study. J Epidemiol Community Health. 1995;49:124–130. doi: 10.1136/jech.49.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duby JJ. Genetic discrimination. Science. 1995;270:1282. [PubMed] [Google Scholar]
- Tibben A. Genetic discrimination in Huntington's disease. Bmj. 2009;338:b1281. doi: 10.1136/bmj.b1281. [DOI] [PubMed] [Google Scholar]
- Bombard Y, Veenstra G, Friedman JM, et al. Perceptions of genetic discrimination among people at risk for Huntington's disease: a cross sectional survey. BMJ. 2009;338:b2175. doi: 10.1136/bmj.b2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penziner E, Williams JK, Erwin C, et al. Perceptions of discrimination among persons who have undergone predictive testing for Huntington's disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147:320–325. doi: 10.1002/ajmg.b.30600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow-Stewart K, Taylor SD, Treloar SA, Stranger M, Otlowski M. Verification of consumers' experiences and perceptions of genetic discrimination and its impact on utilization of genetic testing. Genet Med. 2009;11:193–201. doi: 10.1097/GIM.0b013e318194ee75. [DOI] [PubMed] [Google Scholar]
- Modi AC, Quittner AL, Boyle MP. Assessing disease disclosure in adults with cystic fibrosis: the Adult Data for Understanding Lifestyle and Transitions (ADULT) survey disclosure of disease in adults with cystic fibrosis. BMC Pulm Med. 2010;10:46. doi: 10.1186/1471-2466-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gignac MA, Cao X. ‘Should I tell my employer and coworkers I have arthritis?' A longitudinal examination of self-disclosure in the work place. Arthritis Rheum. 2009;61:1753–1761. doi: 10.1002/art.24889. [DOI] [PubMed] [Google Scholar]