Abstract
Formate is a major metabolite in the anaerobic fermentation of glucose by many enterobacteria. It is translocated across cellular membranes by the pentameric ion channel/transporter FocA that, together with the nitrite channel NirC, forms the formate/nitrite transporter (FNT) family of membrane transport proteins. Here we have carried out an electrophysiological analysis of FocA from Salmonella typhimurium to characterize the channel properties and assess its specificity toward formate and other possible permeating ions. Single-channel currents for formate, hypophosphite and nitrite revealed two mechanistically distinct modes of gating that reflect different types of structural rearrangements in the transport channel of each FocA protomer. Moreover, FocA did not conduct cations or divalent anions, but the chloride anion was identified as further transported species, along with acetate, lactate and pyruvate. Formate, acetate and lactate are major end products of anaerobic mixed-acid fermentation, the pathway where FocA is predominantly required, so that this channel is ideally adapted to act as a multifunctional export protein to prevent their intracellular accumulation. Because of the high degree of conservation in the residues forming the transport channel among FNT family members, the flexibility in conducting multiple molecules is most likely a general feature of these proteins.
Keywords: electrophysiology, planar lipid bilayer, formate channel
The formate/nitrite transporter family of integral membrane transport proteins (FNT; transporter class 2.A.44; ref. 1) comprises the formate channel FocA (2), the nitrite channel NirC (3), the formate uptake permease FdhC (4), and the hydrosulfide channel HSC (5). All members of the family facilitate the translocation of anions across a biological membrane in their respective physiological context. NirC imports nitrite, NO2–, into the cytoplasm. It is commonly found in an operon with the cytoplasmic, assimilatory nitrite reductase NirBD (6) that produces ammonium, NH4+, for subsequent assimilation into biomolecules. Enteric bacteria such as Escherichia coli or Salmonella typhimurium also follow this route for the detoxification of peroxynitrite, produced by the inducible NO synthase in macrophages of their eukaryotic hosts as an aspect of the innate immune response (7, 8). A ΔnirC strain of Salmonella was strongly inhibited for intracellular proliferation in spleen, liver, and lymph tissues, which indicates that NirC plays an important role in enterobacterial pathogenesis and may thus be a suitable target for specific, antimicrobial drugs (9). A recent study on NirC reconstituted in proteoliposomes on solid-supported membranes further indicated that the protein can act as a secondary active H+/NO2– antiporter (10). However, NirC would then actively export nitrite under physiological conditions, a finding that is not obviously consistent with published data. The related formate channel FocA attains a very different physiological role, because it is produced during anaerobic mixed-acid fermentation (2). Here, pyruvate:formate lyase (PFL) converts pyruvate, the product of glycolysis, to acetyl-CoA and releases formate (HCOO–) that must be removed from the cytoplasm to avoid intracellular accumulation. The anion is exported via FocA and is then oxidized to CO2 by a periplasmic formate dehydrogenase, FDH-N or FDH-O, resulting in the generation of proton motive force (11–13). In addition, the complex pathway of mixed-acid fermentation produces acetate, ethanol, lactate, and succinate as further end products (Fig. 1A). FocA acts as a passive exporter for formate anions generated in the cytoplasm, but a remarkable functional switch of transport mode was found for this protein when the pH of the growth medium dropped below 6.8 (14). With ample protons available in the periplasm, the cell switches to active import of formate and again uses FocA for the task. Imported formic acid is then disproportionated into CO2 and H2 by the cytoplasmic formate:hydrogen lyase complex (FHL), and hydrogen gas is either released or used as a low potential electron donor through membrane-bound hydrogenases (15). Recently, high-resolution crystal structures were presented for FocA from E. coli (16) and Vibrio cholerae (17) at high pH, and for S. typhimurium at low pH (18). FocA forms stable pentamers with individual transport channels in each protomer (SI Appendix, Fig. S1) and shares a remarkable structural homology to the (tetrameric) aquaporin and glyceroporin channels (16, 19). The high-pH forms represent the passive channel conformation of FocA, whereas at pH values less than 5, the passive transport of HCOO– is terminated through a pH-dependent gating mechanism that involves structural rearrangements of the N-terminal helices in each protomer of the pentamer. This gating event was shown by direct electrophysiology of FocA reconstituted in planar lipid bilayer membranes (18). We observed voltage-dependent currents, commonly with a small difference in reversal potential from what was expected for the applied gradient of formate, and we now show that this shift was then caused by the presence of chloride anions in the buffer solutions. We conducted a detailed electrophysiological analysis of anion transport by the formate channel FocA, with the result that the protein exclusively transports monovalent anions, albeit with unexpectedly low specificity for the ion itself. Interestingly, the range of molecules transported by FocA largely matches the end products of mixed-acid fermentation.
Fig. 1.
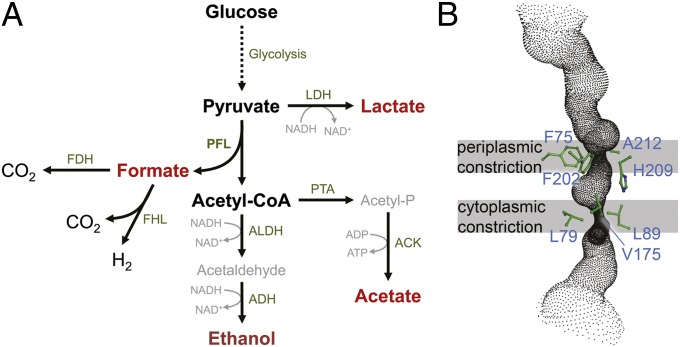
Mixed-acid fermentation and the FocA channel. (A) The metabolic pathway of anaerobic mixed-acid fermentation. Glucose is oxidized along the glycolytic pathway, and the product pyruvate is reduced in a minor degree to lactate via lactate dehydrogenase (LDH). The bulk of pyruvate is cleaved into acetyl-CoA and formate by the key enzyme of the pathway, PFL. Formate is then either exported via the channel FocA for periplasmic reduction by formate dehydrogenases (FDH), or it is disproportionated to H2 and CO2 by the cytoplasmic formate:hydrogen lyase complex (FHL). Approximately half of the generated acetyl-CoA is reduced in two steps by aldehyde dehydrogenase (ALDH) and alcohol dehydrogenase (ADH) to the end product ethanol, whereas the remaining acetyl-CoA is converted to acetate via phosphotransacetylase (PTA) and acetate kinase (ACK). The products formate, lactate, acetate, and ethanol are exported from the cell. (B) Detail of the transport channel of S. typhimurium FocA with the constrictions and the amino acid residues involved in their formation.
Results
Reversal Potential in Electrophysiology Studies of StFocA Indicates Transport of Different Anionic Species.
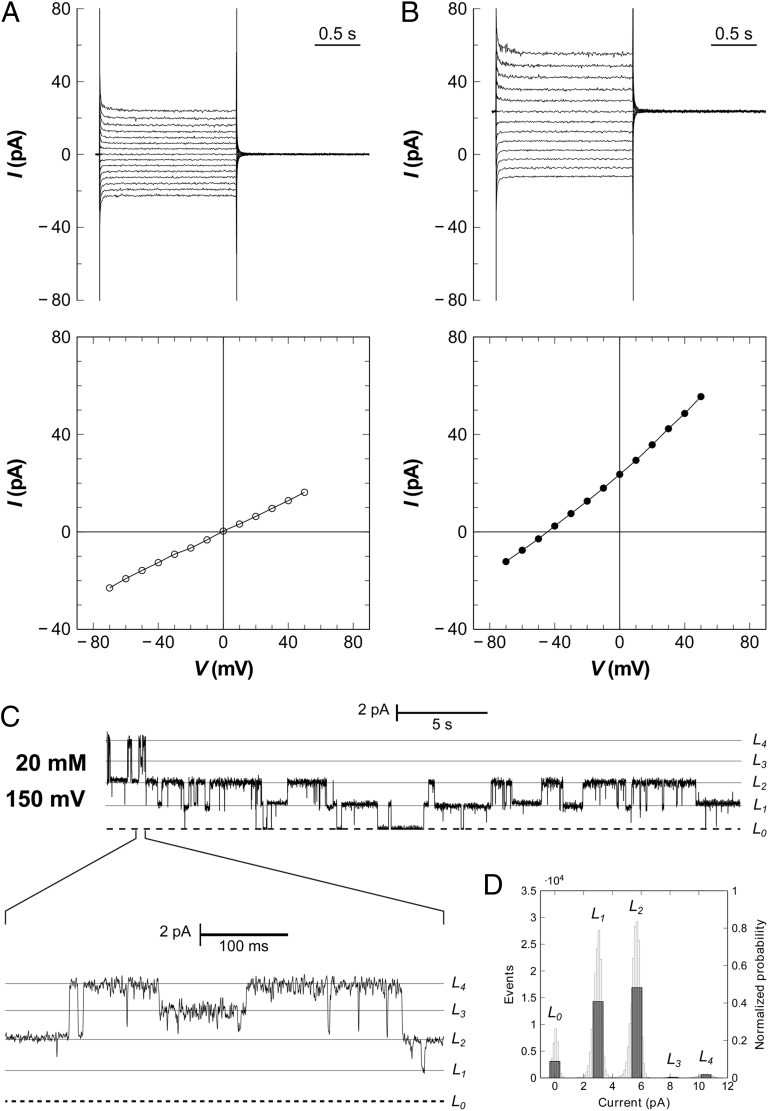
According to the Nernst equation, a gradient of (20/200 mM) of the monovalent anion formate should lead to a reversal potential of –58 mV in a voltage-clamp experiment. In our initial electrophysiological studies of StFocA, the observed reversal potential was slightly, but consistently, lower (18). To investigate a possible interference from other ions such as chloride, experiments were repeated by using 10 mM histidine buffer, thus omitting Tris⋅HCl and NaCl. The sole, negligible source of Cl– was from NaCl present in the added proteoliposomes (commonly 2–8 μL in a 3-mL compartment). The experiments showed current reversal at 0 mV for symmetric formate concentrations of (20/20 mM) (Fig. 2A). When repeated with a gradient of (20/100 mM) formate in the absence of Cl– anions, the currents consistently reversed between –41 and –42 mV, perfectly matching the expected value of –41.4 mV at 298 K (Fig. 2B). As a control, SCN– and I– were tested as known inhibitors of transport in ClC chloride channels (20), and both anions did not inhibit formate transport (SI Appendix, Fig. S2). This experiment confirmed the specificity of FocA for formate and provided evidence that other monovalent anions can traverse the membrane through this channel. Beside the observation of macroscopic currents, the conductance of FocA was sufficiently high to allow the recording of single-channel events (Fig. 2C). The StFocA peptide contains five cysteine residues, of which three (C82, C85, and C176) are located in or around the transport channel, and addition of the thiol-reactive MTSEA (2-aminoethyl methanethiosulfonate) during electrophysiological recordings led to a marked decrease of current (SI Appendix, Fig. S3). A current-voltage diagram derived form single-channel data fully complied with the one obtained from macroscopic currents (SI Appendix, Fig. S4). In various experiments, up to five different levels of conductance were seen that could be attributed to a single pentamer. Notably the fully closed state was only minimally populated, as was an all-open state with five conducting protomers. A representative all-point histogram of the same recording marks the L1 and L2 states as strongly preferred (Fig. 2D), pointing toward interprotomer cooperativity in the gating of individual channels of the FocA pentamer.
Fig. 2.
Electrophysiology of FocA reconstituted in planar lipid bilayers. (A) In the presence of symmetric formate concentrations (20/20 mM), the reversal potential derived from a current-voltage plot is zero. Macroscopic currents are shown above and the respective current–voltage diagram below. (B) Macroscopic currents obtained with a formate gradient of 100 mM (cis side) to 19.6 mM (trans side) yield a reversal potential of –42 mV at 298 K, matching precisely the expected Nernst potential. The membrane for the experiment in A and B contained at least 22 channels, or five pentamers, consistent with the observed single-channel currents. (C) At lower protein concentrations, single-channel opening and closing events are observed, with a current of 2.6 pA per protomer at a membrane potential of +150 mV and symmetric formate concentrations of 20 mM. (D) All-point histogram for the current trace in C. The L1 and L2 states are far higher populated than the others, indicating subunit cooperativity for FocA.
Conductance of FocA Depends on Ion Concentration.
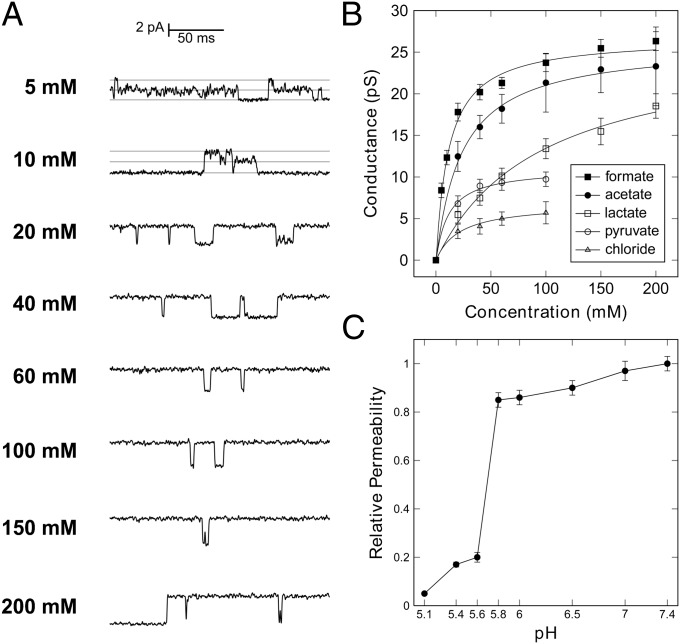
In conditions of symmetric formate concentrations and a constant membrane potential of 150 mV, single-channel events were recorded for formate and the conductance was found to increase with concentration (Fig. 3A), showing a saturation behavior from which a maximum conductance G and an affinity k could be obtained by a hyperbolic fit (Fig. 3B). The recordings for formate yielded GFormate = 26.9 ± 0.5 pS, and kFormate = 11.7 ± 0.1 mM, a value close to the estimated maximal concentration of formate in the medium during mixed-acid fermentation (2, 13). No significant changes in the open probability were observed upon increase of ion concentration (Fig. 3A).
Fig. 3.
Ion conductance through FocA and pH-dependent gating. (A) With varying, symmetric formate concentrations and a holding potential of 150 mV, the conductance of FocA increases with ion concentration. (B) Dependence of channel conductance on ion concentration for formate, acetate, lactate, pyruvate, and chloride. Formate, acetate, and lactate show near identical Gmax values of 26 pS, but differ in affinity, whereas pyruvate has the same affinity as formate, but only reaches a Gmax of 11 pS. (C) The permeability of FocA for formate anions shows specific, reversible gating at pH 5.7, in line with a switch of transport mode from passive channel to active importer (14, 18).
N-Terminal Gating of FocA Is Subject to Precise pH Control.
StFocA was shown to act as a passive channel for formate at pH 7.5. The anion currents ceased at pH 5.1, representing the unique functional switch of this protein from passive to active transport (18). However, whole-cells studies with variations of the pH of the growth medium located the functional switch at a pH value of 6.8 (14). To obtain a precise estimate, we conducted series of electrophysiology experiments at different pH values on the same bilayer membrane, by stepwise addition of acid to the cis chamber. The permeability of FocA remained constant from pH 7.4 to pH 5.8 but dropped steeply at pH 5.6 (Fig. 3C). This specific gating thus occurred at a defined pH, and it can be mechanistically rationalized by the rearrangement of the N-termini of the five protomers, as seen in the structure of StFocA at pH 4 (18). The clear definition of a switching point indicates that the mechanism of pH sensing may involve a protonation event of an amino acid side chain, and given the pH value of approximately 5.7, a histidine may be a suitable candidate. The steepness of the current step itself might well be the result of strong cooperativity between individual subunits, in line with the observed direct interaction of the N-termini of monomers in the intermediate and closed states (18). This gating could be achieved by involving multiple protonable groups, and a series of histidine residues are found in the structures of FocA. Further functional studies on point variants will be required to address this point.
FocA Transport Channel Is Gated and Responds to Variations in the Membrane Potential.
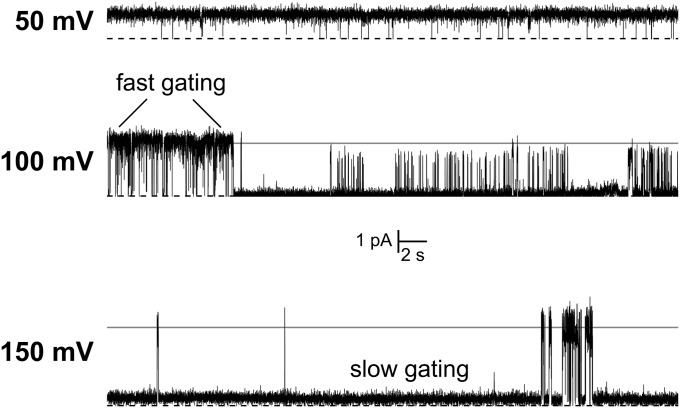
Under high-pH conditions (pH > 5.8), FocA strictly functions as a passive channel. N-terminal gating does not play a role, and structural data on the V. cholerae ortholog indicate that the N-termini of all protomers are flexible in this state (17). Nevertheless, single-channel measurements reveal transient gating that must be based on the structural properties of the transport channel itself (Fig. 2C). Such opening and closing events are typical for ion channels and are commonly explained by small structural rearrangements within the conduction pore that either allow or block the passage of ions. Closer inspection of current traces revealed fast fluctuations of conductance on a millisecond timescale (Fig. 4). This fast, flickering type of gating occurred at various external potentials, but although the current increased with potential, as detailed above, the opening probability decreased significantly. At an applied potential of 50 mV, a channel remained almost continuously in an open state, whereas a nonconducting state was significant at 100 mV and even dominant at 150 mV (Fig. 4). At the same time, macroscopic currents invariably showed a linear current response with increasing holding potentials (Fig. 2 A and B), indicating that FocA is not voltage gated but favors a state of limited conductance (Fig. 4). Note that in the presence of an ion gradient, the strict linear dependence of I on V is lost, but only for the highest potentials examined (Fig. 2B and SI Appendix, Fig. S4B).
Fig. 4.
Membrane potential dependence of single-channel gating. A single FocA channel shows distinct bursts of opening and closing that represent fast structural fluctuations in the protein, likely due to amino acid side-chain rearrangements at the two pore constrictions. The linear increase of current with membrane potential evidences ohmic behavior, but the opening probability of a channel strongly decreased at higher holding potentials.
FocA Is a Low-Selectivity Channel for Monovalent Anions.
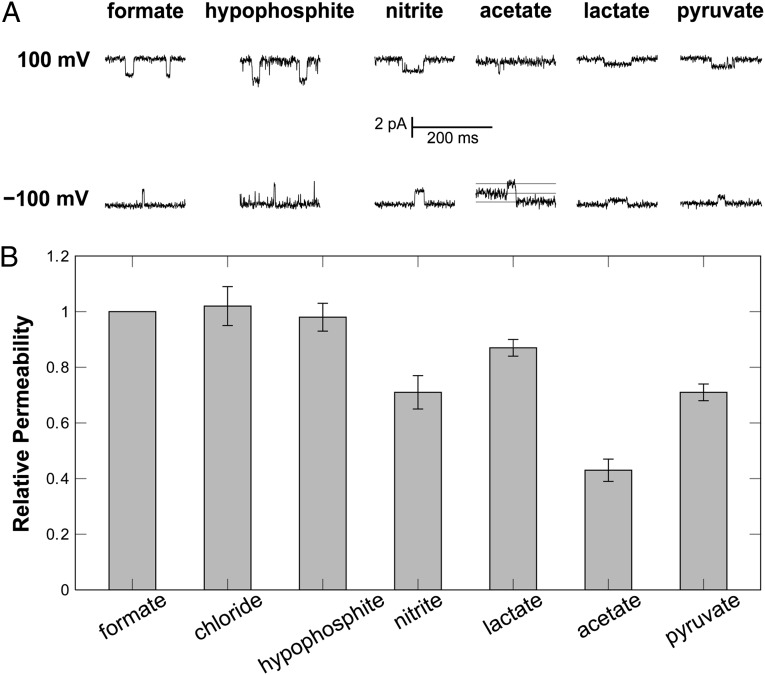
Because FocA also transports Cl– ions, we initiated a screening for further potentially transported molecules by monitoring possible shifts in the reversal potential under biionic conditions (21). The hypophosphite (H2PO2–) and nitrite (NO2–) anions are structurally highly similar to formate, and both were found to be transported by FNT family channels (2, 3). We recorded macroscopic and single-channel currents for both (Fig. 5A and SI Appendix, Fig. S5), and the signals changed polarity upon reversal of the holding potential. The relative permeabilities determined for formate and hypophosphite, but also for chloride, were identical within experimental error. Interestingly, the permeability for nitrite was only 70% relative to formate, and this difference is not straightforward to explain from the structural differences of the two molecules (Fig. 5B). Further trials with alternative ions included the divalent anions sulfate (SO42–) and hydrogen phosphate (HPO42–) and also the trivalent citrate, but in multiple trials, no currents were observed for these ions, indicating that the multivalent species are not suitable for FocA transport. In contrast, other monovalent anions were able to cross the membrane via FocA, even if they were significantly larger than formate. As compounds of physiological relevance, we investigated acetate, lactate, and pyruvate, and FocA efficiently translocated all three (Fig. 5B and SI Appendix, Fig. S5). Acetate and lactate are acidic end products of mixed-acid fermentation and showed permeabilities that amounted to 45% and 85% of the one for formate, respectively (Fig. 5B), whereas the central metabolite pyruvate had 75% permeability relative to formate. Acetate and lactate showed identical conductance maxima within experimental error (Fig. 3B), with GAcetate = 26.2 ± 0.5 pS and GLactate = 26.5 ± 1.8 pS. At the same time, the affinities for the bulkier anions decreased significantly to kAcetate = 23.9 ± 1.7 mM and kLactate = 96.0 ± 14.4 mM. FocA thus only showed 49% of the formate affinity for acetate and 12% for lactate. The situation was different for pyruvate, the product of glycolysis that is not an end product of mixed-acid fermentation. It interacted with FocA with an affinity kPyruvate = 11.6 ± 1.9 mM that closely matched the one for formate, but with a lower GPyruvate of 11.1 ± 0.4 pS (Fig. 3B). This finding may be rationalized by the increased rigidity of the α-ketoacid pyruvate, and it indicates that FocA is not evolutionarily optimized for the transport of pyruvate. Chloride ions yielded weak single-channel currents that were independent of the presence of SCN–, with an apparent affinity kChloride = 21.9 ± 5.1 mM, similar to the one for acetate, but a far lower GChloride of 6.8 ± 0.5 pS (Fig. 3B and SI Appendix, Fig. S4). Although chloride interacts with FocA, it is not efficiently translocated across the membrane, emphasizing that it does not represent a physiologically relevant permeating species.
Fig. 5.
Ion selectivity of FocA. (A) Specific single-channel currents that changed directionality upon reversal of the applied holding potential were recorded for various monovalent anions. No currents derived from single-channel events were observed for any multivalent anion or any cation. (B) FocA showed high permeability for formate, but also for the highly similar hypophosphite, for chloride, and 70% permeability for nitrite. The high permeabilities observed for the organic acids acetate, lactate, and pyruvate match the product profile of mixed-acid fermentation.
Interestingly, the metabolic fluxes in mixed-acid fermentation as determined in a series of chemostat experiments with E. coli (22), a close relative of S. typhimurium, compare strikingly well with the properties of FocA. Pyruvate is split into acetyl-CoA and formate by PFL, and 49.6% of acetyl-CoA were found to be converted to acetate; the rest to ethanol. The alternative route to PFL was lactic acid fermentation by LDH, but here the flux was a mere 3.4% of the one to PFL. Thus, the relative yields of the end products of mixed-acid fermentation match up very well with the relative affinities of FocA for these ions, underlining impressively its multifunctional role as an export channel.
Discussion
FocA is unique among known membrane transport proteins for its remarkable, pH-dependent switch from passive channeling—with a primary physiological function as an anion exporter—to secondary active transport, presumably as a H+/HCOO– importer (14). The protein uses two very different structural principles for these functionalities: At low pH, the N-terminal helix of the FocA protomer changes conformation to form a cytoplasmic gate to the transport channel. It does so in a cooperative manner that imposes one of three possible conformations on the helix, dependent on the state of the neighboring protomers (18). FocA likely works as an active symporter at this point, in an electroneutral transport mode that is not accessible for electrophysiological studies. In the present work, we therefore focused on the high-pH mode of FocA, where the N-terminal helix is flexible and does not play a role in gating transport (17). Here, we directly observed gated single channels that must be controlled in a different manner (Fig. 2C). In closeup, a FocA protomer exhibits a stable closed state, whereas the opening event shows a distinct, flickering gating on a fast timescale (Fig. 4). Such flickering was found in other ion channels, including the inward-rectifying potassium channel Kir2.1 (23) and also the ClC chloride channels (24, 25). The “fast gate” could be a single, flexible amino acid side chain in the transport channel, as is the case with the “Gluex” glutamate residue in ClC channels (25, 26). Its strict conservation within the FNT family makes H209 in the center of the channel a likely candidate (Fig. 1B and Fig. S1), but a final confirmation will require further experimental data.
We found that FocA showed little discrimination as long as the translocated species were monovalent anions. Similar observations were made for other anion channels, including the dimeric ClC chloride channels that also conduct Br–, I–, NO3–, and SCN– (25, 26) and the tetrameric aqua/glyceroporins, for which most recently a broad cargo range from H2O up to the polyalcohol sorbitol was determined (27). Although the transport channel itself is dissimilar in the aqua/glyceroporin and the FNT families, the topology and arrangement of their six transmembrane helices strongly suggests an evolutionary kinship (16). FocA, and most likely other members of the FNT family, discriminate little between small anions of similar size. Although the constrictions in the transport channels are too narrow even for these molecules to pass (16–18), a minor structural rearrangement might be sufficient to allow for passage. In contrast, the larger carboxylates acetate, lactate, and pyruvate are significantly more bulky, and their transport through FocA requires a major degree of flexibility within the pore. This could be interpreted such that the available structures represent a closed state of FocA, or it could mean that the residues forming the constrictions are sufficiently flexible to quickly rearrange for ion passage, and then shift back to a resting state as seen in the structures. We favor the second hypothesis, in particular because a hypothetical “open” state with a pore of sufficient size to allow for the unobstructed passage of a formate or lactate anion would hardly be able to exclude the passage of water. Without the elaborate precautions found in aquaporins (19), this situation would invariably create a proton pathway along a forming water chain that would efficiently degrade the proton motive force across the membrane. Aquaporins avoid this problem through local variations of electrostatic potential in the channel that prevent the formation of a crucial hydrogen bond between two water molecules on opposite sides of the selectivity filter (19).
The ability of FocA to conduct monovalent anions, but exclude multivalent anions and cations, points toward the presence of a single, positively charged residue within the bilayer that compensates the anionic charge and facilitates is translocation across the hydrophobic barrier of the membrane. With both constrictions in the channel formed by hydrophobic residues, the sole conserved amino acid that might be a candidate for this task is H209, a residue that is fully invariant in the FNT family (Fig. 1B and Fig. S1). To provide a positive charge, its side chain would have to be in the protonated imidazolium form. For a solvated histidine side chain, the pKa of this protonation is at 6.5, well within the physiological range, but this value could be significantly altered in the hydrophobic environment of the transport channel. When we analyzed an H209F variant of FocA in electrophysiological experiments, no currents could be detected, indicating this residue is crucial for the function of the channel.
Enteric bacteria and other facultative anaerobes use one of two possible pathways for the fermentation of formate. 2,3-butane diol fermentation is characterized by the typical intermediate acetoin and yields only minor amounts of acidic products. The second pathway, mixed-acid fermentation, is used by genera such as Vibrio, Escherichia, and Salmonella. It yields significant amounts of acetate, lactate, ethanol, and, to a lesser degree, succinate, as well as formate that is then reduced to CO2 by periplasmic formate dehydrogenases. Formate oxidation releases a proton, and two further protons are exported from the cytoplasm in a Q-loop mechanism when formate dehydrogenase transfers the two electrons from formate oxidation to menaquinone. The net export of three protons per formate anion thus is the main cause of the substantial acidification of the growth medium during mixed-acid fermentation (28). FNT proteins are commonly produced when their transported molecules are present in abundance. This is the case for the nitrite channel NirC that is induced by the presence of nitrate and nitrite itself (29), and also for FocA, which is produced during mixed-acid fermentation (2). Here, the bulk of the carbon flux in the cell is through PFL that yields formate and acetyl-CoA, and the activity of PFL, together with the competing reaction of lactate dehydrogenase, counteracts pyruvate efflux through FocA. Note that the focA gene is part of the pfl operon, where it immediately precedes the gene for the enzyme (30). Lactate, formate, and acetate are the predominant end products and will accumulate significantly during anaerobic growth on carbohydrates (28). A single anion channel, FocA, that can keep the intracellular concentrations of these molecules low must constitute a major evolutionary advantage. However, the capacity of FocA to export the central metabolic intermediate pyruvate seems contradictory, in particular because the uptake of pyruvate is an active process (31, 32). Under conditions of mixed-acid fermentation, extracellular pyruvate was detected when glucose was supplied (22), suggesting that intracellular levels of pyruvate may rise to a point where FocA again can serve as an overflow.
In summary, acting as a channel at high extracellular pH, FocA is able to export the main products of mixed-acid fermentation from the cytoplasm. Its seeming lack of specificity thus represents an adaptation to the physiology of this fermentative pathway. Because the properties of the transport channel are likely very similar in the other member of the FNT family, NirC, a similar flexibility toward other monovalent anions can be predicted. The FNT proteins in general should therefore not only be considered a family of channels rather than transporters, as the name suggests, but they should be defined as broad-range monovalent anion channels with an intricate functionality that is governed by their respective metabolic context.
Materials and Methods
Protein Production and Isolation.
StFocA was produced and isolated by following published procedures (18). In short, E. coli BL21 (DE3) C43 cells were transformed with expression plasmid vector pET21a::focA that introduces a C-terminal His6 tag to the full-length protein. Expression of focA was induced at an optical density at 600 nm of 0.8 by addition of 0.2 mM of isopropyl thiogalactoside. After an overnight incubation at 30 °C with agitation, cells were harvested by centrifugation and resuspended in 50 mM Tris⋅HCl buffer at pH 8.0 containing 500 mM NaCl and 10% (vol/vol) of glycerol. After disruption in a microfluidizer (Microfluidics), a membrane extract was prepared by differential centrifugation and the membrane pellet was solubilized with 2% (wt/vol) of n-octyl-β-d-glucopyranoside (OG; Anatrace). The suspension was then cleared by ultracentrifugation and loaded onto a HisTrap column (5 mL of bed volume; GE Healthcare) equilibrated in a loading buffer containing 20 mM Tris⋅HCl at pH 8.0, 150 mM NaCl, 10% (vol/vol) glycerol, and 1.1% (wt/vol) OG. The column was developed with a linear gradient of imidazole, from which FocA eluted at ∼300 mM. The protein was concentrated by ultrafiltration and subjected to size exclusion chromatography (Superdex 200 10/300; GE Healthcare) in 10 mM Tris⋅HCl at pH 8.0, 150 mM NaCl, 10% (wt/vol) glycerol, and 1.1% (wt/vol) OG. The symmetric pentamer peak was collected, concentrated to 10 mg·mL–1 by ultrafiltration, and kept on ice for reconstitution experiments.
Preparation of Planar Lipid Bilayers.
For the creation of planar lipid bilayers, a Delrin bilayer cup with a 200-μm diameter bore was used. The hole was covered with lipids from a stock of E. coli polar lipid extract (Avanti) in decane. After drying, the cup was placed in the chamber holder, buffer was added to both the cis and trans chambers, and the solutions were connected via agar bridges and Ag/AgCl electrodes to a Lipid Bilayer Workstation (Harvard Apparatus). A bilayer was then painted over the hole with an air bubble on a pipette tip previously dipped into lipid solution, and its formation was monitored by the capacitance profile at a holding potential of +100 mV. Initially both chambers contained 10 mM histidine and 20 mM sodium formate at a final pH of 7.5.
Protein Reconstitution in Proteoliposomes and Bilayer Fusion.
Liposomes were prepared from E. coli polar lipid extract (Avanti; 25 mg·mL–1) by following established procedures (18, 33). Lipids and l-α-phosphatidylcholine (Avanti; 25 mg·mL–1) were mixed at a ratio of 3:1 (vol/vol), washed twice with ethanol, and resuspended in buffer containing 20 mM Tris⋅HCl at pH 8.8 and 450 mM NaCl. This mixture was homogenized by sonication, followed by repeated extrusion through a membrane with 200-nm pore size. The resulting liposomes were diluted to 4 mg·mL–1 and destabilized with 48 mM (final concentration) of OG. StFocA was reconstituted into these liposomes at a protein:lipid ration of 1:50 (wt/wt), and OG was removed by using Biobeads (Bio-Rad). Proteoliposomes were collected by centrifugation at 100,000 × g (Beckman Coulter Avanti J-30I, JA30-50 Ti rotor) for 40 min, resuspended in the same buffer to a final concentration of 4 mg·mL–1, and frozen in liquid nitrogen until further use. Proteoliposome fusion with a planar lipid bilayer was achieved by the addition of 2 μL of the proteoliposome solution to the cis chamber close to the bilayer. The system was clamped at a holding potential of 100 mV, and the insertion of protein was identified by the appearance of single-channel events.
Electrophysiology.
All electrophysiological recordings were carried out on a Planar Lipid Bilayer Workstation (Harvard Apparatus) by using an Axon Digidata 1440A digitizer (Molecular Devices) and a Bilayer Clamp BC-535 amplifier (Warner Instruments). Signals were processed with the pClamp 10 software suite (Molecular Devices). Single-channel events were recorded applying symmetric formate concentrations from 20 to 200 mM at varying holding potentials. For all other tested ions, a symmetric concentration of 20 mM was used. Formate gradients were formed by the addition of sodium formate to the cis chamber from an 8 M stock solution. For macroscopic current measurements, holding potentials from –70 to +70 mV were applied. pH was varied through stepwise addition of 0.7 M HCl to the trans side, and the resulting pH value was derived from a calibration curve determined separately. The concentration of chloride ions introduced in the titration was 6.8 mM at pH 5.6 and 8.5 mM at pH 5.1.
Ionic Permeabilities.
Relative permeabilities were determined under biionic conditions (21). Briefly, 20 mM of various anions were added to the cis side of planar lipid bilayers initially prepared with symmetric formate concentrations of 20 mM. The permeability ratios were calculated from the observed reversal potentials by using the Goldman equation (34).
Supplementary Material
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grants EI 520/3 and EI 520/6 (to O.E.), AN 676/1 (to S.L.A.A.), and IRTG 1478.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204201109/-/DCSupplemental.
References
- 1.Saier MH, Jr, et al. Phylogenetic characterization of novel transport protein families revealed by genome analyses. Biochim Biophys Acta. 1999;1422:1–56. doi: 10.1016/s0304-4157(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 2.Suppmann B, Sawers G. Isolation and characterization of hypophosphite—resistant mutants of Escherichia coli: Identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol Microbiol. 1994;11:965–982. doi: 10.1111/j.1365-2958.1994.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 3.Clegg S, Yu F, Griffiths L, Cole JA. The roles of the polytopic membrane proteins NarK, NarU and NirC in Escherichia coli K-12: Two nitrate and three nitrite transporters. Mol Microbiol. 2002;44:143–155. doi: 10.1046/j.1365-2958.2002.02858.x. [DOI] [PubMed] [Google Scholar]
- 4.White WB, Ferry JG. Identification of formate dehydrogenase-specific mRNA species and nucleotide sequence of the fdhC gene of Methanobacterium formicicum. J Bacteriol. 1992;174:4997–5004. doi: 10.1128/jb.174.15.4997-5004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czyzewski BK, Wang DN. Identification and characterization of a bacterial hydrosulphide ion channel. Nature. 2012;483:494–497. doi: 10.1038/nature10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peakman T, et al. Nucleotide sequence, organisation and structural analysis of the products of genes in the nirB-cysG region of the Escherichia coli K-12 chromosome. Eur J Biochem. 1990;191:315–323. doi: 10.1111/j.1432-1033.1990.tb19125.x. [DOI] [PubMed] [Google Scholar]
- 7.Brett PJ, Burtnick MN, Su H, Nair V, Gherardini FC. iNOS activity is critical for the clearance of Burkholderia mallei from infected RAW 264.7 murine macrophages. Cell Microbiol. 2008;10:487–498. doi: 10.1111/j.1462-5822.2007.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakravortty D, Hensel M. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect. 2003;5:621–627. doi: 10.1016/s1286-4579(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 9.Das P, Lahiri A, Lahiri A, Chakravortty D. Novel role of the nitrite transporter NirC in Salmonella pathogenesis: SPI2-dependent suppression of inducible nitric oxide synthase in activated macrophages. Microbiology. 2009;155:2476–2489. doi: 10.1099/mic.0.029611-0. [DOI] [PubMed] [Google Scholar]
- 10.Rycovska A, Hatahet L, Fendler K, Michel H. The nitrite transport protein NirC from Salmonella typhimurium is a nitrite/proton antiporter. Biochim Biophys Acta. 2012;1818:1342–1350. doi: 10.1016/j.bbamem.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Berg BL, Li J, Heider J, Stewart V. Nitrate-inducible formate dehydrogenase in Escherichia coli K-12. I. Nucleotide sequence of the fdnGHI operon and evidence that opal (UGA) encodes selenocysteine. J Biol Chem. 1991;266:22380–22385. [PubMed] [Google Scholar]
- 12.Leonhartsberger S, Korsa I, Böck A. The molecular biology of formate metabolism in enterobacteria. J Mol Microbiol Biotechnol. 2002;4:269–276. [PubMed] [Google Scholar]
- 13.Sawers G. The hydrogenases and formate dehydrogenases of Escherichia coli. Antonie van Leeuwenhoek. 1994;66:57–88. doi: 10.1007/BF00871633. [DOI] [PubMed] [Google Scholar]
- 14.Sawers RG. Formate and its role in hydrogen production in Escherichia coli. Biochem Soc Trans. 2005;33:42–46. doi: 10.1042/BST0330042. [DOI] [PubMed] [Google Scholar]
- 15.Hakobyan M, Sargsyan H, Bagramyan KA. Proton translocation coupled to formate oxidation in anaerobically grown fermenting Escherichia coli. Biophys Chem. 2005;115:55–61. doi: 10.1016/j.bpc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, et al. Structure of the formate transporter FocA reveals a pentameric aquaporin-like channel. Nature. 2009;462:467–472. doi: 10.1038/nature08610. [DOI] [PubMed] [Google Scholar]
- 17.Waight AB, Love J, Wang DN. Structure and mechanism of a pentameric formate channel. Nat Struct Mol Biol. 2010;17:31–37. doi: 10.1038/nsmb.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lü W, et al. pH-dependent gating in a FocA formate channel. Science. 2011;332:352–354. doi: 10.1126/science.1199098. [DOI] [PubMed] [Google Scholar]
- 19.Agre P, et al. Aquaporin water channels—from atomic structure to clinical medicine. J Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rychkov GY, Pusch M, Roberts ML, Jentsch TJ, Bretag AH. Permeation and block of the skeletal muscle chloride channel, ClC-1, by foreign anions. J Gen Physiol. 1998;111:653–665. doi: 10.1085/jgp.111.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeMasurier M, Heginbotham L, Miller C. KcsA: It’s a potassium channel. J Gen Physiol. 2001;118:303–314. doi: 10.1085/jgp.118.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang YT, Bennett GN, San KY. The effects of feed and intracellular pyruvate levels on the redistribution of metabolic fluxes in Escherichia coli. Metab Eng. 2001;3:115–123. doi: 10.1006/mben.2000.0166. [DOI] [PubMed] [Google Scholar]
- 23.Parsons NJ, et al. Lactate enhancement of sialylation of gonococcal lipopolysaccharide and of induction of serum resistance by CMP-NANA is not due to direct activation of the sialyltransferase: Metabolic events are involved. Microb Pathog. 1996;21:193–204. doi: 10.1006/mpat.1996.0054. [DOI] [PubMed] [Google Scholar]
- 24.Poolman B, Knol J. Amplified expression and membrane reconstitution of transport proteins. Biochem Soc Trans. 1999;27:912–917. doi: 10.1042/bst0270912. [DOI] [PubMed] [Google Scholar]
- 25.Miller C. ClC chloride channels viewed through a transporter lens. Nature. 2006;440:484–489. doi: 10.1038/nature04713. [DOI] [PubMed] [Google Scholar]
- 26.Knappe J, Sawers G. A radical-chemical route to acetyl-CoA: The anaerobically induced pyruvate formate-lyase system of Escherichia coli. FEMS Microbiol Rev. 1990;6:383–398. doi: 10.1111/j.1574-6968.1990.tb04108.x. [DOI] [PubMed] [Google Scholar]
- 27.Parsons NJ, et al. Lactic acid is the factor in blood cell extracts which enhances the ability of CMP-NANA to sialylate gonococcal lipopolysaccharide and induce serum resistance. Microb Pathog. 1996;20:87–100. doi: 10.1006/mpat.1996.0008. [DOI] [PubMed] [Google Scholar]
- 28. Fuchs G ed (2007) Allgemeine Mikrobiologie (begr. von Hans G. Schlegel) (Georg Thieme, Stuttgart, Germany), 8th Ed.
- 29.Wu HC, Tyson KL, Cole JA, Busby SJW. Regulation of transcription initiation at the Escherichia coli nir operon promoter: A new mechanism to account for co-dependence on two transcription factors. Mol Microbiol. 1998;27:493–505. doi: 10.1046/j.1365-2958.1998.00699.x. [DOI] [PubMed] [Google Scholar]
- 30.Sawers G, Böck A. Anaerobic regulation of pyruvate formate-lyase from Escherichia coli K-12. J Bacteriol. 1988;170:5330–5336. doi: 10.1128/jb.170.11.5330-5336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kornberg HL, Smith J. Genetic control of the uptake of pyruvate by Escherichia coli. Biochim Biophys Acta. 1967;148:591–592. doi: 10.1016/0304-4165(67)90167-5. [DOI] [PubMed] [Google Scholar]
- 32.Lang VJ, Leystra-Lantz C, Cook RA. Characterization of the specific pyruvate transport system in Escherichia coli K-12. J Bacteriol. 1987;169:380–385. doi: 10.1128/jb.169.1.380-385.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knol J, Sjollema K, Poolman B. Detergent-mediated reconstitution of membrane proteins. Biochemistry. 1998;37:16410–16415. doi: 10.1021/bi981596u. [DOI] [PubMed] [Google Scholar]
- 34.Goldman DE. Potential, impedance, and rectification in membranes. J Gen Physiol. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.