Abstract
The Na-Cl cotransporter (NCC), which is the target of inhibition by thiazides, is located in close proximity to the chloride-absorbing transporter pendrin in the kidney distal nephron. Single deletion of pendrin or NCC does not cause salt wasting or excessive diuresis under basal conditions, raising the possibility that these transporters are predominantly active during salt depletion or in response to excess aldosterone. We hypothesized that pendrin and NCC compensate for loss of function of the other under basal conditions, thereby masking the role that each plays in salt absorption. To test our hypothesis, we generated pendrin/NCC double knockout (KO) mice by crossing pendrin KO mice with NCC KO mice. Pendrin/NCC double KO mice displayed severe salt wasting and sharp increase in urine output under basal conditions. As a result, animals developed profound volume depletion, renal failure, and metabolic alkalosis without hypokalemia, which were all corrected with salt replacement. We propose that the combined inhibition of pendrin and NCC can provide a strong diuretic regimen without causing hypokalemia for patients with fluid overload, including patients with congestive heart failure, nephrotic syndrome, diuretic resistance, or generalized edema.
Keywords: diuretics, kidney tubules, nephrogenic diabetes insipidus
The thiazide-sensitive Na-Cl cotransporter (NCC) (SLC12A3) and the Cl−/HCO3− exchanger pendrin (SLC26A4) are expressed on apical membranes of distal cortical nephron segments and mediate salt absorption, with pendrin working in tandem with the epithelial Na channel and NCC working by itself (1–6). Pendrin is expressed on the apical membrane of intercalated cells in late distal convoluted tubule (DCT), connecting tubule (CNT), and the cortical collecting duct (CCD) (7–9). The thiazide-sensitive NCC is primarily expressed on the apical membrane of DCT cells (10, 11).
Single deletion of pendrin or NCC does not cause salt wasting or excessive diuresis under basal conditions (5, 12–15). Indeed, even a mild degree of salt wasting has not been demonstrated in these two genetically engineered mouse models at steady state. Kidney functions, including sodium and chloride excretion, urine output, and blood urea nitrogen (BUN) levels in mutant mice are comparable to wild-type (WT) animals (12–15). Both pendrin KO and NCC KO mice, however, show signs of volume depletion or develop hypotension during salt restriction (12, 14). These findings have led investigators to conclude that pendrin and NCC are predominantly active during salt depletion (and or in response to increased aldosterone levels), and their contribution to salt reabsorption at baseline conditions is small.
We hypothesized that NCC and pendrin may compensate for loss of the other under basal conditions, thereby masking the role that each plays in salt reabsorption. To test this hypothesis, we generated double knockout of pendrin and NCC mice by crossing animals with single deletion for NCC and pendrin. The double KO mice show significant salt and fluid wasting, along with volume depletion and renal failure under baseline conditions. We propose that pendrin could be a target for a diuretic that, in conjunction with thiazide, can be an effective regimen for patients with fluid overload, such as congestive heart failure, nephrotic syndrome, diuretic resistance, or advanced chronic kidney disease.
Results
Generation of Pendrin/NCC Double KO Mice.
The double NCC/pendrin KO mice were generated by crossing mice with single deletion of pendrin or NCC with each other. Fig. 1 A and B demonstrates the generation of double NCC/pendrin KO mice, as verified by tail genotyping and Northern hybridization. Fig. 1C shows that 8-wk-old double KO mice are smaller than either single KOs or WT animals (Fig. 1C), with the average body weight being ∼40% lower in age-matched double KO mice vs. WT or single KO mice (Fig. 1D) (P < 0.01; n = 5 in each group). Table 1 depicts body weights of WT and double KO mice at the time of weaning (end of 3 wk) and at 8 wk of age. The double KO mice were smaller at 3 wk and remained small at 8 wk of age. The failure to thrive was evident in both sexes. The double KO mice were born in the expected Mendelian distribution. Double KO mice survive and live for at least 6 mo, with comparable survival rates to those in WT or single KO mice. (Longer-term survival analysis has not been performed.)
Fig. 1.
Generation and examination of NCC/pendrin double KO mice. (A) Generation of double pendrin/NCC KO mice. Tail genotyping was used to verify the generation of double KO mice. (B) Northern hybridization showing the generation of double Pendrin/NCC KO mice. Pendrin/NCC double KO mice lack the expression of pendrin and NCC in their kidneys. (C) Double pendrin/NCC KO mice display growth retardation. Double pendrin/NCC KO mice are small and have growth retardation. (D) Body weights of WT and mutant animals. Double pendrin/NCC KO mice fail to thrive and are ∼40% smaller than either single KOs or WT animals at 8 wk after birth. *Significant difference (see Results for more information).
Table 1.
Body weights of WT and double KO mice at 3 and 8 wk of age
| 3 wk | 8 wk | |
| WT body weight, g | 12.2 ± 1.2 | 22.6 ± 2 |
| dKO body weight, g | 7.2 ± 1.00 | 14.6 ± 2 |
| P | <0.05 | <0.05 |
The double KO (dKO) mice were significantly smaller at 3 and 8 wk of age (n = 6 in each group). Both male and female mice are included in calculations.
Pendrin/NCC Double KO Mice Have Profound Renal Water and Salt Wasting.
Animals were placed in metabolic cages, and, after acclamation for 2 d, balanced studies (daily food intake, water intake, urine volume, and body weight) were performed for 4 consecutive days. Results in Fig. 2 depict the collections on day 3, which is representative of daily experiments, and demonstrate the presence of profound polyuria (increased urine output), along with polydipsia (increased water intake), in double pendrin/NCC KO mice but not in single KO mice (Fig. 2 A and B). Fig. 2C demonstrates that urine osmolality is reduced by ∼70% in double KO mice vs. the other genotypes. Fig. 2D shows sharp increases in sodium and chloride excretion in double pendrin/NCC KO mice. Double KO mice were eating normally; however, when adjusted for body weights, they showed increased food intake vs. WT mice (0.163 and 0.207 g food intake/g of body weight in WT and double KO, respectively; P < 0.05, n = 5). Food consumption in single KO mice (pendrin or NCC) was comparable to WT mice.
Fig. 2.
Fluid balance and salt excretion in WT and mutant animals. (A) Urine output in WT and mutant animals. Double pendrin/NCC KO mice display profound polyuria (increased urine output). (B) Water intake in WT and mutant animals. Double pendrin/NCC KO mice have significant polydipsia (increased water intake). (C) Urine osmolality in WT and mutant animals. Double pendrin/NCC KO mice have low urine osmolality relative to WT and single KO mice. (D) Salt excretion in WT and mutant animals. Double pendrin/NCC KO mice display a sharp increase in sodium and chloride wasting compared with other genotypes. *Significant difference (see Results for more information).
Pendrin/NCC Double KO Mice Have Severe Volume Depletion, Renal Failure, and Metabolic Alkalosis.
To determine whether excessive salt wasting causes volume depletion in double KO mice, we examined the kidney expression of renin in WT and mutant mice. As shown in Fig. 3A (Upper gels), mRNA expression and protein abundance of renin robustly increased in kidneys of double KO mice relative to single KO or WT mice, with enhanced mRNA expression and protein abundance by ∼7-fold (P < 0.0001 vs. WT; n = 4) and >10-fold (P < 0.001 vs. WT; n = 5), respectively. These results indicate that the double KO mice are in a state of severe volume depletion.
Fig. 3.
Double pendrin/NCC KO mice have severe volume depletion and display renal failure and metabolic alkalosis. (A) mRNA expression and protein abundance of renin in kidneys of WT and mutant animals. The mRNA expression levels of renin (Left and Center) increased by ∼700% in double pendrin/NCC KO mice relative to WT or single KO mice (P < 0.0001). The renin protein (Right) was induced in kidneys of double KO mice (P < 0.00001). (B) BUN levels in WT and mutant animals. Double pendrin/NCC KO mice demonstrate significant elevation in BUN level relative to other genotypes, consistent with prerenal failure. (C) Acid–base parameters in WT and mutant mice. Double pendrin KO/NCC KO mice have profound metabolic alkalosis vs. WT mice. Mice with NCC or pendrin deletion demonstrate normal or borderline elevation in their serum bicarbonate. (D) Blood pressure measurement in WT and mutant animals. Pendrin/NCC double KO mice show significant hypotension (P < 0.01 vs. WT). *Significant difference (see Results for more information).
To determine whether salt wasting (Fig. 2) and the subsequent volume depletion (Fig. 3A) impair the kidney function, BUN levels were measured in WT and mutant mice. Results demonstrated a sharp increase in BUN levels in double KO mice relative to other genotypes, consistent with significant prerenal failure (Fig. 3B), which signifies impaired kidney function subsequent to decreased perfusion as a result of volume depletion. Double KO animals have normal serum sodium and potassium concentrations (Table 2).
Table 2.
Serum sodium and potassium concentrations in WT and double KO mice
| WT | double KO | |
| Na+, mEq/L | 136 ± 1.2 | 138 ± 1.5 |
| K+, mEq/L | 4.6 ± 0.3 | 4.2 ± 0.4 |
Serum sodium and potassium concentrations were not different between WT and double KO mice.
To ascertain the impact of severe volume depletion and the subsequent renal failure on acid base balance, systemic acid base parameters in various genotypes were examined (Experimental Procedures). As indicated, double pendrin/NCC KO mice have profound metabolic alkalosis vs. WT mice, as determined by elevated serum bicarbonate and arterial pH (Fig. 3C; P < 0.01 vs. WT mice). Mice with NCC deletion have normal serum bicarbonate levels, and mice with pendrin deletion demonstrate mild elevation in serum bicarbonate (12, 15). Blood pressure measurement by the computerized tail cuff method indicated that double KO mice are hypotensive (Fig. 3D; P < 0.01 vs. WT).
Effect of Salt Replacement on Volume Depletion, Renal Failure, and Metabolic Alkalosis in Pendrin/NCC Double KO Mice.
To ascertain the role of volume depletion in the pathogenesis of renal failure and metabolic alkalosis, double pendrin/NCC KO and WT mice were placed on high-salt (7%) diet for 7 d. Fig. 4A (Left and Right) demonstrates that the mRNA expression levels of renin decreased by ∼90% in kidneys of double KO mice on high-salt intake relative to normal diet. The above results demonstrate that salt replacement significantly improves the vascular volume depletion in double KO mice. Fig. 4B shows that BUN levels, although mildly higher in double KO vs. WT mice, are significantly decreased in double KO mice on high-salt diet relative to normal diet (comparison vs. Fig. 3B). Fig. 4C indicates significant improvement in the severity of metabolic alkalosis, as verified by a sharp reduction in serum bicarbonate levels in double KO mice on high-salt vs. normal-salt diet (P < 0.001; n = 4).
Fig. 4.
Effect of increased dietary salt intake on vascular volume, kidney function, and acid–base parameters in double pendrin/NCC KO mice. (A) Expression of renin in kidneys of WT and mutant animals. The mRNA expression levels of renin (Upper and Lower) were decreased by 90% in kidneys of double pendrin/NCC KO mice on high-salt relative to normal-salt diet. (B) BUN levels in WT and mutant animals. In salt-replete animals, the increase in BUN levels in pendrin/NCC double KO mice is significantly blunted relative to baseline conditions. (C) Acid–base status in WT and mutant mice. The magnitude of metabolic alkalosis was significantly improved in double pendrin KO/NCC KO mice on high-salt vs. normal diet. *Significant difference (see Results for more information).
Pendrin/NCC Double KO Mice Have Impaired Kidney Response to Exogenous Vasopressin.
The presence of low urine osmolality, despite severe volume depletion, suggests either the impairment in arginine vasopressin (AVP) secretion or dysregulation in the collecting duct aquaporin 2 (AQP-2) and/or thick-limb Na-K-2Cl cotransporter (NKCC2). Our results demonstrate the down-regulation of AQP2 in medullary collecting duct in pendrin/NCC double KO mice (Fig. S1 A and B). The expression levels of AVP mRNA in the brain were enhanced in double KO mice (Fig. S1D), consistent with increased circulating AVP levels. NCC double KO mice failed to increase their urine osmolality significantly 24 h after 1-desamino-8-d-AVP (dDAVP) (an AVP analog) injection, consistent with the impaired response to AVP in double KO mice (Fig. S1E). The expression of NKCC2, the apical Na-K-2Cl cotransporter in the thick limb, increased (Fig. S1C), presumably to minimize the salt wasting.
In the last series of experiments, the expression of sodium-dependent Cl−/HCO3− exchanger (NDCBE) (Slc4a8) and sodium channel (ENaC) subunits were examined in kidneys of four WT, single KO, and double KO mice. Fig. S2A is a Northern hybridization and shows that the expression of NDCBE increased significantly in kidneys of pendrin KO mice and NCC KO mice but not in double KO mice. Examination of ENaC subunits indicated a consistent increase in the expression levels of α, β, and γ subunits only in NCC KO mice. In kidneys of double KO mice, the expression of ENaC α and β subunits achieved statistically significant up-regulation vs. WT animals (P < 0.05; n = 4) (Fig. S2B). Western blots showed significant up-regulation of ENaC β and γ subunits in kidneys of double KO mice vs. WT animals (Fig. S2C; n = 4 in each group; P < 0.05 for each subunit). Antibodies against NDCBE (Experimental Procedures) did not yield any specific bands on Western blots.
Discussion
Thiazides are the specific inhibitors of NCC in the distal tubule and the most widely used diuretic in the world, in large part because they are considered mild agents and do not cause severe salt wasting (16, 17). The DCT is responsible for the reabsorption of 7–10% of filtered salt, and published reports indicate that majority of this process is mediated via NCC (18, 19). The absorption of the remaining filtered sodium in DCT is estimated to occur via sodium channel, with the residual component being absorbed via the Na+/H+ exchanger (20, 21). The mild salt-wasting effect of thiazides is disproportionate to the magnitude of NCC inhibition, suggesting that other salt-absorbing transporters in the proximity of NCC might be compensating and, therefore, blunting the diuretic effect of thiazides. However, despite the presence of several sodium-absorbing molecules (above), the identity of chloride-absorbing transporter(s) in the distal nephron, which might become active in the setting of NCC inactivation, remains speculative.
Pendrin, which was first identified by linkage analysis in patients with Pendred syndrome (22), can function in Cl−/HCO3− exchange mode (4) and works in conjunction with the sodium channel in the kidney (6). Mice with the genetic deletion of pendrin, and human beings with inactivating mutation of pendrin, do not display any evidence of salt wasting under baseline conditions (5, 15). Similar to pendrin KO mice, NCC-null mice do not demonstrate any noticeable salt wasting under baseline conditions (12). Both mutant mouse models, pendrin and NCC KO mice, show signs of volume depletion and salt wasting or develop hypotension in response to salt restriction (12, 14). Both NCC and pendrin are regulated by excess aldosterone (6, 12, 14, 23–26). These findings have led investigators to conclude that pendrin and NCC are predominantly active during salt depletion and/or in response to aldosterone, and their contribution to salt reabsorption at baseline conditions is insubstantial.
Two lines of evidence support an important role for pendrin in compensatory salt absorption in response to NCC inactivation. First, pendrin expression increases significantly in kidneys of NCC KO mice (25, 27). Second, treatment with hydrochlorothiazide causes increased fluid loss in pendrin KO mice relative to WT littermates (27). It should be noted, however, that neither NCC KO mice nor pendrin KO mice display any evidence of salt wasting, volume depletion, or renal failure under baseline conditions (12–15).
The most salient feature of the present studies is the profound fluid and salt wasting that are observed subsequent to the combined deletion of pendrin and NCC, strongly suggesting that each transporter masks the absence of the other under baseline conditions. The fluid and electrolyte loss resulted in massive volume depletion with subsequent prerenal failure and metabolic alkalosis in double KO mice. This picture is completely distinct from that in mice with single deletion of pendrin or NCC, which do not display any significant electrolyte or fluid loss under baseline conditions. This report highlights the essential role of pendrin in salt and fluid reabsorption in the distal nephron in the setting of NCC inactivation.
The double knockout of NCC and pendrin caused significant volume depletion, as verified by a robust increase in renin expression levels in the kidney and a sharp increase in BUN levels in mutant mice (Results). Interestingly, the serum potassium levels in double KO mice remained normal (Results), which is intriguing by and in itself, given the fact that similar magnitudes of volume depletion, salt wasting, and renin/aldosterone activation in subjects treated with loop diuretics (such as furosemide) is usually associated with hypokalemia, subsequent to potassium secretion into the lumen in exchange for sodium absorption via the sodium channel in the CNTs and collecting ducts. One plausible explanation could be that pendrin works in conjunction with the sodium channel (ENaC) to absorb NaCl, and the inhibition of pendrin would blunt the activity of ENaC, therefore impairing the secretion of potassium via K+ channels in exchange for sodium absorption through ENaC.
A recent study showed that hydrochlorothiazide treatment resulted in severe volume depletion, along with metabolic alkalosis and decreased kidney function, in a patient with Pendred syndrome resulting from an inactivating mutation in Pendred Syndrome (PDS) gene (28). This presentation is extremely unusual for thiazides and closely mimics the clinical picture in our double KO mice and supports the conclusion that a functional pendrin blunts the diuretic effect of NCC inhibition subsequent to treatment with thiazides by compensatory absorption of salt in the distal nephron.
The pendrin/NCC double KO mice have low urine osmolality despite severe volume depletion (Figs. 2 and 3), along with the down-regulation of AQP2 and impaired response to exogenous vasopressin analog (dDAVP) (Fig. S1), consistent with the generation of nephrogenic diabetes insipidus, which is extremely unusual in humans or animals with massive volume depletion and elevation of vasopressin and activation of renin–angiotensin–aldosterone axis. The impaired urine-concentrating ability indicates that factors unknown at the present blunt the action of endogenous vasopressin and, as a result, down-regulate AQP2 expression and activity in pendrin/NCC double KO mice.
Salt replacement corrected almost all physiologic abnormalities observed in pendrin/NCC double KO mice, as verified by >85% correction in the severity of volume depletion (improvement in renin expression levels), renal failure (reduction in BUN levels), and metabolic alkalosis (decrease in serum bicarbonate concentration). These results strongly indicate that salt and water wasting with the subsequent volume depletion are the central events in pendrin/NCC double KO mice, and their correction with salt replacement minimizes the severity of the volume depletion.
A recent publication indicated that pendrin could work in tandem with NDCBE in B-intercalated cells to absorb sodium and chloride (29). Such an interconnecting mechanism in the intercalated cells can provide an ENaC-independent salt-reabsorption pathway in the collecting duct, which is electroneutral and not associated with a net K+ transport. It is plausible that the interruption of this pathway plays an important role in the prevention of hypokalemia in NCC/pendrin double KO mice (Table 2). These observations predict that crossing the NDCBE KO mice (29) with the NCC KO mice may result in severe salt wasting with normal serum potassium in NCC/NDCBE double KO mice, a phenotype similar to that observed in NCC/pendrin double KO mice.
Whether specific inhibitors of pendrin can adversely affect the function of inner ear and thyroid, two organs with abundant expression of pendrin (22, 30), remains speculative. Pendrin is essential for the embryonic development of structures involved in normal hearing (cochlea, the vestibular labyrinth, and the endolymphatic sac of the inner ear) (22, 30), and its mutation in humans or in its deletion in mice causes structural abnormalities in the above organs during their embryonic stage, which results in deafness (30). It is, therefore, highly plausible that the inhibition of pendrin after the normal development of inner-ear structures is completed will not interfere with normal hearing. The impact of pendrin inhibitors on thyroid function remains speculative at the present.
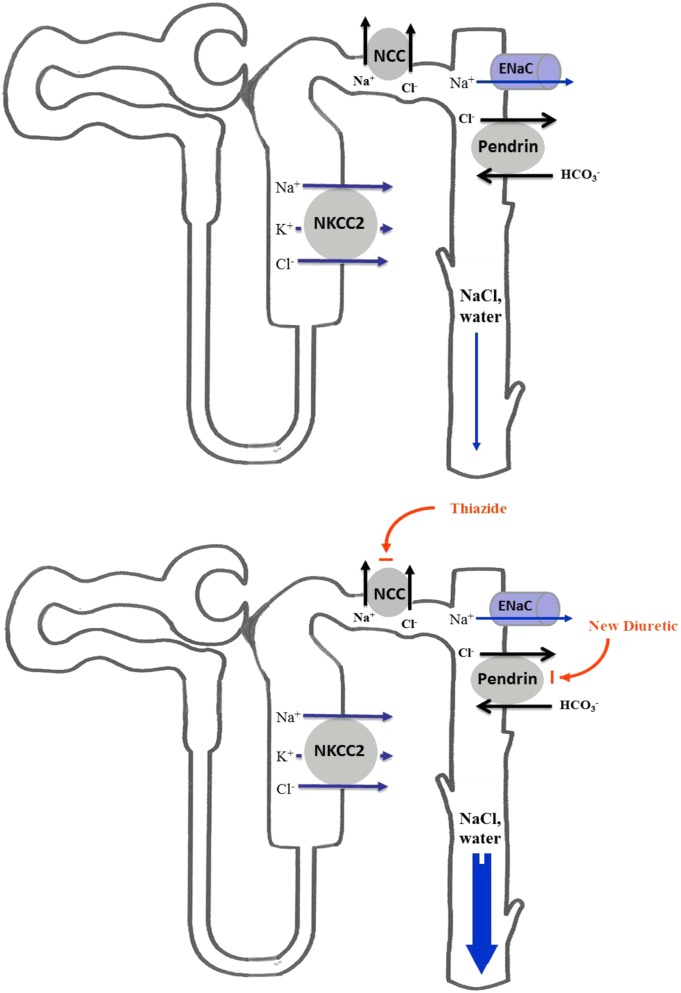
In conclusion, we propose that the combined inhibition of pendrin and NCC can provide a strong diuretic regimen without causing hypokalemia for patients with fluid overload (schematic diagram in Fig. 5 Upper and Lower), such as those with congestive heart failure, nephrotic syndrome, diuretic resistance, or generalized edema. Whether the combination of thiazides and mineralocorticoid antagonists can be as effective as the combination of thiazides and pendrin inhibitors remains speculative. Furthermore, whether alterations in the milieu of the CCD subsequent to pendrin inhibition (such as acidic luminal pH) are important determinants of the salt wasting phenotype in pendrin/NCC double KO mice (and by inference pendrin inhibitor/thiazide combination) is currently unknown.
Fig. 5.
Proposed schematic diagram depicting the synergistic diuretic effects of thiazides and inhibitors of pendrin in the distal nephron. The combined inhibition of NCC and pendrin (Lower) can cause significant salt and water wasting vs. control (Upper).
Experimental Procedures
Animal Models.
Details of generation of Slc26a4 (pendrin) and the thiazide-sensitive cotransporter (NCC)-null mice have been reported by our group (12, 15). Double pendrin/NCC KO mice were generated by crossing pendrin KO mice with NCC KO mice. The WT, single KO, and double KO mice have a mixed BALB/c and C57BL/6J background.
Tail DNA Genotyping for NCC KO, pendrin KO, and Pendrin/NCC Double KO Mice.
The details of tail DNA genotyping are included in SI Experimental Procedures.
Antibodies, Western Blotting, and Immunofluorescence Labeling.
Details of antibodies, Western blots, and immunofluorescence labeling are included in SI Experimental Procedures.
RNA Isolation and Northern Hybridization.
Details regarding RNA isolation, hybridization, and gene-specific PCR fragments are included in SI Experimental Procedures.
Blood Composition and Urine Electrolytes Analysis.
Details of blood composition and urine electrolyte analysis are included in SI Experimental Procedures.
Blood Pressure Monitoring.
Details of blood pressure measurements are included in SI Experimental Procedures.
Vasopressin Analog Injection.
Animals (WT and double KO mice) were placed in metabolic cages, and their urine output and water intake, along with food intake, were measured before and 24 h after s.c. injection with the vasopressin 2 (V2)-receptor–specific vasopressin analog dDAVP at 5 μg/100 g of body weight.
Statistical Analysis.
The results for chloride excretion, sodium excretion, urine volume, urine osmolarity, and blood pressure are presented as means ± SE. Statistical significance between WT and double KO mice was determined by Student’s unpaired t test or ANOVA, and P < 0.05 was considered significant. Unless indicated, at least four separate animals from each group (WT, pendrin KO, NCC KO, or double KO) were used for determination of expression levels (Northern, Western, or immunofluorescence) and balanced studies.
Supplementary Material
Acknowledgments
This work was supported by a Merit Review award from the Department of Veterans Affairs, funds from the Center on Genetics of Transport and Epithelial Biology at the University of Cincinnati, National Institutes of Health Award R56DK62809, and grants from US Renal Care and Dialysis Clinic, Inc. (DCI) (to M.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202671109/-/DCSupplemental.
References
- 1.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- 2.Ellison DH. The thiazide-sensitive Na-Cl cotransporter and human disease: Reemergence of an old player. J Am Soc Nephrol. 2003;14:538–540. doi: 10.1681/ASN.V142538. [DOI] [PubMed] [Google Scholar]
- 3.Delpire E, Kaplan MR, Plotkin MD, Hebert SC. The Na-(K)-Cl cotransporter family in the mammalian kidney: Molecular identification and function(s) Nephrol Dial Transplant. 1996;11:1967–1973. doi: 10.1093/oxfordjournals.ndt.a027081. [DOI] [PubMed] [Google Scholar]
- 4.Soleimani M, et al. Pendrin: An apical Cl-/OH-/HCO3- exchanger in the kidney cortex. Am J Physiol Renal Physiol. 2001;280:F356–F364. doi: 10.1152/ajprenal.2001.280.2.F356. [DOI] [PubMed] [Google Scholar]
- 5.Royaux IE, et al. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA. 2001;98:4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verlander JW, et al. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: Role of pendrin in mineralocorticoid-induced hypertension. Hypertension. 2003;42:356–362. doi: 10.1161/01.HYP.0000088321.67254.B7. [DOI] [PubMed] [Google Scholar]
- 7.Kim YH, et al. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol. 2002;283:F744–F754. doi: 10.1152/ajprenal.00037.2002. [DOI] [PubMed] [Google Scholar]
- 8.Wall SM, et al. Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol. 2003;284:F229–F241. doi: 10.1152/ajprenal.00147.2002. [DOI] [PubMed] [Google Scholar]
- 9.Petrovic S, Wang Z, Ma L, Soleimani M. Regulation of the apical Cl-/HCO-3 exchanger pendrin in rat cortical collecting duct in metabolic acidosis. Am J Physiol Renal Physiol. 2003;284:F103–F112. doi: 10.1152/ajprenal.00205.2002. [DOI] [PubMed] [Google Scholar]
- 10.Câmpean V, Kricke J, Ellison D, Luft FC, Bachmann S. Localization of thiazide-sensitive Na(+)-Cl(-) cotransport and associated gene products in mouse DCT. Am J Physiol Renal Physiol. 2001;281:F1028–F1035. doi: 10.1152/ajprenal.0148.2001. [DOI] [PubMed] [Google Scholar]
- 11.Delpire E, Mount DB. Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu Rev Physiol. 2002;64:803–843. doi: 10.1146/annurev.physiol.64.081501.155847. [DOI] [PubMed] [Google Scholar]
- 12.Schultheis PJ, et al. Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na+-Cl- cotransporter of the distal convoluted tubule. J Biol Chem. 1998;273:29150–29155. doi: 10.1074/jbc.273.44.29150. [DOI] [PubMed] [Google Scholar]
- 13.Loffing J, et al. Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman’s syndrome. J Am Soc Nephrol. 2004;15:2276–2288. doi: 10.1097/01.ASN.0000138234.18569.63. [DOI] [PubMed] [Google Scholar]
- 14.Wall SM, et al. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: Role in Cl- conservation. Hypertension. 2004;44:982–987. doi: 10.1161/01.HYP.0000145863.96091.89. [DOI] [PubMed] [Google Scholar]
- 15.Amlal H, et al. Deletion of the anion exchanger Slc26a4 (pendrin) decreases apical Cl(-)/HCO3(-) exchanger activity and impairs bicarbonate secretion in kidney collecting duct. Am J Physiol Cell Physiol. 2010;299:C33–C41. doi: 10.1152/ajpcell.00033.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sica DA, Carter B, Cushman W, Hamm L. Thiazide and loop diuretics. J Clin Hypertens (Greenwich) 2011;13:639–643. doi: 10.1111/j.1751-7176.2011.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reilly RF, Peixoto AJ, Desir GV. The evidence-based use of thiazide diuretics in hypertension and nephrolithiasis. Clin J Am Soc Nephrol. 2010;5:1893–1903. doi: 10.2215/CJN.04670510. [DOI] [PubMed] [Google Scholar]
- 18.Wang XY, et al. The renal thiazide-sensitive Na-Cl cotransporter as mediator of the aldosterone-escape phenomenon. J Clin Invest. 2001;108:215–222. doi: 10.1172/JCI10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glover M, Zuber AM, O’Shaughnessy KM. Hypertension, dietary salt intake, and the role of the thiazide-sensitive sodium chloride transporter NCCT. Cardiovasc Ther. 2011;29:68–76. doi: 10.1111/j.1755-5922.2010.00180.x. [DOI] [PubMed] [Google Scholar]
- 20.Gesek FA, Friedman PA. Sodium entry mechanisms in distal convoluted tubule cells. Am J Physiol. 1995;268:F89–F98. doi: 10.1152/ajprenal.1995.268.1.F89. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Hropot M, Aronson PS, Giebisch G. Role of NHE isoforms in mediating bicarbonate reabsorption along the nephron. Am J Physiol Renal Physiol. 2001;281:F1117–F1122. doi: 10.1152/ajprenal.2001.281.6.F1117. [DOI] [PubMed] [Google Scholar]
- 22.Everett LA, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 23.van der Lubbe N, et al. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 2011;79:66–76. doi: 10.1038/ki.2010.290. [DOI] [PubMed] [Google Scholar]
- 24.Hadchouel J, et al. Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension. Proc Natl Acad Sci USA. 2010;107:18109–18114. doi: 10.1073/pnas.1006128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallet M, et al. Pendrin regulation in mouse kidney primarily is chloride-dependent. J Am Soc Nephrol. 2006;17:2153–2163. doi: 10.1681/ASN.2005101054. [DOI] [PubMed] [Google Scholar]
- 26.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl- cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol. 2009;297:F704–F712. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amlal H, Barone SL, Xu J, Soleimani M. Pendrin confers partial resistance to the diuretic effect of loop and thiazide diuretics in mice. J Am Soc Nephrol. 2010;21:480A. [Google Scholar]
- 28.Pela I, Bigozzi M, Bianchi B. Profound hypokalemia and hypochloremic metabolic alkalosis during thiazide therapy in a child with Pendred syndrome. Clin Nephrol. 2008;69:450–453. doi: 10.5414/cnp69450. [DOI] [PubMed] [Google Scholar]
- 29.Leviel F, et al. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120:1627–1635. doi: 10.1172/JCI40145. and correction (2011) 121:1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wangemann P. The role of pendrin in the development of the murine inner ear. Cell Physiol Biochem. 2011;28:527–534. doi: 10.1159/000335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.