Abstract
Due to recent population emigration movements, an epidemic of Chagas disease is currently menacing most developed countries. The authors report the case of a 53-year-old Brazilian woman living in Europe for the last 10 years who developed heart failure symptoms, having a previous symptomatic sinus node disease with a pacemaker implant at age of 40 years. The diagnosis was based on serology and myocardial biopsy and the patient was treated with nifurtimox. The authors emphasize the need of a high level of suspicion in patients with suggestive epidemiology and the need of populational screening of specific high risk groups. New treatment options are also discussed.
Keywords: Chagas disease, Chagas cardiomyopathy, Dysrhythmia, Emigration, Non-endemic countries, Benznidazole, Nifurtimox
INTRODUCTION
Chagas disease (CD) is one of the parasitic diseases with a high economic burden throughout Latin America. It affects around 10 million people and has now become global due to recent population movements and migration[1]. Being one of the 17 neglected tropical diseases, it still afflicts the poor and “promotes poverty”. Its presence outside Latin America is primarily the result of migration but it has also been reported among travellers and in adopted children. Important transmission routes in Europe are transfusion, vertical or organ transplantation[2]. The challenge in treatment of chronic CD is due to limitations of the currently available specific treatments, benznidazole and nifurtimox, and also to the lack of biological markers for the early evaluation of antiparasitic drug efficacy and clinical response[3]. Chronic chagasic cardiomyopathy (CCC) is the most serious and common etiology of CCC in Latin America and occurs in 20%-30% of infected subjects, usually 10-30 years after infection by Trypanosoma cruzi[4].
In this report, we emphasize the implications of a very great latent time until CD diagnosis and emergent opportunities in treatment and surveillance programs. A brief presentation of new therapeutic options is also presented at the end of the paper.
CASE REPORT
In January 2011, a 53-year-old Brazilian woman was referred for assessment in our Cardiology Clinic and Infectious Diseases Department due to complaints of heart failure and after taking into account her epidemiological background.
She had experienced worsening dyspnea with marked limitation of activity and orthopnea for the last 3 mo, and was currently graded as class III New York Heart Association (NYHA). She denied any gastrointestinal symptoms or constitutional symptoms.
Her comorbidities were diabetes and hypertension. Moreover, in 2002 she was diagnosed with symptomatic (syncopal) sinus node disease, presenting with sinus bradycardia of 40 beats/min and frequent sinus pauses and non-sustained ventricular tachycardia with runs of less than 5 beats. At that time, echocardiography revealed normal left ventricular systolic function and no further relevant changes. A DDD-R permanent pacemaker was then implanted and some years later she was lost to follow-up. She was currently medicated with metformin/sitagliptin, an angiotensin receptor blocker and nitrates.
She had lived in a rural area in the Goiás state of Brazil, located in the central part of the country, until the age of 12 years. Her family house was a typical one of suburban areas in Goiás, with plenty of palm trees in the vicinity. No bed nets were used for protection. She had one blood transfusion during her first delivery (age 24 years). Her mother died when she was 60 years old with the diagnosis of CD. Additionally, she reported two sisters and one aunt living in Brazil, also diagnosed with CD recently, and her father with the diagnosis of gastrointestinal CD. She had been living in Portugal for the last 10 years.
On physical examination, a systolic grade II/VI murmur and basal rales were found. Her electrocardiography (ECG) revealed atrial pacing and ventricular sensing with normal QRS morphology and duration alongside frequent ventricular premature beats. On chest X-ray cardiomegaly could be easily spotted. Transthoracic echocardiogram showed a dilated (59 mm diastolic diameter/1.62 m2 body surface = 36.42 mm/m2) and hypokinetic LV was found. Ejection fraction was estimated to be 35% using Simpson method and a grade II-III/IV mitral regurgitation was found (Figure 1). On 24-h Holter monitoring, occasional non-sustained ventricular tachycardia runs (4 beats) were found. She had a normal coronary angiogram and an elevated (28 mmHg) mean pulmonary artery pressure was recorded on catheterization. Left ventricle (LV) angiogram confirmed a dilated LV with generalized hypokinesia, blood stasis and grade III mitral regurgitation.
Figure 1.
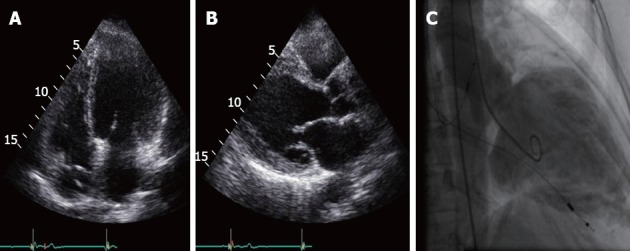
Transthoracic echocardiogram: apical 4-chamber view (A), parasternal long-axis (B); and (C) left ventricle angiogram showing a dilated and aneurysmatic left ventricle (36.37 mm/m2 end diastolic and 31.44 mm/m2 end systolic diameter) with generalized hypokinesia.
A myocardial biopsy was performed and showed plenty of pseudocysts full of amastigotes compatible with T. cruzi without inflammatory infiltrate (Figure 2). Anti-T. cruzi antibodies were detected by the indirect immunofluorescent antibody test (IFAT; MarDX Diagnostics, Inc.) and by enzyme-linked immunosorbent assay (ELISA; bioELISA Recombinant antigens V 3.0). Polymerase chain reaction (PCR) for T. cruzi was not performed at this stage.
Figure 2.
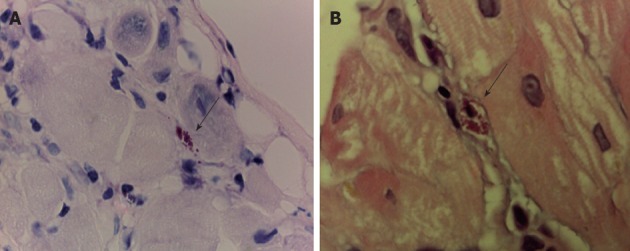
T. cruzi pseudocyst (arrow). A: Heart muscle, Giemsa staining, 100×; B: Heart muscle, hematoxylin-eosin staining, 100×.
She was implanted with a cardioversor defibrillator. A beta-blocker, warfarin and amiodarone were added to her usual medication.
Her two sons (30 and 31 years old, respectively) were screened for CD using ELISA and IFAT and they were both negative. The patient started antitrypanosomal therapy with nifurtimox 10 mg/kg per day, administered orally in four divided doses, for 90 d. Side-effects were significant: 12 kg weight loss, decreased short-term memory, nausea, headache, dizziness and mood changes. Despite this, the patient strictly complied with her treatment. Monitoring of blood count, hepatic enzymes, serum creatinine, and urea was performed before, monthly and at end of treatment. One month after the end of treatment anti-T. cruzi antibodies were still detected by IFAT and ELISA and nested-PCR for T. cruzi was inconclusive.
No improvement was observed in the patient’s ejection fraction, which remained stable at 12-mo follow-up. She is currently graded as NYHA II on regular follow-up at the Cardiology and Infectious Diseases clinics in our hospital. She has been advised not to donate blood or organs in the future and not to undergo further pregnancies due to the risk of transmission.
DISCUSSION
In Portugal, CD is an emerging imported parasitosis. According to the World Health Organization (WHO) Working Group on Chagas disease, there are an estimated 1255 people infected with T. cruzi (prevalence rate of 1%) in Portugal, but only 8 cases have been diagnosed by laboratory testing[1]. Unfortunately, all these cases were lost to follow-up. Our patient is the ninth to be diagnosed. This means an index of underdiagnosis of 99.4%. The index of underdiagnosis of the other eight European countries that participated in that surveillance study shows that, in general, between 95% and 96% of expected cases are not diagnosed[1]. This enormous percentage is probably due to illegal immigrants and due to the fact of limited experience in the detection and management of CD of most European health professionals.
In the Northern Goiás State of Brazil, where our patient was born, the presence of triatomides in dwellings or evidence of triatomide colonization was found to be statistically correlated with seropositivity in children[5]. Our case report could be a vector-borne transmission supported by the very typical correlation with mud houses, such as the one inhabited by our patient during her childhood. Less likely, it could have been a blood-borne infection due to transfusion. It seems unlikely to be a congenital transmission since the mortality rate among such infected children is very high, mainly due to acute meningoencephalitis and myocarditis, and this transmission is greatly related to abortion and low-birth weight[6].
The most frequent ECG finding in CD individuals is the presence of right bundle branch block alone or in association with left anterior fascicular block. Other ECG changes are also frequent, such as atrial and ventricular premature beats, intraventricular or atrioventricular conduction disturbances, and primary ST-T wave changes[7]. Sinus node disease, as an early presentation in a patient with no extensive myocardial involvement (and subsequent LV systolic function compromise), may suggest an abnormality in the innervation of the sinus node[8]. A 10% to 45% prevalence of sick sinus disease according to the level (more prevalent in advanced forms) of chagasic cardiomyopathy has been reported[9]. Other authors have reported prevalence rates ranging from 26.8% (in subjects with normal LV systolic function) to 83.3% in those having compromise[10]. Auto-antibodies resulting from a molecular mimicry mechanism may also be involved in the pathogenesis of sick sinus disease[10]. Sinus bradycardia and chronotropic incompetence are therefore common in these patients. In more advanced disease states, marked atrial remodeling and dilatation alongside atrial arrhythmias may also occur[11].
Congestive heart failure and sudden cardiac death are the main causes of death among chagasic patients. The average prevalence of malignant arrhythmias in these patients is unknown, but risk factors have been identified: regional contractile abnormalities and mild left ventricle dysfunction[12], apical lesions[13], moderate or severe LV systolic dysfunction[14], exercise-induced ventricular arrhythmia[15], non-sustained ventricular arrhythmia[16], New York Heart Association functional class III-IV and absence of β-blocker treatment[17].
According to the I Latin American guidelines for the diagnosis and treatment of Chagas cardiomyopathy[18], the patient’s drug therapy would have the following classes of recommendation and levels of evidence: angiotensin receptor blocker (I-C); β-blocker, oral anticoagulants (I-C); amiodarone (I-B); and nitrate (IIa-C). Further therapy can be introduced in the future if the patient develops congestive symptoms and deteriorates into III or IV NYHA class: spironolactone (I-B); diuretic (I-C) for congestive symptoms; and digitalis (IIa-C).
Digitalis, angiotensin-converting enzyme inhibitors[19] and beta-blockers[20] have proven to be useful in reducing neurohormonal activation in patients with advanced Chagas cardiomyopathy.
The implantable cardiac defibrillator (ICD) has proven to be effective in terminating life-threatening arrhythmias, in a registry including mostly patients under secondary prevention[21]. These patients have a very high incidence of electrical storms, reaching 15.7% in an ICD registry[21].
Patients should be selected for this type of therapy according to the international guidelines[22]. However, some authors propose that patients with non-sustained ventricular arrhythmia without straight indication for ICD should be referred for programmed ventricular stimulation[23]. Radiofrequency ablation is another option for treating these patients, but sometimes an epicardial approach may be necessary in order to more effectively target the arrhythmia substrate[24].
According to the scoring system defined by Rassi et al[25], this patient would be assigned 15 points based on: NYHA III class (5 points), cardiomegaly on chest X-ray (5 points), LV systolic dysfunction on echocardiography (3 points) and non-sustained ventricular tachycardia on 24-h Holter monitoring (2 points). This score puts her in the high-risk category (risk of death in the next 10 years of 84%).
PCR for T. cruzi was not performed initially because serology is the gold-standard for diagnosis of chronic CD[26,27], despite being affected by cross-reactions with antibodies induced by other parasites, namely Leishmania and T. rangeli[28]. Due to this, two different serologic tests were performed to confirm the results. PCR is used for post-treatment follow-up to look for failure of therapy in achieving parasitological response, but its variability in sensitivity may be explained by the intermittent presence and quantity of circulating parasites at the time of blood collection[29].
Our patient was not cured as antibody titres did not decrease significantly and did not become negative. However, this result is often not observed until 8 to 10 years post-treatment and then only in approximately 15% of treated adult subjects[27]. The main impact of the great latent time until the diagnosis of CCC is the lack of efficacy of the two approved drugs for antitrypanosomal therapy in chronically-infected patients. Conversely, in acute disease the cure rate is reported to be around 85%[30] and in congenital transmission it can reach 100%[6]. It is clear that efficacy declines markedly with the duration of infection[3]. Uncertainty remains regarding the degree of efficacy, mainly because of the lack of a reliable test of cure, as explained above.
The reason why this patient was treated with nifurtimox was due to WHO stock shortage of benznidazole, which seems to be a better tolerated drug[30]. The Benznidazole Evaluation for Interrupting Trypanosomiasis, a large, multicenter, double-blind, randomized, placebo-controlled trial of benznidazole for patients with mild-to-moderate CCC, is ongoing and the results will not be known until end of 2012[31]. Despite most physicians often being reluctant to treat patients with CCC who are above 50 years of age because of the frequent side effects, the inability to confirm cure conclusively, and lack of efficacy of the two currently available drugs, some authors have shown a reduction in the rate of progression toward advanced cardiopathy compared to untreated patients[3,4,30,31]. It is obvious that new treatments are urgently needed, and the antifungal triazoles, namely posaconazole, have demonstrated potential for therapeutic switching[3,30,32]. Unfortunately, only one clinical case has been published of a previous treatment failure with benznidazole and subsequent successful treatment with posaconazole using PCR as follow-up[3].
In the face of relative unsuitability of Chagas patients for heart transplant due to the risks of increased parasitemia when under immunosuppression, the possibility of using stem cells to treat chagasic cardiomyopathy has been viewed with enthusiasm[33].
In 2004, Soares et al[34] demonstrated that intravenous injection of bone marrow cells into chronic chagasic mice resulted in migration to the heart and a significant reduction in inflammatory infiltrates and interstitial fibrosis. Guarita-Souza et al[35] have administered autologous skeletal myoblasts and mesenchymal stem cells from bone marrow to Wistar rats and observed a significant improvement in LV ejection fraction and reduction in LV end-systolic and end-diastolic function. Similar changes were found by another group using bone marrow cells in mice[36]. It has been recently proposed that the beneficial immunomodulatory effect of this therapy may be related to transcriptomic recovery, a measure of the genes that are either up- or downregulated by the presence of the disease[37]. The first clinical trial was conducted by the group of Vilas-Boas and colleagues in 28 patients using autologous bone marrow cells. A significant improvement during an 180-d follow-up was observed in LV ejection fraction (20.1% ± 6.8% to 28.3% ± 7.9%; P < 0.03), NYHA functional class (3.1 ± 0.3 to 1.8 ± 0.5; P < 0.001), Minnesota quality of life questionnaire (50.9 ± 11.7 to 25.1 ± 15.9; P < 0.001) and 6-min walk test[38]. Nevertheless, a larger randomized, double-blind, placebo-controlled clinical trial is needed.
Chagas disease is associated with heart failure, the presence of left ventricular apical aneurysm, left atrial dysfunction, cardiac arrhythmias, a proinflammatory status and several other factors that predispose to stroke[39,40]. Furthermore, the presence of Chagas disease is in itself an independent risk factor for stroke[41]. The possible existence of a prothrombotic disease state is still controversial[42,43]. Sometimes, stroke can be the first sign of Chagas disease in asymptomatic patients or in those with mild systolic dysfunction[44]. Recurrence is estimated to be around 20%, so secondary prevention is recommended[39]. No randomized studies have been conducted so far regarding the role of anticoagulation for primary prevention of thromboembolism in Chagas disease. However, Sousa et al[45] have developed a cardioembolic risk score including 4 variables: systolic dysfunction (2 points), apical aneurism (1 point), primary alteration on ventricular repolarization (1 point) and age > 48 years (1 point). According to this score, our patient would achieve 4 points, placing her under a risk category that has an annual stroke rate of 4.4% and therefore has benefit from oral anticoagulation. The decision to use aspirin or warfarin to prevent thromboembolism in these patients remains an open and challenging question[39].
Another interesting issue regarding chagasic therapy is the role of supplementation with selenium (Se), which is an essential micronutrient and an antioxidant at the cellular level[46]. Data from Souza et al[47] indicate that Se treatment prevents right ventricular chamber increase and thus can be proposed as an adjuvant therapy for cardiac alterations already established by T. cruzi infection. Moreover, this author had previously demonstrated that a Se-deficient diet contributed to an increased susceptibility to this infection in experimental models and higher mortality during T. cruzi infection[48], whereas oral Se supplementation at low doses alleviated heart damage[49].
The index of underdiagnosis estimated for eight European countries with culture and linguistic proximity to Latin American countries is a good indicator of the limited epidemiological impact of CD in the context of European health and surveillance systems. Since subjects with CD have low economic resources, the pharmaceutical companies are not particularly interested in supporting research and development of new drugs. Delay in diagnosis may result in progression of cardiac disease. New therapeutic options are promising a better future for CD, but their efficacy and safety still need to be tested. The priority should be the implementation of screening programs for target populations (women of childbearing age and a high suspicion level in young patients with heart failure or dysrhythmias at risk of having been infected earlier in endemic countries) and the training of professionals in the detection of possible cases, as well as implementation of rigorous protocols for blood and organ donation, are essential to limit the impact of Chagas disease in countries where there is no vector transmission.
ACKNOWLEDGMENTS
The authors would like to thank Olga Matos, PhD, for technical assistance.
Footnotes
Peer reviewers: Jacob Joseph, MBBS, MD, Associate Professor of Medicine, Boston University School of Medicine,VA Boston Healthcare, Cardiology Section, 1400 VFW Parkway, West Roxbury, MA 02132, United States; Pietro Amedeo Modesti, MD, PhD, Professor of Internal Medicine, Department Critical Care Medicine, University of Florence, Viale Morgagni 85, 50124 Florence, Italy
S- Editor Cheng JX L- Editor Logan S E- Editor Li JY
References
- 1.Basile L, Jansa JM, Carlier Y, Salamanca DD, Angheben A, Bartoloni A, Seixas J, Van Gool T, Canavate C, Flores-Chavez M, et al. Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill. 2011;16:pii 19968. [PubMed] [Google Scholar]
- 2. Available from: www.who.int/neglected_diseases/2010report/en/
- 3.Pinazo MJ, Espinosa G, Gállego M, López-Chejade PL, Urbina JA, Gascón J. Successful treatment with posaconazole of a patient with chronic Chagas disease and systemic lupus erythematosus. Am J Trop Med Hyg. 2010;82:583–587. doi: 10.4269/ajtmh.2010.09-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rassi A, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 5.Telleria J, Tibayrenc M. American Trypanosomiasis: Chagas Disease One Hundred Years of Research (Elsevier Insights). 1st ed. Amsterdam : Elsevier. 2010:531–532. [Google Scholar]
- 6.Flores-Chávez M, Faez Y, Olalla JM, Cruz I, Gárate T, Rodríguez M, Blanc P, Cañavate C. Fatal congenital Chagas’ disease in a non-endemic area: a case report. Cases J. 2008;1:302. doi: 10.1186/1757-1626-1-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elizari MV, Chiale PA. Cardiac arrhythmias in Chagas’ heart disease. J Cardiovasc Electrophysiol. 1993;4:596–608. doi: 10.1111/j.1540-8167.1993.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 8.Caeiro T, Iosa D. Chronic Chagas’ disease: possible mechanism of sinus bradycardia. Can J Cardiol. 1994;10:765–768. [PubMed] [Google Scholar]
- 9.Carrasco HA, Mora R, Inglessis G, Contreras JM, Marval J, Fuenmayor A. [Study of sinus node function and atrioventricular conduction in patients with chagas disease (author’s transl)] Arch Inst Cardiol Mex. 1982;52:245–251. [PubMed] [Google Scholar]
- 10.Altschüller MB, Pedrosa RC, Pereira Bde B, Corrêa Filho WB, Medeiros AS, Costa PC, de Carvalho AC. [Chronic Chagas disease patients with sinus node dysfunction: is the presence of IgG antibodies with muscarinic agonist action independent of left ventricular dysfunction?] Rev Soc Bras Med Trop. 2007;40:665–671. doi: 10.1590/s0037-86822007000600014. [DOI] [PubMed] [Google Scholar]
- 11.Benchimol-Barbosa PR, Barbosa-Filho J. Atrial mechanical remodeling and new onset atrial fibrillation in chronic Chagas’ heart disease. Int J Cardiol. 2008;127:e113–e115. doi: 10.1016/j.ijcard.2007.04.103. [DOI] [PubMed] [Google Scholar]
- 12.Terzi FV, Siqueira Filho AG, Nascimento EM, Pereira Bde B, Pedrosa RC. [Regional left ventricular dysfunction and its association with complex ventricular arrhythmia, in chagasic patients with normal or borderline electrocardiogram] Rev Soc Bras Med Trop. 2010;43:557–561. doi: 10.1590/s0037-86822010000500017. [DOI] [PubMed] [Google Scholar]
- 13.Gurgel CB, Ferreira MC, Mendes CR, Coutinho E, Favoritto P, Carneiro F. [Apical lesions in Chagas’ heart disease patients: an autopsy study] Rev Soc Bras Med Trop. 2010;43:709–712. doi: 10.1590/s0037-86822010000600022. [DOI] [PubMed] [Google Scholar]
- 14.Sarabanda AV, Marin-Neto JA. Predictors of mortality in patients with Chagas’ cardiomyopathy and ventricular tachycardia not treated with implantable cardioverter-defibrillators. Pacing Clin Electrophysiol. 2011;34:54–62. doi: 10.1111/j.1540-8159.2010.02896.x. [DOI] [PubMed] [Google Scholar]
- 15.Pedrosa RC, Salles JH, Magnanini MM, Bezerra DC, Bloch KV. Prognostic value of exercise-induced ventricular arrhythmia in Chagas’ heart disease. Pacing Clin Electrophysiol. 2011;34:1492–1497. doi: 10.1111/j.1540-8159.2011.03171.x. [DOI] [PubMed] [Google Scholar]
- 16.Silva RM, Távora MZ, Gondim FA, Metha N, Hara VM, Paola AA. Predictive value of clinical and electrophysiological variables in patients with chronic chagasic cardiomyopathy and nonsustained ventricular tachycardia. Arq Bras Cardiol. 2000;75:33–47. doi: 10.1590/s0066-782x2000000700004. [DOI] [PubMed] [Google Scholar]
- 17.Flores-Ocampo J, Nava S, Márquez MF, Gómez-Flores J, Colín L, López A, Celaya M, Treviño E, González-Hermosillo JA, Iturralde P. [Clinical predictors of ventricular arrhythmia storms in Chagas cardiomyopathy patients with implantable defibrillators] Arch Cardiol Mex. 2009;79:263–267. [PubMed] [Google Scholar]
- 18.Andrade JP, Marin-Neto JA, Paola AA, Vilas-Boas F, Oliveira GM, Bacal F, Bocchi EA, Almeida DR, Fragata Filho AA, Moreira Mda C, et al. [I Latin American guidelines for the diagnosis and treatment of Chagas cardiomyopathy] Arq Bras Cardiol. 2011;97:1–48. [PubMed] [Google Scholar]
- 19.Khoury AM, Davila DF, Bellabarba G, Donis JH, Torres A, Lemorvan C, Hernandez L, Bishop W. Acute effects of digitalis and enalapril on the neurohormonal profile of chagasic patients with severe congestive heart failure. Int J Cardiol. 1996;57:21–29. doi: 10.1016/s0167-5273(96)02776-3. [DOI] [PubMed] [Google Scholar]
- 20.Dávila DF, Angel F, Arata de Bellabarba G, Donis JH. Effects of metoprolol in chagasic patients with severe congestive heart failure. Int J Cardiol. 2002;85:255–260. doi: 10.1016/s0167-5273(02)00181-x. [DOI] [PubMed] [Google Scholar]
- 21.Muratore CA, Batista Sa LA, Chiale PA, Eloy R, Tentori MC, Escudero J, Lima AM, Medina LE, Garillo R, Maloney J. Implantable cardioverter defibrillators and Chagas’ disease: results of the ICD Registry Latin America. Europace. 2009;11:164–168. doi: 10.1093/europace/eun325. [DOI] [PubMed] [Google Scholar]
- 22.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 23.Bestetti RB, Cardinalli-Neto A. Sudden cardiac death in Chagas’ heart disease in the contemporary era. Int J Cardiol. 2008;131:9–17. doi: 10.1016/j.ijcard.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 24.Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 25.Rassi A, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, Rassi GG, Hasslocher-Moreno A, Sousa AS, Scanavacca MI. Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med. 2006;355:799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 26.Rassi A, Rassi A, Franco-Paredes C. A Latin American man with palpitations, dizziness, episodes of nonsustained ventricular tachycardia, and an apical aneurysm. PLoS Negl Trop Dis. 2011;5:e852. doi: 10.1371/journal.pntd.0000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viotti R, Vigliano C, Alvarez MG, Lococo B, Petti M, Bertocchi G, Armenti A, De Rissio AM, Cooley G, Tarleton R, et al. Impact of aetiological treatment on conventional and multiplex serology in chronic Chagas disease. PLoS Negl Trop Dis. 2011;5:e1314. doi: 10.1371/journal.pntd.0001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deborggraeve S, Coronado X, Solari A, Zulantay I, Apt W, Mertens P, Laurent T, Leclipteux T, Stessens T, Dujardin JC, et al. T. cruzi OligoC-TesT: a simplified and standardized polymerase chain reaction format for diagnosis of Chagas disease. PLoS Negl Trop Dis. 2009;3:e450. doi: 10.1371/journal.pntd.0000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Mejia Jaramillo AM, Cura C, Auter F, Veron V, Qvarnstrom Y, et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis. 2011;5:e931. doi: 10.1371/journal.pntd.0000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bern C. Antitrypanosomal therapy for chronic Chagas’ disease. N Engl J Med. 2011;364:2527–2534. doi: 10.1056/NEJMct1014204. [DOI] [PubMed] [Google Scholar]
- 31.Marin-Neto JA, Rassi A, Morillo CA, Avezum A, Connolly SJ, Sosa-Estani S, Rosas F, Yusuf S. Rationale and design of a randomized placebo-controlled trial assessing the effects of etiologic treatment in Chagas’ cardiomyopathy: the BENznidazole Evaluation For Interrupting Trypanosomiasis (BENEFIT) Am Heart J. 2008;156:37–43. doi: 10.1016/j.ahj.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro I, Sevcsik AM, Alves F, Diap G, Don R, Harhay MO, Chang S, Pecoul B. New, improved treatments for Chagas disease: from the R& amp; D pipeline to the patients. PLoS Negl Trop Dis. 2009;3:e484. doi: 10.1371/journal.pntd.0000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muratore CA, Baranchuk A. Current and emerging therapeutic options for the treatment of chronic chagasic cardiomyopathy. Vasc Health Risk Manag. 2010;6:593–601. doi: 10.2147/vhrm.s8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soares MB, Lima RS, Rocha LL, Takyia CM, Pontes-de-Carvalho L, de Carvalho AC, Ribeiro-dos-Santos R. Transplanted bone marrow cells repair heart tissue and reduce myocarditis in chronic chagasic mice. Am J Pathol. 2004;164:441–447. doi: 10.1016/s0002-9440(10)63134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guarita-Souza LC, Carvalho KA, Woitowicz V, Rebelatto C, Senegaglia A, Hansen P, Miyague N, Francisco JC, Olandoski M, Faria-Neto JR, et al. Simultaneous autologous transplantation of cocultured mesenchymal stem cells and skeletal myoblasts improves ventricular function in a murine model of Chagas disease. Circulation. 2006;114:I120–I124. doi: 10.1161/CIRCULATIONAHA.105.000646. [DOI] [PubMed] [Google Scholar]
- 36.Goldenberg RC, Jelicks LA, Fortes FS, Weiss LM, Rocha LL, Zhao D, Carvalho AC, Spray DC, Tanowitz HB. Bone marrow cell therapy ameliorates and reverses chagasic cardiomyopathy in a mouse model. J Infect Dis. 2008;197:544–547. doi: 10.1086/526793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soares MB, Lima RS, Souza BS, Vasconcelos JF, Rocha LL, Dos Santos RR, Iacobas S, Goldenberg RC, Lisanti MP, Iacobas DA, et al. Reversion of gene expression alterations in hearts of mice with chronic chagasic cardiomyopathy after transplantation of bone marrow cells. Cell Cycle. 2011;10:1448–1455. doi: 10.4161/cc.10.9.15487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vilas-Boas F, Feitosa GS, Soares MB, Pinho-Filho JA, Mota AC, Almeida AJ, Andrade MV, Carvalho HG, Oliveira AD, Ribeiro-dos-Santos R. Bone marrow cell transplantation in Chagas’ disease heart failure: report of the first human experience. Arq Bras Cardiol. 2011;96:325–331. doi: 10.1590/s0066-782x2011005000028. [DOI] [PubMed] [Google Scholar]
- 39.Carod-Artal FJ, Gascon J. Chagas disease and stroke. Lancet Neurol. 2010;9:533–542. doi: 10.1016/S1474-4422(10)70042-9. [DOI] [PubMed] [Google Scholar]
- 40.Mancuso FJ, Almeida DR, Moisés VA, Oliveira WA, Mello ES, Poyares D, Tufik S, Carvalho AC, Campos O. Left atrial dysfunction in chagas cardiomyopathy is more severe than in idiopathic dilated cardiomyopathy: a study with real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2011;24:526–532. doi: 10.1016/j.echo.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Paixão LC, Ribeiro AL, Valacio RA, Teixeira AL. Chagas disease: independent risk factor for stroke. Stroke. 2009;40:3691–3694. doi: 10.1161/STROKEAHA.109.560854. [DOI] [PubMed] [Google Scholar]
- 42.Pinazo MJ, Tàssies D, Muñoz J, Fisa R, Posada Ede J, Monteagudo J, Ayala E, Gállego M, Reverter JC, Gascon J. Hypercoagulability biomarkers in Trypanosoma cruzi -infected patients. Thromb Haemost. 2011;106:617–623. doi: 10.1160/TH11-04-0251. [DOI] [PubMed] [Google Scholar]
- 43.Melo LM, Souza GE, Valim LR, Moreira LF, Damico EA, Rocha TR, Barretto AC, Strunz CM, Bocchi EA, Ramires JA. Study of pro-thrombotic and pro-inflammatory factors in Chagas cardiomyopathy. Arq Bras Cardiol. 2010;95:655–662. doi: 10.1590/s0066-782x2010005000146. [DOI] [PubMed] [Google Scholar]
- 44.Carod-Artal FJ, Vargas AP, Falcao T. Stroke in asymptomatic Trypanosoma cruzi-infected patients. Cerebrovasc Dis. 2011;31:24–28. doi: 10.1159/000320248. [DOI] [PubMed] [Google Scholar]
- 45.Sousa AS, Xavier SS, Freitas GR, Hasslocher-Moreno A. Prevention strategies of cardioembolic ischemic stroke in Chagas’ disease. Arq Bras Cardiol. 2008;91:306–310. doi: 10.1590/s0066-782x2008001700004. [DOI] [PubMed] [Google Scholar]
- 46.Jelicks LA, de Souza AP, Araújo-Jorge TC, Tanowitz HB. Would selenium supplementation aid in therapy for Chagas disease? Trends Parasitol. 2011;27:102–105. doi: 10.1016/j.pt.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Souza AP, Jelicks LA, Tanowitz HB, Olivieri BP, Medeiros MM, Oliveira GM, Pires AR, Santos AM, Araújo-Jorge TC. The benefits of using selenium in the treatment of Chagas disease: prevention of right ventricle chamber dilatation and reversion of Trypanosoma cruzi-induced acute and chronic cardiomyopathy in mice. Mem Inst Oswaldo Cruz. 2010;105:746–751. doi: 10.1590/s0074-02762010000600003. [DOI] [PubMed] [Google Scholar]
- 48.de Souza AP, Melo de Oliveira G, Nève J, Vanderpas J, Pirmez C, de Castro SL, Araújo-Jorge TC, Rivera MT. Trypanosoma cruzi: host selenium deficiency leads to higher mortality but similar parasitemia in mice. Exp Parasitol. 2002;101:193–199. doi: 10.1016/s0014-4894(02)00134-0. [DOI] [PubMed] [Google Scholar]
- 49.de Souza AP, de Oliveira GM, Vanderpas J, de Castro SL, Rivera MT, Araújo-Jorge TC. Selenium supplementation at low doses contributes to the decrease in heart damage in experimental Trypanosoma cruzi infection. Parasitol Res. 2003;91:51–54. doi: 10.1007/s00436-003-0867-9. [DOI] [PubMed] [Google Scholar]


