Abstract
During the primary response, the commitment of the CD8+ T cell to Blimp-1 expression and the terminal differentiation that Blimp-1 induces must be timed so as not to impair the process of clonal expansion. We determined whether the Hippo pathway, which links cell–cell contact to differentiation in other cell lineages, controls Blimp-1 expression. Activating the CD8+ T cell with antigen and IL-2 causes expression of the core Hippo pathway components, including the pivotal transcriptional cofactor Yap. Contact between activated CD8+ T cells induces Hippo pathway-mediated Yap degradation and Blimp-1 expression; a Hippo-resistant, stable form of Yap suppresses Blimp-1 expression. Cytotoxic T lymphocyte antigen 4 (CTLA-4) and CD80 comprise the receptor–ligand pair that mediates contact-dependent Hippo pathway activation. In vivo, CD8+ T cells expressing Hippo resistant-Yap or lacking CTLA-4 have diminished expression of the senescence marker, KLRG1, during a viral infection. The CTLA-4/Hippo pathway/Blimp-1 system may couple terminal differentiation of CD8+ T cell with the magnitude of clonal expansion.
During the first week of a viral infection, virus-specific CD8+ T cells must coordinate two interdependent but mutually exclusive processes: rapid replication to generate the precursors of effector cells and development of effector cells from these precursors. These two cellular responses must be appropriately timed, because full effector differentiation, so-called terminal differentiation, is associated with the diminution or loss of replicative function. If terminal differentiation occurs too early during the process of clonal expansion, then paradoxically, the rate at which effector cells would be generated would be insufficient for host defense. The ability of the CD8+ T cell to suspend terminal differentiation until clonal expansion has occurred is documented by the observation that CD62Llo, CD127lo CD8+ T cells specific for Listeria monocytogenes did not appear until day 4 postinfection (1). Conversely, inappropriate delay of terminal differentiation would also impair control of an infection.
Recent studies that have focused on identifying the determinants of terminal differentiation of the CD8+ T cell have advanced our understanding of this developmental step. The transcriptional repressor, Blimp-1, and the T-box transcription factors, T-bet (2) and Eomesodermin (Eomes) (3), have been shown to regulate the differentiation of effector CD8+ T cells. Absence of Blimp-1 is associated with virus-specific CD8+ T cells having impaired cytolytic function, lower expression of the senescence-associated marker killer cell lectin-like receptor G1 (KLRG1), and enhanced development of the central memory phenotype (4, 5). The acquisition of effector functions [but maintenance of replicative function in both CD4+ (6) and CD8+ (7) T cells] is consistent with the capacity of Blimp-1–deficient CD8+ T cells to express IFN-γ and cytolytic activities, although at suboptimal levels. These loss-of-function studies (4, 5) indicate that Blimp-1 is required for terminal differentiation by the activated CD8+ T cell, which it for two other lymphocytic lineages, the B (8) and NK (9) cells. T-bet promotes Blimp-1 expression in the natural killer (NK) cell (9), and a similar role may be expected in the CD8+ cell, because T-bet– (2) and Blimp-1–deficient (4, 5) CD8+ T cells have similar differentiation phenotypes. Moreover, in vitro studies have shown that T-bet expression enables signaling by the IL-2 receptor to induce Blimp-1 transcription (10). Although T-bet levels are always higher in terminally differentiated, KLRG1+ cells than at other stages of differentiation, it may be that the ratio of T-bet and Eomes is also critical (11). Eomes shares with T-bet a capacity to mediate the development of effector function (12), but it differs from T-bet in being necessary for the development of memory CD8+ T cells having secondary proliferative capability (3) in response to the mammalian homolog of the target of rapamycin (mTOR) (13) or T-cell factor (TCF-1) (14) signaling.
The extracellular signals that regulate the expression of these transcription factors include antigen and the cytokines IL-2, IL-12, IL-21, and IL-27 (15–19). The inflammatory cytokines, IL-12 and IL-27, are produced by dendritic cells and cells of the innate immune system that have been stimulated by microbial products, whereas IL-2 and IL-21 are secreted by antigen-stimulated CD4+ and CD8+ T cells. Although both types of cytokines may increase during the course of an infection and therefore, provide temporal elements by which the CD8+ T cell could time its commitment to terminal differentiation, these signals also would be coupled to the extent of microbial replication, which may not reflect the magnitude of clonal expansion. IL-2, acting in a paracrine manner, could meet the requirement of being directly correlated with the number of antigen-stimulated T cells, but this cytokine has dual functions in promoting the development of both terminally differentiated effector cells and replication-competent memory cells (20–24). Thus, neither inflammatory- nor T-cell–derived cytokines are likely to be the ultimate determinants of Blimp-1 expression and terminal differentiation.
In multiple cell types of invertebrates and vertebrates, terminal differentiation is directed by a highly conserved developmental system, termed the Hippo pathway. The Hippo pathway is triggered when replicating cells come into contact with each other, enabling a receptor–ligand pair to activate a serine/threonine kinase cascade (25). This activation results in the phosphorylation and proteosomal degradation of the transcriptional cofactor, Yap, which permits differentiation to proceed (26). A potential role for the Hippo pathway in regulating the expression of Blimp-1 and Eomes is suggested by the independent observations that Blimp-1 and the Hippo pathway, respectively, are required for the normal differentiation of skin (27, 28) and that Yap, by interacting with the transcription factor TEA domain (TEAD), induces Eomes expression in trophoectoderm (29, 30). Because cell–cell contact triggers the Hippo pathway, the likelihood of which would be proportional to the relative frequency of the interacting cells, this pathway would fulfill the hypothetical requirement of linking the magnitude of clonal expansion by activated CD8+ T cells to their commitment to terminal differentiation. Accordingly, we have examined the role of the Hippo pathway in regulating Blimp-1 expression by the CD8+ T cell.
Results
Kinase Cascade of the Hippo Pathway Is Assembled by the Activated CD8+ T Cell and Regulates the Expression of Blimp-1.
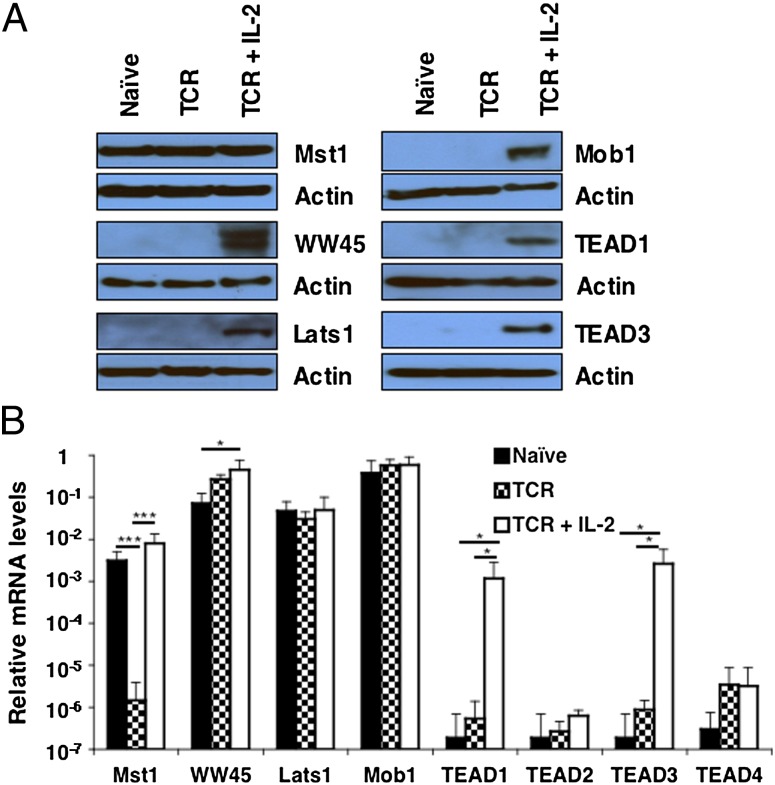
Purified OT-I cells were incubated with SIINFEKL peptide alone or IL-2 for 24 h and then assessed for expression of the components of the Hippo pathway. Resting CD8+ T cells expressed only Mst1 protein, although mRNA for WW45, Lats1, and Mob1 was detected. The presence of WW45, Lats1, and Mob1 protein required signaling both through the T-cell receptor (TCR) and IL-2 receptor, indicating IL-2–dependent posttrancriptional regulation of these components. Transcription and translation of TEAD1 and TEAD3, two transcription factors of the Hippo pathway, occurred in cells receiving both TCR and IL-2 receptor signals (Fig. 1).
Fig. 1.
Expression of core components of the Hippo pathway by OT-I cells. (A) Immunoblot analysis of components of the Hippo pathway in whole-cell lysates prepared from naïve OT-I cells and OT-I cells stimulated for 24 h with 0.1 nM SIINFEKL peptide alone or with IL-2 (TCR + IL-2). (B) Bar graph showing the mRNA levels of these components in identically treated samples of OT-I cells relative to those levels for CD3ε (given an arbitrary level of one). *P < 0.05; ***P < 0.001. Data presented are the mean ± SEM (n = 3).
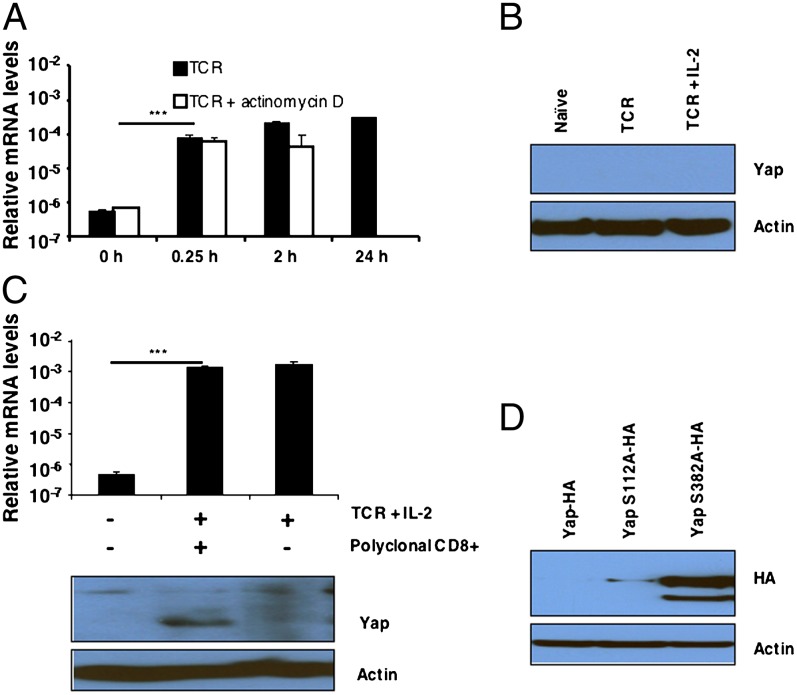
The expression of Yap was more complex insofar as stimulating OT-I cells with TCR and IL-2 induced the rapid appearance of mRNA but not the protein (Fig. 2 A and B). The appearance of Yap mRNA after only 15 min TCR stimulation suggested that it was released from a preformed pool, which was consistent with actinomycin D having no inhibitory effect (Fig. 2A). We considered whether the absence of Yap protein in antigen and IL-2–stimulated cells could be caused by its degradation secondary to contact between activated cells. Adding resting, polyclonal naïve CD8+ T cells to interfere with this process permitted the detection of Yap in the activated OT-I cells (Fig. 2C). Yap mRNA in the recovered CD8+ T cells was not increased by the addition of naïve T cells, suggesting that the appearance of Yap protein reflected its stabilization rather than increased production (Fig. 2C). To show that the absence of Yap in contacting activated CD8+ T cells was caused by its phosphorylation at serine-382 of the phosphedegron domain, we transduced activated OT-I cells with hemagglutin (HA) -tagged WT Yap-HA, Yap S112A-HA, in which the serine mediating cytoplasmic retention after phosphorylation was mutated to alanine, and Yap S382A-HA, respectively. After additional culture of the transduced cells for 48 h in IL-2, only Yap in which serine-382 had been substituted with alanine was present (Fig. 2D). Therefore, as in other cell types, contact between activated CD8+ T cells triggers the serine–kinase cascade that leads to phosphorylation and degradation of Yap.
Fig. 2.
The regulation of Yap expression in OT-I cells. (A) Bar graph showing the mRNA levels, relative to the levels for CD3ε, for Yap in naive OT-I cells and OT-I cells stimulated for timed intervals with 0.1 nM SIINFEKL peptide in the absence or presence of actinomycin D. Data presented are the mean ± SEM. (B) Immunoblot analysis of Yap in whole-cell lysates prepared from naïve OT-I cells and OT-I cells stimulated for 24 h with 0.1 nM SIINFEKL peptide alone (TCR) or together with IL-2 (TCR + IL-2). (C) Levels of Yap protein (Lower) and Yap mRNA levels (Upper) in whole-cell lysates prepared from naïve Thy1.2+ OT-I cells and Thy1.2+ OT-I cells that had been stimulated with SIINFEKL peptide and IL-2 for 24 h alone or in the presence of 10-fold excess of nonactivated Thy1.1+ C57BL/6 CD8+ T cells. The Thy1.2+ OT-I cells were recovered by MACS purification, and Yap protein and mRNA were assessed. Data presented are the mean ± SEM. (D) Immunoblot analysis using antihemagglutin (anti-HA) of whole-cell lysates prepared from retrovirally transduced OT-I cells that expressed ectopic WT Yap tagged with HA (Yap-HA) and Yap in which serine 112 had been mutated to alanine (Yap S112A-HA) or serine-382 had been mutated to alanine (Yap S382A-HA). Lysates were cultured for 48 h with IL-2. ***P < 0.001.
CTLA-4–CD80 Is the Receptor–Ligand Pair Mediating Activation of the Hippo Pathway.
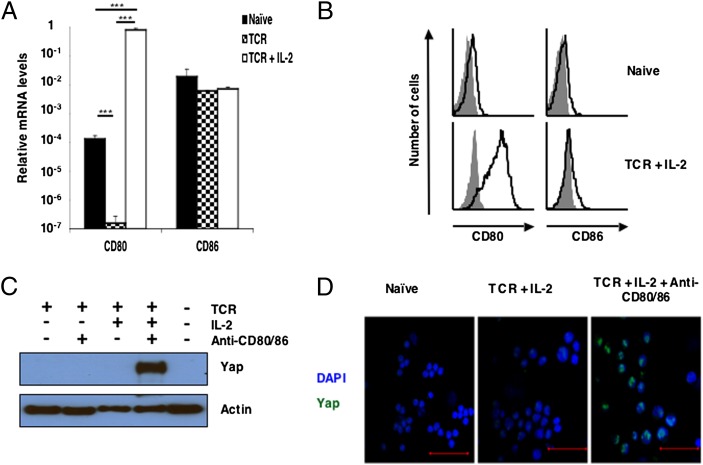
The receptor and its ligand that triggers the Hippo pathway are expressed by activated but not resting CD8+ T cells (Fig. 2C). Among the membrane proteins induced by activation of the CD8+ T cell are CD80 and its receptor, CTLA-4. We assessed the requirements for the expression of the preferred ligand for CTLA-4, CD80 (31), by culturing OT-I cells with SIINFEKL peptide alone and combined with IL-2. Although a low level of CD80 transcription and protein was found in the naïve, resting CD8+ T cell, TCR and IL-2 signaling increased CD80 mRNA by more than 1,000-fold, leading to robust expression of CD80 protein (Fig. 3 A and B). The other potential ligand for CTLA-4, CD86, was relatively unaffected by T-cell activation. The possibility that CD80 and CD86 had a role in activation of the Hippo pathway was examined by including blocking antibodies to these proteins in cultures of activated OT-I CD8+ T cells. Yap protein was shown by immunoblot analysis in lysates of OT-I cells that had been activated with antigen and IL-2 in the presence of these blocking antibodies but not lysates of similarly activated cells in the absence of anti-CD80/86 (Fig. 3C). Blocking CD80/86 did not lead to the appearance of Yap protein in OT-I cells activated by the TCR alone, indicating that expression of the protein required both TCR and IL-2 stimulation. The stabilization of Yap protein and inhibition of Hippo pathway activation by blocking CD80/86 was confirmed by confocal analysis of the activated CD8+ cells, in which nuclear Yap was shown (Fig. 3D). Because all CD80 in these cultures was on CD8+ T cells, with no contaminating MHC class II-expressing cells (SI Appendix, Fig. S1A), we conclude that Yap stability was controlled by interaction between the activated OT-I cells.
Fig. 3.
The role of CD80/86 in triggering the Hippo pathway during contact between activated CD8+ T cells. (A) Bar graph showing the mRNA levels of CD80 and CD86 relative to the levels of CD3ε in naïve OT-I cells and OT-I cells stimulated for 24 h with 0.1 nM SIINFEKL peptide alone (TCR) or together with IL-2 (TCR + IL-2). Data presented are the mean ± SEM. (B) Naïve and TCR + IL-2–stimulated OT-I cells were also assessed by FACS for membrane expression of CD80 and CD86 (shaded histogram, isotype control; black line, specific antibody). (C) Immunoblot analysis of Yap in cell lysates prepared from naïve OT-I cells or OT-I cells that had been stimulated for 24 h with SIINFEKL peptide in the absence or presence IL-2 and the presence of isotype control antibodies or blocking antibodies to CD80 and CD86. (D) Analysis by confocal microscopy of Yap expression by naïve OT-I cells or OT-I cells that had been stimulated for 24 h with SIINFEKL peptide and IL-2 in the presence of isotype control antibodies or blocking antibodies to CD80 and CD86 (green, anti-Yap; blue, DAPI). (Scale bars: 50 μm.) ***P < 0.001.
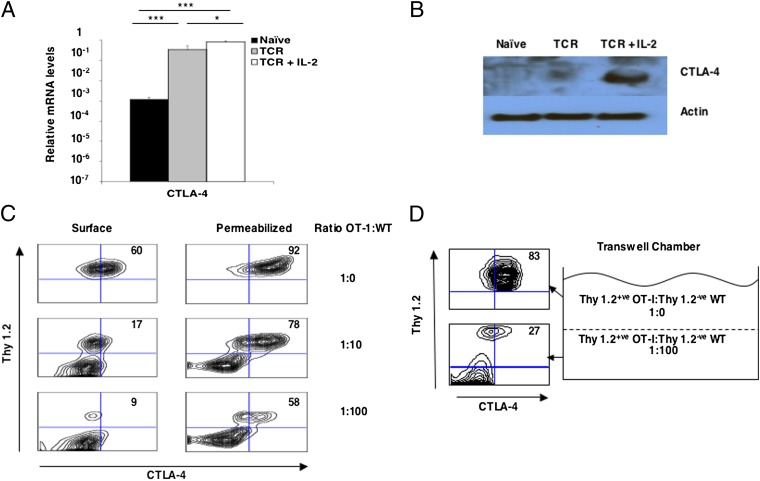
CD80 and CD86 have two receptors, CD28 and CTLA-4. We excluded CD28 from having a role in Hippo pathway activation by showing that its ligation on activated CD8+ T cells, on which CD80/86 had been blocked, did not lead to the loss of Yap protein (SI Appendix, Fig. S1B). We focused, then, on the requirements for the expression at the plasma membrane of the other receptor for CD80, CTLA-4. Although ligation of the TCR on OT-I cells induced a greater than 100-fold increase in CTLA-4 mRNA, optimal CTLA-4 protein, as assessed by immunoblot analysis of cell lysates, required the addition of IL-2 (Fig. 4 A and B). Expression at the plasma membrane of CTLA-4 also is a regulated event and can be suppressed by the addition of nonactivated CD8+ T cells (Fig. 4C). The role of soluble factors secreted by activated CD8+ T cells in regulating plasma membrane expression of CTLA-4 was excluded by the use of transwell chambers. When noncontacting, activated CD8+ T cells were separated by a semipermeable membrane from contacting, activated CD8+ T cells, the former maintained low plasma membrane expression of CTLA-4, despite the latter having high expression (Fig. 4D).
Fig. 4.
Plasma membrane expression of CTLA-4 requires contact between activated CD8+ T cells. (A) Bar graph showing the mRNA levels of CTLA-4 relative to the levels for CD3ε in naïve OT-I cells and OT-I cells stimulated for 24 h with 0.1 nM SIINFEKL peptide alone (TCR) or together with IL-2 (TCR + IL-2). Data presented are the mean ± SEM. (B) Immunoblots showing CTLA-4 and actin protein in lysates of naïve OT-I cells and OT-I cells stimulated for 24 h with 0.1 nM SIINFEKL peptide alone (TCR) or together with IL-2 (TCR + IL-2). (C) FACS analysis of CTLA-4 expression by TCR- and IL-2–stimulated Thy1.2+ OT-I cells that had been cocultured for 30 h alone or with increasing numbers of resting polyclonal Thy1.1+ CD8+ T cells. The numbers represent the proportion of intact and detergent-permeabilized Thy1.2+ cells, respectively, showing specific staining with anti–CTLA-4. (D) FACS analysis of CTLA-4 expression by TCR and IL-2–stimulated Thy1.2+ OT-I cells that had been cultured in the top compartment of a transwell chamber in which the bottom compartment contained activated Thy1.2+ OT-I cells cocultured with 100-fold excess polyclonal CD8+ T cells. The numbers represent the proportion of intact, nonpermeabilized Thy1.2+ cells staining with anti–CTLA-4. *P < 0.05; ***P < 0.001.
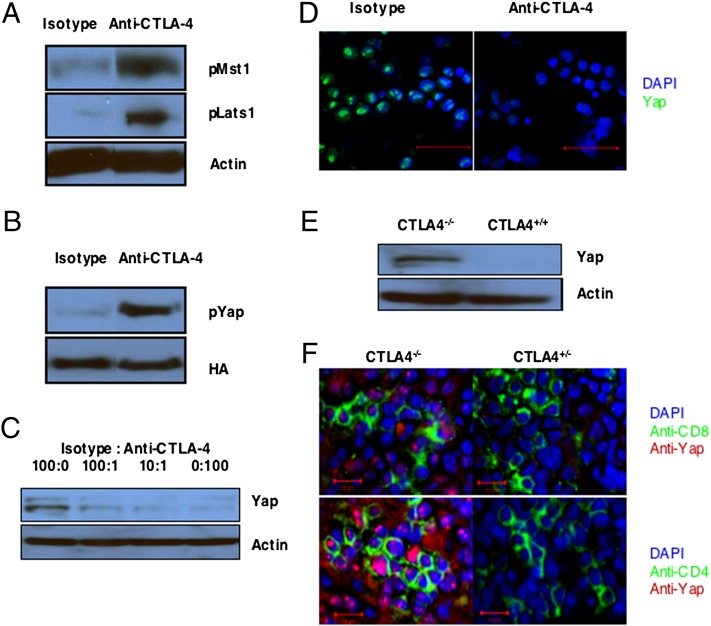
Having determined the conditions for optimal expression of CTLA-4, we could assess whether its specific ligation by beads coated with CTLA-4 antibody activated the Hippo pathway in CD8+ T cells, in which this triggering event was otherwise blocked by anti-CD80/86 antibodies. Ligation of CTLA-4 by this means induced serine and threonine phosphorylation of Mst1 and Lats1, respectively (Fig. 5A). To evaluate phosphorylation of Yap by activated Lats1, we retrovirally introduced the degradation-resistant Yap S382A mutant into CD8+ T cells. Ligation of CTLA-4 by the immobilized anti–CTLA-4 induced phosphorylation of serine-112 in these cells (Fig. 5B). Ligation of CTLA-4 also caused, in a dose–response manner, the loss of Yap protein, which was shown both by immunoblot analysis of cell lysates and confocal analysis of anti-Yap–stained cells (Fig. 5 C and D). The essential role of CTLA-4 in Hippo pathway activation was confirmed with loss-of-function experiments by the use of CTLA-4−/− OT-I cells, which had been purified from healthy, young mice and thus, had a naïve, CD62Lhi phenotype (SI Appendix, Fig. S2). Activation of these cells with antigen and IL-2 did not induce the loss of Yap protein, which was observed with WT OT-I cells (Fig. 5E). The possibility that the inability of CTLA-4−/− T cells to activate the Hippo pathway may contribute to the immunopathology associated with this deficiency is supported by the finding that, in CTLA-4−/− mice, the proliferating CD4+ and CD8+ T cells in lymph nodes showed nuclear Yap protein (Fig. 5F).
Fig. 5.
Activation of the Hippo pathway by CTLA-4 in vitro and in vivo. (A) Immunoblot analysis of lysates from activated OT-I cells cultured in the presence of antibodies to CD80 and CD86 and incubated for 1 h with beads bearing isotype control antibody or antibody to CTLA-4. Blots were developed with antibodies specific for phosphothreonine-183 of Mst1 and phosphoserine-909 of Lats1. Staining of actin is shown as a loading control. (B) Immunoblot analysis with antiphosphoserine-112 of Yap of lysates from activated OT-I cells expressing Yap S382A-HA that had been cultured in the presence of blocking antibodies to CD80 and CD86 and incubated for 1 h with beads bearing isotype control antibody or antibody to CTLA-4. HA staining is shown as a loading control. (C) Immunoblot analysis of WT Yap in cell lysates from OT-I cells activated in the presence of antibodies to CD80 and CD86 and incubated for 3 h with beads bearing various ratios of isotype control antibody and antibody to CTLA-4. Staining of actin is shown as a loading control. (D) Analysis by confocal microscopy of anti-Yap–stained OT-I cells that had been activated in the presence of blocking antibodies to CD80 and CD86 and incubated for 3 h with beads bearing isotype control antibody or antibody to CTLA-4 (green, anti-Yap; blue, DAPI). (Scale bars: 50 μm.) (E) Immunoblot analysis of Yap protein in lysates from CTLA-4−/− OT-I cells and WT OT-I cells that had been activated with SIINFEKL peptide and IL-2 for 24 h. (F) Analysis by confocal microscopy of Yap protein in CD8+ and CD4+ cells from the lymph nodes of CTLA-4−/− and CTLA-4−/+ 16- to 20-d-old mice. (Scale bars: 10 μm.)
Blocking the Hippo Pathway Suppresses CD8+ T-Cell Differentiation.
We assessed the role of the Hippo pathway on the expression of two transcription factors that regulate the differentiation of the CD8+ T cell. Eomes is necessary for the maintenance of the replicative potential of memory CD8+ T cells in secondary responses, and Eomes expression is maintained in activated CTLA-4−/− CD8+ T cells (32). In contrast, Blimp-1 is required for terminal differentiation and replicative senescence of the CD8+ T cell (4, 5). The effects of Hippo pathway activation in other cell types can be prevented by ectopic expression of Yap 5SA, a form of the protein in which the five serine targets of the Lats1/2 kinase have been mutated to alanine. Indeed, the stabilization of endogenous Yap in activated CD8+ T cells by blocking CD80/86 and ectopically introducing Yap 5SA into CD8+ T cells led to comparable intracellular levels of Yap protein (SI Appendix, Fig. S3). Therefore, the finding that ectopic Yap 5SA maintained the levels of Eomes protein (Fig. 6A) and mRNA (SI Appendix, Fig. S4A) in activated CD8+ T cells, without affecting the levels of T-bet mRNA (SI Appendix, Fig. S4B), indicates that the Hippo pathway negatively regulates Eomes, which may impair the replicative potential of the antigen-experienced CD8+ T cell.
Fig. 6.
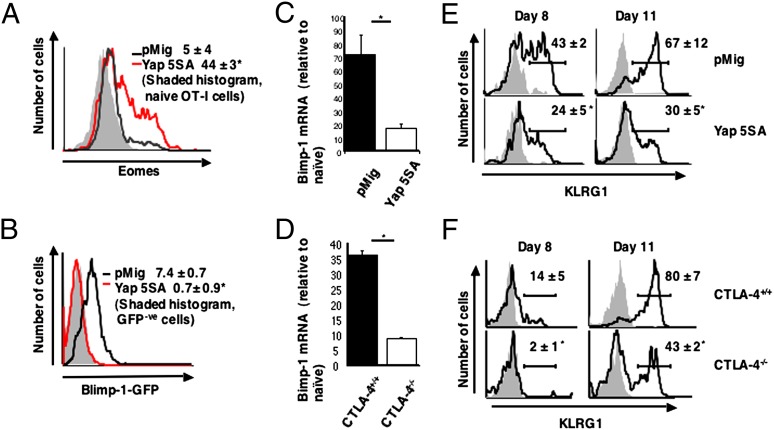
Preventing Hippo pathway activation in CD8+ T cells affects Blimp-1 expression and differentiation. (A) OT-I CD8+ T cells were transduced with the Yap 5SA-expressing retrovirus or the pMig vector control and analyzed for Eomes protein by staining of permeabilized cells with anti-Eomes relative to naïve, unstimulated OT-I cells 16 h after restimulation with IL-2 and SIINFEKL peptide. Numbers represent the Eomes-specific mean fluorescence intensity ± SEM. (B) Prdm1gfp/+ CD8+ T cells that had been transduced with control pMig (black line) or the pMig vector-expressing Yap 5SA (red line) and WT CD8+ T cells transduced with pMig (shaded) were restimulated for 48 h with anti-CD3ε and IL-2 and assessed for GFP fluorescence. Numbers are the GFP-specific mean fluorescence intensities ± SEM. (C and D) OT-I cells transduced with a retrovirus expressing the Lats-resistant Yap 5SA or pMig control vector (C) and CTLA-4+/+ or CTLA-4−/− OT-I cells (D) were analyzed for Blimp-1 mRNA 48 h after restimulation with IL-2 and SIINFEKL peptide. The levels of Blimp-1 mRNA relative to the levels of naïve unstimulated OT-I cells are shown. Data presented are the mean ± SEM. (E and F) Thy1.2+ OT-I cells that had been transduced with the Yap 5SA-expressing retrovirus or control pMig (E), and Thy1.2+ CTLA-4−/− OT-I cells or CTLA-4+/+ OT-I cells (F) were adoptively transferred to Thy1.1+ C57BL/6 mice, and 2 d later, the mice were infected with γ-MHV-68/OVA. On days 8 and 11 postinfection, peripheral blood Thy1.2+ OT-I cells were assessed for expression of KLRG1. Numbers represent the percentage KLRG1+ cells (means ± SEM). *P < 0.05.
We assessed the role of the Hippo pathway in regulating Blimp-1 by the use of Prdm1gfp/+ CD8+ T cells, in which the expression of Blimp-1 is reported by the GFP-modified Prdm1 allele. Ectopically expressing Yap 5SA in these cells repressed the appearance of GFP+ cells after in vitro activation, which contrasts the response of activated cells that had been transduced with the control vector that became GFP+ (Fig. 6B). The capacity of Yap 5SA to suppress Blimp-1 transcription was confirmed by the demonstration of a fourfold lower level of Blimp-1 mRNA in activated CD8+ T cells expressing Yap 5SA than the vector control cells (Fig. 6C).
The capacity of Yap 5SA to maintain Eomes expression and suppress Blimp-1 expression by CD8+ T cells in vitro predicts that it may suppress terminal differentiation of the activated CD8+ T cell in vivo. To assess this prediction, we infected mice with a modified form of murine γ-herpesvirus-68 that expresses ovalbumin (γ-MHV-68/OVA). This virus induces senescent Blimp-1+ cells that can be distinguished from nonsenescent Blimp-1− antigen-experienced cells by their expression of KLRG1 (SI Appendix, Fig. S4C) (33, 34). OT-I cells, which had been transduced with a control vector or retrovirus expressing Yap 5SA, were adoptively transferred into mice that were then infected with γ-MHV-68/OVA. Terminal differentiation of the transferred CD8+ T cells during the peak of the primary response at day 11 was suppressed by Yap 5SA, which was indicated by the diminished expression of KLRG1 (Fig. 6E) and the enhanced expression of CD127 (SI Appendix, Fig. S4D) without a change in the magnitude of their clonal expansion (SI Appendix, Fig. S4E).
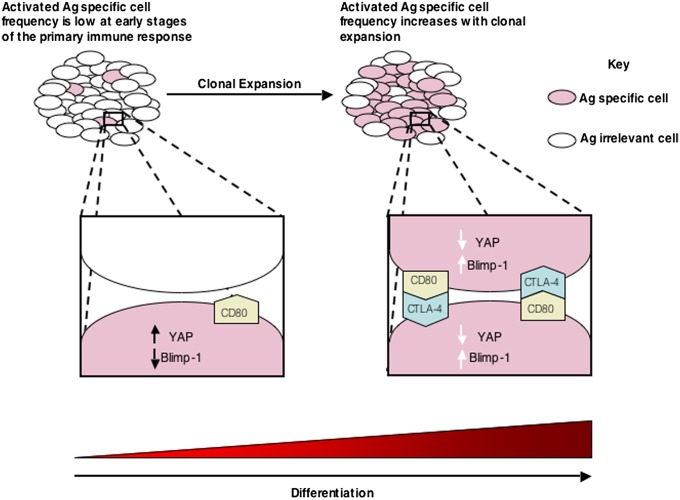
Because the ligation of CTLA-4 triggers the Hippo pathway, CTLA-4−/− T cells should resemble WT T cells expressing Yap 5SA in their response to stimuli that induce Blimp-1. Indeed, activation of the purified, naïve CTLA-4−/− OT-I cell with antigen and IL-2 was associated with diminished expression of Blimp-1 relative to the response of WT OT-I cells (Fig. 6D). Moreover, these cells, after adoptive transfer to WT mice, also had diminished expression of KLRG1 (Fig. 6F) and enhanced expression of CD127 (SI Appendix, Fig. S4F) at the peak of the primary response to γ-MHV68/OVA infection without a change in the magnitude of their clonal expansion (SI Appendix, Fig. S4G). Thus, maintaining the expression of endogenous Yap in the activated CD8+ T cell by deletion of the Hippo pathway receptor, CTLA-4, has the same phenotypic consequences as introduction of the Hippo pathway-resistant Yap 5SA. The present finding that the Hippo pathway links the expression of a gene that mediates differentiation, Blimp-1, to the population density of activated CD8+ T cells is reminiscent of quorum sensing in bacteria and allows us to propose the following model. In the early phase of the immune response, replication will drive clonal expansion, because the low frequency of antigen-specific CD8+ T cells makes contact between them improbable and CTLA-4 is not surface-expressed. Only when replication has increased their numbers will contact between activated cells and triggering of the Hippo pathway be likely to occur, which then will create a steady state wherein the rates of differentiation and replication are balanced.
Discussion
The present study finds that the Hippo pathway provides the activated CD8+ T cell with a means for regulating Blimp-1 expression that is independent of other cell types. Because triggering of the Hippo pathway is mediated by contact between activated CD8+ T cells, this mechanism for controlling Blimp-1 expression would be linked to the frequency of activated CD8+ T cells within a larger population of nonactivated T cells, which would be a measure of the magnitude of clonal expansion in, for example, a lymph node. Thus, the Hippo pathway has the requisite properties to allow replicating CD8+ T cells to select the appropriate time for Blimp-1 expression and terminal differentiation.
The naïve CD8+ T cell differs from other cell types in which the Hippo pathway has been studied in that Mst1 is the only member of the core components of the pathway that is expressed constitutively (Fig. 1A). The need of the naïve CD8+ T cell to express Mst1 is consistent with other findings that Mst1 has biological activities independent of the Hippo pathway (35). WW45, Lats1, Mob1, and Yap were all posttranscriptionally regulated, requiring both antigen and IL-2 for their expression (Figs. 1 and 2), indicating that the growth factors of clonal expansion rapidly establish the core Hippo pathway in the CD8+ T cell. The essential role for IL-2 in the protein translation of the core Hippo components and the CTLA-4/CD80 receptor–ligand pair (Figs. 3 and 4) indicates that IL-2, by inducing both Yap and the serine kinases involved in its degradation and nuclear exclusion, can have the opposing functions of suppressing or permitting Blimp-1 expression. This dual role for IL-2 in the Hippo pathway is reminiscent of its capacity to promote the development of both memory and terminally differentiated CD8+ T cells (20–24).
The Hippo pathway was activated in purified CD8+ T cells that were cultured in the presence of antigen and IL-2, which was indicated by the loss of Yap protein secondary to its phosphorylation at serine-382 (Fig. 2D), indicating that no other cell types were required for this process. The appearance of Yap protein in activated CD8+ T cells after the addition of nonactivated CD8+ T cells (Fig. 2C) suggested that contact between the activated CD8+ T cells triggers the Hippo pathway.
Two independent studies reported that activation of lymphocyte function-associated antigen 1 (LFA-1) in lymphocytes involved Mst1 (36) and CTLA-4 (37), respectively, which raised the possibility that a Hippo core component and this lymphocyte receptor were in a common pathway. A triggering role for CTLA-4 was consistent with the up-regulation of CD80 by antigen- and IL-2–stimulated CD8+ T cells (Fig. 3 A and B), which induced Hippo pathway activation, and the absence of CD80 and CD86 on resting CD8+ T cells, which did not have this function (Fig. 2C). More importantly, blocking antibodies to CD80 and CD86 suppressed Yap degradation in contacting activated CD8+ T cells (Fig. 3 C and D), which identified CD28 and CTLA-4 as the only two candidates for the Hippo pathway receptor. CTLA-4 was formally shown to be a Hippo pathway receptor by the ability of immobilized anti–CTLA-4, an unambiguous ligand for CTLA-4, to induce the phosphorylation of Mst1, Lats1, and Yap (Fig. 5 A and B); the degradation of Yap (Fig. 5 C and D) by the stability of Yap in activated CTLA-4−/− CD8+ T cells in vitro (Fig. 5E) and in vivo (Fig. 5F); and the inability of agonistic anti-CD28 to induce degradation of Yap (SI Appendix, Fig. S1B).
Two interactions between activated CD8+ T cells, therefore, may be required for Hippo pathway activation. The first interaction involves undefined membrane components and leads to CTLA-4 expression at the plasma membrane of the antigen- and IL-2–stimulated cells (Fig. 4 C and D), which may indicate how, during the early phase of clonal expansion when the low frequency of activated CD8+ T cells would make contact between them unlikely, the ligation of CD28 is favored over CTLA-4 by CD80 and CD86 on antigen-presenting cells. The second interaction involves CD80 and possibly, CD86 on one cell and CTLA-4 on the other cell, which initiates the Hippo pathway kinase cascade. Effects of T-cell–T-cell interactions involving CD80 and CTLA-4 have been observed in vivo (38) and suggested previously to induce anergy (39). Furthermore, these activated T-cell–T-cell interactions have been directly visualized in vivo and shown to occur through stable, multifocal synapse-like structures (40) to which the microtubule-organizing complex polarizes. Of interest in this regard is the observation that CTLA-4 localizes to the microtubule-organizing complex (41).
Blocking the transcriptional consequences of Hippo pathway activation by expressing Yap 5SA at physiological levels (SI Appendix, Fig. S3) enhanced expression of Eomes and suppressed expression of Blimp-1 (Fig. 6 A–C), indicating that the Hippo pathway enables contact between activated CD8+ T cells to regulate the expression of these two transcription factors that are involved in terminal differentiation. Two prior studies examining the in vivo phenotype of Blimp-1−/− CD8+ T cells both showed impaired KLRG1 and enhanced CD127 expression, despite having conflicting conclusions regarding secondary memory responses (4, 5). The present finding that Yap 5SA or CTLA-4 deficiency suppresses the development of the terminally differentiated KLRG1+/CD127lo CD8+ T cell during a viral infection (Fig. 6 E and F and SI Appendix, Fig. S4 D and F) is consistent with the ability of Yap 5SA or CTLA-4 deficiency to suppress Blimp-1 expression in vitro.
The relationship between the finding that CTLA-4 is the triggering receptor of the Hippo pathway leading to Blimp-1 expression and the phenotypic characteristics of the CTLA-4−/− mouse may be complex. For example, the CTLA-4−/− mouse has massive lymphoproliferation (42, 43), and maintaining Yap expression in hepatocytes causes an apparently similar phenotype of marked hepatomegaly (25). However, the Yap 5SA-expressing CD8+ T cell and the CTLA-4−/− CD8+ T cell responding to a viral infection showed mainly impaired markers of terminal differentiation (Fig. 6 E and F and SI Appendix, Fig. S4 D and F) and not excessive clonal expansion (SI Appendix, Fig. S4 E and G). The marked lymphoproliferation of CTLA-4−/− mice, however, may reflect the effects of persistent stimulation of effector cells by self-antigen caused by the loss of regulatory function by CD4+ Foxp3+ T cells, whereas control of a viral infection would diminish the availability of antigen and terminate clonal expansion. Furthermore, with regard to whether the primary role of the Hippo pathway is to control proliferation or differentiation, recent reports emphasize the latter function. Overexpression of Yap in murine intestine or chick neural tubes results in loss of differentiated cells (44, 45), and loss of WW45 leads to defective terminal differentiation in skin, intestine, and lung epithelia (46). Our results, moreover, are consistent with a previous study of CTLA-4–deficient CD8+ T cells in mixed bone marrow chimeric mice, which reported no difference in clonal expansion between WT and CTLA-4−/− CD8+ T cells (47, 48); unfortunately, markers of terminal differentiation, such as KLRG1 and CD127, were not evaluated. The relevance of the present studies to the question of the function of CTLA-4 on the CD4+ Foxp3+ T cell may be that, in its absence, there is impaired Hippo pathway-mediated terminal differentiation and acquisition of regulatory function, which has been shown for Blimp-1−/− regulatory T cells (17). At the very least, the present finding of a cell-intrinsic function for CTLA-4 in the activation of a conserved, ancient developmental pathway, when coupled with the recent demonstration of its cell extrinsic function of transendocytosis of CD80 and CD86 (49), is additional evidence of the range of biological activities of CTLA-4 that account for its central role in the immune system.
Materials and Methods
The details of the mice strains used and their maintenance are contained in S1 Materials and Methods. Similarly, the preparation of tissue samples and cell culture, the antibodies used, the retroviral transduction, and the γ-MHV-68/OVA infection are described there. Also included in this section are the protocols fro the qRT-PCR, immunoblot, flow cytometry and confocal assays.
Supplementary Material
Acknowledgments
We thank F. Randow and E. Roberts for their helpful discussions, F. Randow and A. Betz for their review of the manuscript, and L. Walker for providing lymph nodes from CTLA-4 mutant mice. M.B.-W. was supported by Sonderforschungsbereich Grant 854. This research was supported by the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 13152 (volume 109, number 33).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209115109/-/DCSupplemental.
References
- 1.Khanna KM, McNamara JT, Lefrançois L. In situ imaging of the endogenous CD8 T cell response to infection. Science. 2007;318:116–120. doi: 10.1126/science.1146291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee A, et al. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutishauser RL, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 7.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner CA, Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 9.Kallies A, et al. A role for Blimp1 in the transcriptional network controlling natural killer cell maturation. Blood. 2011;117:1869–1879. doi: 10.1182/blood-2010-08-303123. [DOI] [PubMed] [Google Scholar]
- 10.Yeo CJ, Fearon DT. T-bet-mediated differentiation of the activated CD8+ T cell. Eur J Immunol. 2011;41:60–66. doi: 10.1002/eji.201040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi NS, et al. Increased numbers of preexisting memory CD8 T cells and decreased T-bet expression can restrain terminal differentiation of secondary effector and memory CD8 T cells. J Immunol. 2011;187:4068–4076. doi: 10.4049/jimmunol.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearce EL, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 13.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, et al. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol. 2007;178:242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- 16.Pipkin ME, et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cretney E, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 18.Kwon H, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat Immunol. 2011;12:327–334. doi: 10.1038/ni.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obar JJ, et al. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci USA. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalia V, et al. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 24.Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nat Immunol. 2011;12:908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlegelmilch K, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnúsdóttir E, et al. Epidermal terminal differentiation depends on B lymphocyte-induced maturation protein-1. Proc Natl Acad Sci USA. 2007;104:14988–14993. doi: 10.1073/pnas.0707323104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishioka N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Russ AP, et al. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404:95–99. doi: 10.1038/35003601. [DOI] [PubMed] [Google Scholar]
- 31.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Hegel JK, Knieke K, Kolar P, Reiner SL, Brunner-Weinzierl MC. CD152 (CTLA-4) regulates effector functions of CD8+ T lymphocytes by repressing Eomesodermin. Eur J Immunol. 2009;39:883–893. doi: 10.1002/eji.200838770. [DOI] [PubMed] [Google Scholar]
- 33.Voehringer D, et al. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 34.Heffner M, Fearon DT. Loss of T cell receptor-induced Bmi-1 in the KLRG1(+) senescent CD8(+) T lymphocyte. Proc Natl Acad Sci USA. 2007;104:13414–13419. doi: 10.1073/pnas.0706040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi J, et al. Mst1-FoxO signaling protects Naïve T lymphocytes from cellular oxidative stress in mice. PLoS One. 2009;4:e8011. doi: 10.1371/journal.pone.0008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–928. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 37.Schneider H, Valk E, da Rocha Dias S, Wei B, Rudd CE. CTLA-4 up-regulation of lymphocyte function-associated antigen 1 adhesion and clustering as an alternate basis for coreceptor function. Proc Natl Acad Sci USA. 2005;102:12861–12866. doi: 10.1073/pnas.0505802102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor PA, et al. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions [corrections] J Immunol. 2004;172:34–39. doi: 10.4049/jimmunol.172.1.34. [DOI] [PubMed] [Google Scholar]
- 39.Chai JG, Vendetti S, Amofah E, Dyson J, Lechler R. CD152 ligation by CD80 on T cells is required for the induction of unresponsiveness by costimulation-deficient antigen presentation. J Immunol. 2000;165:3037–3042. doi: 10.4049/jimmunol.165.6.3037. [DOI] [PubMed] [Google Scholar]
- 40.Sabatos CA, et al. A synaptic basis for paracrine interleukin-2 signaling during homotypic T cell interaction. Immunity. 2008;29:238–248. doi: 10.1016/j.immuni.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 42.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 43.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 44.Camargo FD, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 45.Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22:3320–3334. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JH, et al. A crucial role of WW45 in developing epithelial tissues in the mouse. EMBO J. 2008;27:1231–1242. doi: 10.1038/emboj.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bachmann MF, et al. Normal pathogen-specific immune responses mounted by CTLA-4-deficient T cells: A paradigm reconsidered. Eur J Immunol. 2001;31:450–458. doi: 10.1002/1521-4141(200102)31:2<450::aid-immu450>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 48.Bachmann MF, et al. Normal responsiveness of CTLA-4-deficient anti-viral cytotoxic T cells. J Immunol. 1998;160:95–100. [PubMed] [Google Scholar]
- 49.Qureshi OS, et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]