Abstract
In vivo recycling of nitrate (NO3−) and nitrite (NO2−) is an important alternative pathway for the generation of nitric oxide (NO) and maintenance of systemic nitrate–nitrite–NO balance. More than 25% of the circulating NO3− is actively removed and secreted by salivary glands. Oral commensal bacteria convert salivary NO3− to NO2−, which enters circulation and leads to NO generation. The transporters for NO3− in salivary glands have not yet been identified. Here we report that sialin (SLC17A5), mutations in which cause Salla disease and infantile sialic acid storage disorder (ISSD), functions as an electrogenic 2NO3−/H+ cotransporter in the plasma membrane of salivary gland acinar cells. We have identified an extracellular pH-dependent anion current that is carried by NO3− or sialic acid (SA), but not by Br−, and is accompanied by intracellular acidification. Both responses were reduced by knockdown of sialin expression and increased by the plasma membrane-targeted sialin mutant (L22A-L23A). Fibroblasts from patients with ISSD displayed reduced SA- and NO3−-induced currents compared with healthy controls. Furthermore, expression of disease-associated sialin mutants in fibroblasts and salivary gland cells suppressed the H+-dependent NO3− conductance. Importantly, adenovirus-dependent expression of the sialinH183R mutant in vivo in pig salivary glands decreased NO3− secretion in saliva after intake of a NO3−-rich diet. Taken together, these data demonstrate that sialin mediates nitrate influx into salivary gland and other cell types. We suggest that the 2NO3−/H+ transport function of sialin in salivary glands can contribute significantly to clearance of serum nitrate, as well as nitrate recycling and physiological nitrite-NO homeostasis.
Keywords: pH, proton
The anions NO3− and NO2− were once thought to be inert end products of NO metabolism. However, it is now evident that nitrate and nitrite can be recycled in vivo to form NO, and thus these anions complement the nitric oxide synthase (NOS)-dependent activity (1). The nitrate-nitrite-NO pathway is emerging as a potential therapeutic target in such diseases as myocardial infarction, stroke, gastric ulcers, and pulmonary hypertension (1, 2). There are two major sources of nitrate and nitrite: the l-arginine–NO synthase pathway and diet. Dietary intake of nitrate leads to a relatively rapid increase in NO3− concentration in serum. Although a large part of the anion is excreted via the kidneys, up to 25% of the circulating nitrate is actively taken up by the salivary glands and concentrated ∼10-fold in the saliva secreted from the glands (3–5). Conditions that compromise salivary gland function have been linked to decreased NO3− secretion from the salivary glands and increased NO3− levels in the serum and urine (5, 6). Although nitrate can be reduced to nitrite by the commensal bacteria in the oral cavity, most of the salivary nitrite escapes gastric conversion to NO and enters the systemic circulation, where it generates NO. Thus, salivary nitrate is recycled back to NO2− and is critical for the maintenance of physiological levels of NO and NO2− in the serum (1, 2, 7).
Nitrate uptake into salivary glands represents the key initial step in NO3− clearance from the serum; however, the mechanism mediating transport of NO3− in salivary gland epithelial cells has not yet been established. In this study, we examined NO3− influx in salivary gland cells. Here we report that the sialic acid (SA)/H+ cotransporter, sialin (SLC17A5), mutations in which result in Salla disease and ISSD, is involved in nitrate uptake into salivary glands. Our data suggest a similar function for sialin in several other cell types as well, including fibroblasts. We show that sialin is endogenously localized in the lysosomes as well as in the plasma membrane of salivary gland cells, where it functions as an electrogenic 2NO3−/H+ cotransporter mediating influx of nitrate into the cell. We also provide evidence that plasma membrane sialin is a multifunctional anion transporter that can mediate electrogenic A−/H+ cotransport of anions such as SA, glutamate, and aspartate. Importantly, we have assessed the function of sialin in vivo in pig salivary glands and provide evidence for the physiological relevance of sialin in mediating nitrate uptake NO3− influx into pig salivary glands. In aggregate, our findings suggest that sialin is a versatile anion transporter, and that functional defects in the protein may have a deleterious impact on several critical physiological functions.
Results
Coupled NO3− Currents and Intracellular Acidification in Human Submandibular Gland Cells.
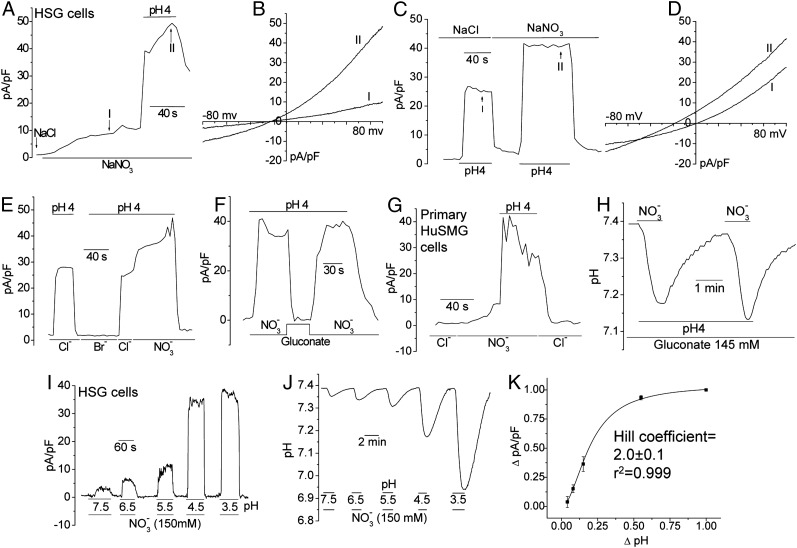
Human submandibular gland cell line (HSG) cells did not display constitutive currents in standard extracellular solution. Replacement of Cl− with 150 mM NO3− produced a relatively slow, but significant, spontaneous increase in the outward current that was dramatically enhanced by decreasing the external pH from 7.4 to 4.0 (Fig. 1A); the current density in the 150 mM NO3− solution was 42.4 ± 5.4 pA/pF (n = 21). Both the constitutive and low pH-induced currents had similar outwardly rectifying characteristics with a reversal potential of −15 ± 2 mV (n = 19) (Fig. 1B). Low pH also increased the outward current in normal standard extracellular solution (Cl−-containing), although the amplitude of the current was smaller than that seen with NO3− (23.1 ± 4.3 pA/pF; n = 18) (Fig. 1C), and the current reversed at 0 mV (Fig. 1D). Decreasing the external pH in Br−- or gluconate-containing medium did not generate any detectable current (Fig. 1 E and F). Similar findings were observed in primary human submandibular gland (huSMG) cells (Fig. 1G), human parotid gland ductal cells, and freshly dispersed acini cells prepared from mouse salivary glands. Cl− channels in salivary gland cells are quite permeable to NO3− and Br−, unlike the activity described here (8–11). Nonetheless, NO3− conductance was inhibited by several anion channel blockers that block various Cl− channels (Fig. S1). Furthermore, unlike known Cl− channels in salivary gland cells, NO3− conductance was not regulated by cAMP, intracellular or extracellular Ca2+, or muscarinic or purinergic receptor agonists (Fig. S2). Replacing Na+ in the medium with Cs+ also had no affect on the current (Fig. S3 A and B). Relatively lower (and likely more physiological) NO3− concentrations (0.05–0.5 mM) also induced currents in HSG cells, with channel densities of 2.3 ± 0.3 pA/pF (n = 5) and 3.4 ± 0.5 pA/pF (n = 6), respectively (Fig. S3C). Taken together, these findings demonstrate the unique properties of the NO3− conductance detected in salivary gland cells.
Fig. 1.
Coupled NO3− currents and intracellular acidification in HSG cells. NO3− currents in HSG cells (A–F) and primary huSMG cells (G) measured by the whole-cell patch-clamp technique. NaCl was replaced with NaNO3, NaBr, or Na-gluconate as indicated. Changes in extracellular pH are shown in the traces (bar). I-V curves are shown in B and D. (H) External pH and NO3− (5 mM)-dependent acidification of HSG cells measured using BCECF fluorescence. (I) pH dependence of NO3− currents in HSG cells. (J) Intracellular pH changes under the same experimental conditions as shown in I. (K) Data from I and J were used to determined the relationship of NO3− currents and intracellular acidification (Hill coefficient: 2.0 ± 0.1).
To examine the modulation of NO3− conductance by pH, we monitored intracellular pH using 2′-7′-bis(carboxyethyl)-5(6)-carboxyfluorescein (BCECF). Decreasing the external pH in gluconate-containing medium (ambient [Cl−] in gluconate-containing medium was <10 mM) did not change intracellular pH, whereas including 5 mM NO3− induced rapid acidification that was reversed by replacing NO3− with gluconate in the medium (Fig. 1H). When external pH was varied, keeping [NO3−] constant at 5 mM, the outward current was changed as a function of external pH (Fig. 1I). Importantly, measurement of intracellular pH under the same conditions demonstrated pH- and NO3−-dependent intracellular acidification (Fig. 1J). The data were fitted (R2 = 0.999) using the Hill equation, yielding a Hill coefficient of 2.0 ± 0.1 (Fig. 1K), consistent with the electrophysiological data. These data strongly suggest that an electrogenic 2NO3−/H+ cotransporter is involved in mediating NO3− uptake in salivary gland epithelial cells.
Involvement of Sialin in 2NO3−/H+ Cotransport.
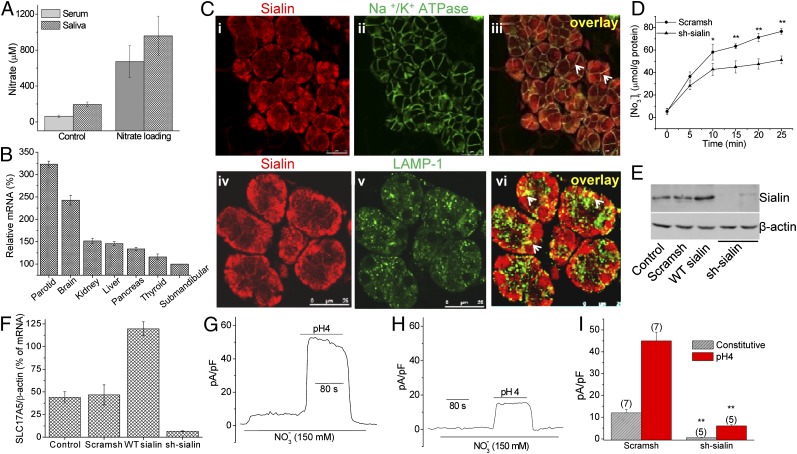
To examine nitrate transport via salivary gland, [NO3−] was measured in the serum and saliva of miniature pigs fed with regular or nitrate-rich fodder. In both cases, the [NO3−] level in saliva exceeded that in serum within 1 h after feeding (Fig. 2A), suggesting that salivary glands take up and secrete nitrate. The nitrate transporters identified to date belong to the highly conserved major facilitator superfamily (MFS) (11–15). To determine the molecular component mediating 2NO3−/H+ cotransport in salivary glands, we analyzed the expression of 127 MFS genes (11, 16, 17) in human parotid and submandibular glands (n = 3) using Affymetrix U133Plus 2.0 microarrays. SLC17A5 (which encodes sialin) was expressed at high levels in both types of salivary glands (Table S1). Sialin functions as a lysosomal SA/H+ transporter involved in SA efflux, although it is detected in the plasma membrane of neurons (18, 19) where it mediates aspartate and glutamate transport. The protein is widely expressed in such tissues as brain, heart, lung, liver, kidney, and mouse submandibular glands (20–22). Importantly, mutations in sialin are causative factors in the neurodegenerative disorders Salla disease and ISSD (18, 23). We validated the expression of sialin in various human tissues by qPCR (Fig. 2B) and by Western blot analysis using samples of various tissues from miniature pigs (Fig. S4). Sialin was highly expressed in salivary glands, and liver, with lower levels of expression in brain, spleen and kidney, and even lower in muscle and pancreas. Sialin was localized in the basolateral regions of human parotid gland biopsies and in lysosomes as detected by its colocalization with the respective markers Na+/K+ ATPase and LAMP-1 (Fig. 2C).
Fig. 2.
Involvement of sialin in 2NO3−/H+ cotransport. (A) Saliva and serum [NO3−] in miniature pigs fed on regular fodder or supplemented with 100 mg/kg of nitrate. (B) Validation of gene expression of SLC17A5 based on the MFS gene expression pattern (Table S1). (C) Detection of sialin (i and iv) in human salivary gland. Na/K-ATPase (ii) or LAMP-1 (v) are markers for basolateral membrane and lysosomes, respectively. Colocalization of the two proteins is shown (iii and vi, yellow, indicated by arrows). (D) Nitrate uptake in HSG cells transfected with sh-sialin or scram-sh. The values indicated by * or ** are significantly different from the unmarked values. (P < 0.05 or P < 0.01; n ≥ 3). (E and F) Knockdown of sialin by sh-sialin and overexpression of WT-sialin. (G and H) Effect of sialin knockdown on constitutive and low-pH–induced NO3− current. Levels of current and intracellular pH in control HSG cells transfected with scram-sh (G) or sh-sialin (H) were as described in Fig. 1. (I) Average data obtained from the experiments shown in G and H. The number of cells tested is indicated. Statistically significant differences are indicated by ** (P < 0.01).
Based on the expression and function of known solute carrier (SLC) transporters (Table S1) and the channel activity described in Fig. 1, we assessed the involvement of sialin in 2NO3−/H+ cotransport. HSG cells accumulated NO3−, and this function was significantly decreased in cells expressing sh-sialin compared to cells expressing scrambled shRNA (scram-sh) (Fig. 2D; protein expression and mRNA levels are shown in Fig. 2 E and F). Intracellular levels of K+, Na+, and Cl− were not changed by sh-sialin expression. Importantly, NO3− current in HSG cells maintained at normal or low pH (Fig. 2 G–I) was significantly reduced in sh-sialin–treated cells.
In addition, incubation of HSG and other cell types, including human colon carcinoma (RKO), human gastric mucosal epithelial cells (GES-1), and human umbilical vein endothelial cells (HE-CV-304), in medium containing NO3− resulted in increased levels of intracellular NO as detected by diaminofluorescein (DAF) (Fig. S5 A and B); nitrite was not detected in the nitrate loading solution. Athough HSG cells displayed nitrate conductance when exposed to physiological levels of nitrate (50–1,000 μM), indicating that these cells have an influx pathway that can transport nitrate at relatively low [NO3−], generation of NO and cGMP was detected only at relatively high, nonphysiological levels of the anions (≥15 mM nitrate and 3 mM NO2−, respectively) (Fig. S5 C and D). Note that other tissues, such as liver, can effectively metabolize both anions at lower concentrations (Fig. S5E). This indicates that direct nitrate uptake via sialin is not a physiological pathway for NO generation in these cell types; rather, once secreted, salivary nitrate is metabolized to nitrite by the action of commensal bacteria in the oral cavity. This is not a completely unexpected finding, given that the physiological function of salivary gland cells is to mediate transepithelial NO3− transport and concentrate the anion in saliva. A similar conclusion was reached in an earlier study, which showed that salivary glands deliver NO3− from the serum into the oral cavity with minimal metabolism (2).
Sialin Mediates NO3−/H+ and SA/H+ Cotransport in HSG Cells.
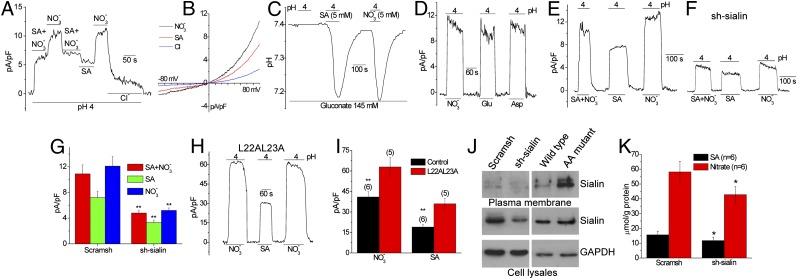
Inclusion of SA, a major substrate for sialin, in the external medium also induced currents when ambient pH was decreased. Although NO3− and SA individually (5 mM each) generated outward currents at low external pH (Fig. 3A), the amplitude with NO3− (11.2 ± 2.2 pA/pF; n = 15) was greater than that with SA (5.4 ± 0.7 pA/pF; n = 7). When both anions were present together (10.6 ± 0.7 pA/pF; n = 9), the current amplitude was slightly more than that seen with SA alone, although it was not a sum of the individual currents. The characteristics of currents were similar in all of the conditions (Fig. 3B). As seen with NO3−, decreasing extracellular pH in the presence of 5 mM SA also led to intracellular acidification (Fig. 3C). In addition, proton-dependent aspartate and glutamate currents, similar to NO3− current, were detected in these cells (Fig. 3D), consistent with a previous study indicating that sialin transports both of these anions (20). Sialin knockdown decreased low-pH stimulated currents in HSG cells perfused with media containing SA + NO3−, SA, or NO3− (Fig. 3 E–G). Importantly, expression of the sialin mutant L22A-L23A, which reportedly is targeted to the plasma membrane (24, 25), increased the currents by approximately twofold with either NO3− or SA in the external solution (Fig. 3 H and I). This increase was also detected when the anions were used at low concentrations (5 mM). Surface biotinylation confirmed the presence of endogenous sialin in the plasma membrane, which was decreased by the expression of sh-sialin and increased by expression of sialin L22A-L23A (Fig. 3J). NO3− and SA were transported into HSG cells (NO3− uptake > SA uptake), and uptake of both anions was reduced when sialin expression was suppressed by sh-sialin (Fig. 3K).
Fig. 3.
Sialin mediates NO3−/H+ as well as SA/H+ cotransporter in HSG cells. (A) Current measurement in cells perfused with medium at pH 4.0 containing 5 mM SA, NO3−, or both. (B) I-V curves of the current (Erev= 0 mV for 5 mM SA or NO3−) obtained under the different conditions shown in A. (C) Intracellular pH in medium of pH 4.0 containing either SA or NO3−. (D) Currents generated by substituting NO3− with glutamate (Glu) or aspartate (Asp), with other anions transported by sialin. (E and F) SA and NO3− currents induced at pH 4.0 in control HSG cells (E) and cells treated with sh-sialin (F). The y-axis scale is the same in E and F. (G) Average of data from the experiments shown in E and F. (H) Effect of expression of plasma membrane-targeted mutant of sialin L22A-L23A on NO3− and SA currents. (I) Average data and statistical evaluation for H. (J) Surface expression of sialin in WT cells and in cells transfected with scram-sh, sh-sialin, or L22A-L23A mutant (AA). (K) NO3− and SA uptake measured at 10 min after loading in HSG cells transfected with sh-sialin or scram-sh. *Values significantly different from the unmarked values (P < 0.05; n = 6).
Sialin also transports NO2−, as detected by activation of low-pH currents when either NO3− or NO2− was included in the bath solution (Fig. S3D). NO2− current (7.5 ± 1.0 pA/pF; n = 8) was smaller than NO3− current (11.2 ± 2.2 pA/pF; n = 15), but was not additive in solutions containing both anions (12.8 ± 2.4 pA/pF; n = 5). Nitrite uptake was confirmed in cells for which including nitrite or nitrate in the medium led to intracellular increases in the accumulation of the respective anion, and in cells for which including SA with the anions did not significantly alter the uptake of either anion (Fig. S3E).
Assessment of Sialin-Mediated NO3− Transport in Fibroblasts from Patients with ISSD and Effect of the Salla Disease and ISSD Sialin Mutations on Anion Transport.
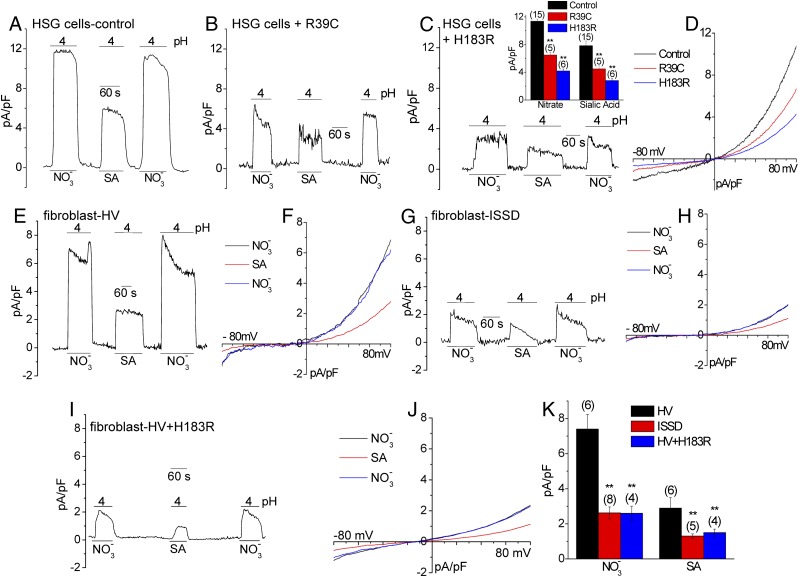
To establish the link between sialin and nitrate transport, we examined the effect of two nonfunctional sialin mutants that have been associated with Salla disease (R39C) and ISSD (H183R) (23–25). Expression of each of these mutants in HSG cells induced dominant suppression of both NO3− and SA− currents without altering the current-voltage (I-V) characteristics (Fig. 4 A–D). A major finding of the present study was that fibroblasts from patients with ISSD displayed a substantial reduction in pH-dependent NO3− or SA currents compared with the current amplitudes in cells from healthy volunteers (Fig. 4 E and G) with no change in the I-V relationship (Fig. 4 F and H). Note that a relatively higher [NO3−] level was required to detect NO3− conductance in fibroblasts. Although we could not induce recovery of function in cells obtained from patients with ISSD by expressing the WT-sialin (likely due to a dominant negative effect of the endogenously expressed mutant), expression of sialinH183R in cells from healthy volunteers strongly suppressed transporter function to levels seen in the cells from patients with ISSD (Fig. 4 I–K). Although we appreciate that including data demonstrating salivary deficiencies in patients would have strengthened our findings, several major issues preclude conducting such a study at the present time. There are very few patients with Salla disease or ISSD worldwide. In Salla disease, found primarily in Finland, newborns develop intellectual impairment gradually. ISSD, although not geographically restricted, is more severe, and patients generally die early in childhood or even in utero. Both disorders cause developmental delays. The majority of patients are children with severe developmental defects and poor overall health. Thus, although assessment of saliva in patients might yield significant data, this is beyond the scope of the present study. Despite lack of such data, the findings presented herein establish a strong link between nitrate transport and sialin. We further demonstrate that disease-causing mutants of sialin also decreases NO3− transport.
Fig. 4.
Assessment of sialin-mediated NO3− transport in fibroblasts from patients with ISSD, showing the effect of the Salla disease and ISSD sialin mutations on anion transport. (A–D) NO3− and SA (5 mM each) currents in HSG cells transfected with sialinR39C (B) or sialinH183R (C). (C, Insert) Average data and statistical evaluation. (D) I-V curve of the NO3− current shown in A and B. (E–H) Sialin-mediated current (150 mM SA or NO3−) in fibroblasts from healthy volunteers (HV) (E and F) and patients with ISSD (G and H). (I and J) Effect of sialinH183R expression on anion transport in fibroblasts from patients with ISSD. (K) Average data from the experiments with cells from patients.
Effect of Adenovirus-Dependent Expression of Sialinh183r Mutant in Vivo in Pig Salivary Glands on Salivary Nitrate Secretion.
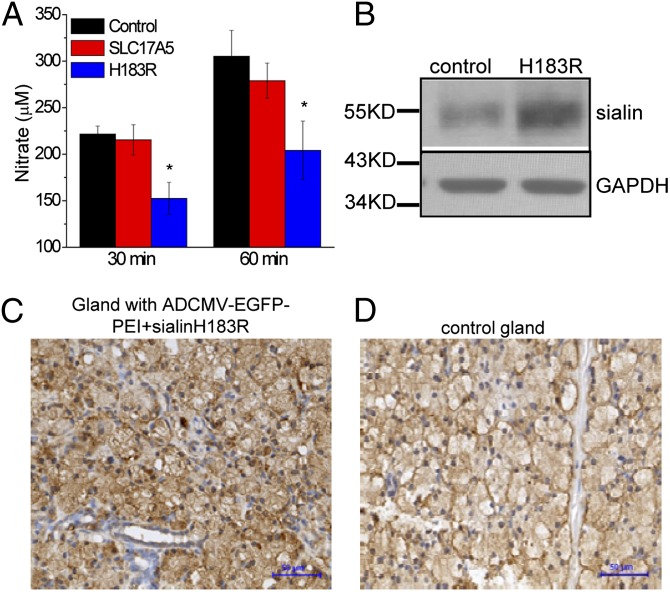
To provide further evidence that sialin mediates salivary gland nitrate transport, we used adenoviral vector containing cytomegalovirus promoter (AdCMV)-EGFP-polyethylenimine (PEI) complex as a carrier to deliver sialinH183R or WT-sialin vector into pig salivary glands. At 3 d after infection, the animals were fed a NO3−-rich diet for 30 min and then stimulated with pilocarpine to induce saliva secretion. Compared with control animals or those that received WT-sialin, the pigs receiving sialinH183R displayed relatively lower levels of NO3− in the saliva (152.5 ± 17.3 μM vs. 221.6 ± 8.5 μM at 30 min and 204.1 ± 31.5 vs. 305.3 ± 27.8 μM after 60 min of feeding; P < 0.05; data obtained from fours pigs and eight parotid glands in each group) (Fig. 5A). [NO3−] in the saliva from animals overexpressing WT-sialin was not significantly different than that from control animals, and although serum and urine [NO3−] levels were higher after feeding, there was no difference between the two groups. Western blot analysis (Fig. 5B) revealed greater sialin expression in parotid glands in pigs receiving the sialinH183R plasmid compared with controls (receiving AdCMV-EGFP-PEI alone). Furthermore, immunohistochemistry demonstrated a higher sialin signal in glands receiving sialinH183R compared with those in the control group (Fig. 5 C and D). Taken together with the data shown in Fig. 4, these data strongly suggest that nitrate transport in salivary glands could be potentially altered in patients with ISSD or Salla disease.
Fig. 5.
Effect of in vivo suppression of sialin function in salivary glands on salivary nitrate secretion. (A) Salivary nitrate concentrations at 30 min and 60 min after feeding a nitrate-rich diet to miniature pigs after in vivo delivery of plasmids encoding WT-sialin or sialinH183R (Methods). *Values significantly different from the control values (P < 0.05). (B) Sialin expression in control parotid glands and glands receiving the plasmid-PEI-Ad complex as determined by Western blot analysis. (C and D) Detection of sialin in salivary glands at 3 d after transduction with AdCMV-EGFP-PEI plus sialinH183R vector (C) or in control parotid gland (D). (Scale bar: 50 μM.)
Discussion
Herein we report a novel function for the lysosomal SA/H+ cotransporter, sialin (SLC17A5), which has been linked to Salla disease and ISSD. We show that sialin can also function as an electrogenic 2NO3−/H+ cotransporter in the plasma membrane. Other findings include that (i) sialin mutants associated with Salla disease and ISSD induce dominant suppression of 2NO3−/H+ cotransporter activity, (ii) cotransporter activity is relatively low in fibroblasts obtained from patients with ISSD compared with healthy volunteers, and (iii) expression of the ISSD-associated sialin mutant (H183R) suppresses cotransporter function in cells from healthy volunteers. Taken together, these data provide strong evidence that sialin mediates H+-dependent NO3− uptake into cells. Although it is well established that the lysosomal H+/SA− transport function mediated by sialin contributes to lysosomal recycling of SA, further studies are needed to determine the exact physiological function of nitrate transport mediated by sialin in fibroblasts and other cell types. However, it has been well established that salivary glands serve as a major route for NO3− clearance from the serum. Approximately 25% of the circulating NO3− is taken up by the salivary glands, where it is concentrated and secreted (at millimolar levels) in the saliva. Thus, it is likely that transepithelial flux of NO3− occurs across the salivary gland cells as the anion is taken up from the serum and secreted into saliva.
We propose that sialin can function as the NO3− uptake system in salivary gland cells. Consistent with this proposal, we found that the protein is localized in the basal and lateral regions. Further studies are needed to identify the NO3− efflux pathway likely to be localized in the apical membrane of the cells. The physiological relevance of sialin in NO3− transport via the salivary gland is confirmed by our finding that in vivo expression of the dominant negative sialin mutant (sialinH183R) in pig parotid glands reduced NO3− secretion in the saliva in response to a NO3−-rich diet compared with the function in control animals and those expressing WT-sialin in the glands. Together with our findings demonstrating that sialin mediates NO3− uptake into salivary gland cells, these data provide evidence for a physiological role of sialin in nitrate uptake into these glands. In addition, sialin appears to be a versatile anion transporter that also has the ability to mediate H+-dependent transport of SA, NO2−, aspartate, or glutamate. Given the protein’s relatively wide distribution in different tissues, its function in the plasma membrane as well as lysosomes can have a significant impact on cell function, including SA recycling as well as nitrate–NO balance. In neuronal tissues, there might be additional consequences related to sialin’s ability to transport glutamate and aspartate.
In summary, our data provide insight into the function of sialin and demonstrate its unique function as a nitrate transporter. In mammals, the diet is a major source of NO3−; absorption of dietary NO3− results in an increase in serum NO3−. Approximately 25% of the circulating NO3− is taken up into salivary gland and secreted via saliva, where it is reduced to nitrite by the action of commensal bacteria in the oral cavity and then converted to NO in the stomach. NO is suggested to have an important role in the protection of gastric tissues from stress-induced injury; however, a large amount of ingested nitrite survives hydrolysis in the stomach and enters the systemic circulation, where it is reduced to NO and other bioactive nitrogen oxides (1, 2). Thus, salivary nitrate transport provides an alternative, noncanonical pathway for the generation of nitrite and NO. This pathway appears to be particularly significant under conditions of hypoxia and acidosis. Taken together, these findings suggest that NO3− secretion via the gland can significantly impact the nitrate–nitrite–NO balance in the serum, which is of major importance in such conditions as high blood pressure, platelet aggregation, and vascular damage (26–30). We suggest that disruption of sialin function, as seen in patients with Salla disease or ISSD, can have significant effects on the nitrate–nitrite–NO balance, in addition to the previously recognized impairment in SA storage and aspartergic neurotransmission. Furthermore, loss of salivary gland secretory activity as a result of radiation treatment for head and neck cancers or autoimmune disease (e.g., Sjogren’s syndrome) can have potential systemic consequences owing to an imbalance of nitrite–NO homeostasis. Together, the findings presented herein demonstrate that sialin, as a nitrate cotransporter in the salivary glands, can play an important role in the physiological regulation of systemic nitrate–nitrite–NO balance.
Methods
All reagents and detailed methods are described in SI Methods. These include cell culture, electrophysiology, confocal imaging, and biochemical techniques such as surface biotinylation and western blotting. Descriptions of experiments with miniature pigs are also provided in SI Methods.
Supplementary Material
Acknowledgments
We thank Dr. Jim Turner, Dr. Shmuel Muallem, Dr. Jing Jiang, and Professor Liangbiao Chen for their invaluable help during the course of this work. This study was supported by the National Nature Science Foundation of China (Grants 30430690, 30125042, and 81170975), the National Basic Research Program of China (Grants 2007CB947304 and 2010CB944801), and the Divisions of Intramural Research of the National Institute of Dental and Craniofacial Research and the National Human Genome Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.G. is a guest editor invited by the Editorial Board.
See Commentary on page 13144.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116633109/-/DCSupplemental.
References
- 1.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 2.Jansson EA, et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–417. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 3.Djekoun-Bensoltane S, Kammerer M, Larhantec M, Pilet N, Thorin C. Nitrate and nitrite concentrations in rabbit saliva: Comparison with rat saliva. Environ Toxicol Pharmacol. 2007;23:132–134. doi: 10.1016/j.etap.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Pannala AS, et al. The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic Biol Med. 2003;34:576–584. doi: 10.1016/s0891-5849(02)01353-9. [DOI] [PubMed] [Google Scholar]
- 5.Xia DS, Deng DJ, Wang SL. Destruction of parotid glands affects nitrate and nitrite metabolism. J Dent Res. 2003;82:101–105. doi: 10.1177/154405910308200205. [DOI] [PubMed] [Google Scholar]
- 6.Xia D, Deng D, Wang S. Alterations of nitrate and nitrite content in saliva, serum, and urine in patients with salivary dysfunction. J Oral Pathol Med. 2003;32:95–99. doi: 10.1034/j.1600-0714.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin N, et al. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 8.Arreola J, Melvin JE. A novel chloride conductance activated by extracellular ATP in mouse parotid acinar cells. J Physiol. 2003;547:197–208. doi: 10.1113/jphysiol.2002.028373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbier-Brygoo H, et al. Anion channels in higher plants: Functional characterization, molecular structure and physiological role. Biochim Biophys Acta. 2000;1465:199–218. doi: 10.1016/s0005-2736(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 10.Gong X, Linsdell P. Mutation-induced blocker permeability and multiion block of the CFTR chloride channel pore. J Gen Physiol. 2003;122:673–687. doi: 10.1085/jgp.200308889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pao SS, Paulsen IT, Saier MH., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo FQ, Wang R, Crawford NM. The Arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is regulated by auxin in both shoots and roots. J Exp Bot. 2002;53:835–844. doi: 10.1093/jexbot/53.370.835. [DOI] [PubMed] [Google Scholar]
- 13.Jia W, Tovell N, Clegg S, Trimmer M, Cole J. A single channel for nitrate uptake, nitrite export and nitrite uptake by Escherichia coli NarU and a role for NirC in nitrite export and uptake. Biochem J. 2009;417:297–304. doi: 10.1042/BJ20080746. [DOI] [PubMed] [Google Scholar]
- 14.Martín Y, Navarro FJ, Siverio JM. Functional characterization of the Arabidopsis thaliana nitrate transporter CHL1 in the yeast Hansenula polymorpha. Plant Mol Biol. 2008;68:215–224. doi: 10.1007/s11103-008-9363-z. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, Liu D, Crawford NM. The Arabidopsis CHL1 protein plays a major role in high-affinity nitrate uptake. Proc Natl Acad Sci USA. 1998;95:15134–15139. doi: 10.1073/pnas.95.25.15134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown S, Chang JL, Sadée W, Babbitt PC. A semiautomated approach to gene discovery through expressed sequence tag data mining: Discovery of new human transporter genes. AAPS PharmSci. 2003;5:E1. doi: 10.1208/ps050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2008;23:22–44. doi: 10.2133/dmpk.23.22. [DOI] [PubMed] [Google Scholar]
- 18.Aula N, Jalanko A, Aula P, Peltonen L. Unraveling the molecular pathogenesis of free sialic acid storage disorders: Altered targeting of mutant sialin. Mol Genet Metab. 2002;77:99–107. doi: 10.1016/s1096-7192(02)00124-5. [DOI] [PubMed] [Google Scholar]
- 19.Aula N, Kopra O, Jalanko A, Peltonen L. Sialin expression in the CNS implicates extralysosomal function in neurons. Neurobiol Dis. 2004;15:251–261. doi: 10.1016/j.nbd.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Miyaji T, et al. Identification of a vesicular aspartate transporter. Proc Natl Acad Sci USA. 2008;105:11720–11724. doi: 10.1073/pnas.0804015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarovaya N, et al. Sialin, an anion transporter defective in sialic acid storage diseases, shows highly variable expression in adult mouse brain, and is developmentally regulated. Neurobiol Dis. 2005;19:351–365. doi: 10.1016/j.nbd.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 22.He M, et al. Postnatal expression of sialin in the mouse submandibular gland. Arch Oral Biol. 2011;56:1333–1338. doi: 10.1016/j.archoralbio.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Verheijen FW, et al. A new gene, encoding an anion transporter, is mutated in sialic acid storage diseases. Nat Genet. 1999;23:462–465. doi: 10.1038/70585. [DOI] [PubMed] [Google Scholar]
- 24.Morin P, Sagné C, Gasnier B. Functional characterization of wild-type and mutant human sialin. EMBO J. 2004;23:4560–4570. doi: 10.1038/sj.emboj.7600464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wreden CC, Wlizla M, Reimer RJ. Varied mechanisms underlie the free sialic acid storage disorders. J Biol Chem. 2005;280:1408–1416. doi: 10.1074/jbc.M411295200. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg JO, Carlström M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res. 2011;89:525–532. doi: 10.1093/cvr/cvq325. [DOI] [PubMed] [Google Scholar]
- 27.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 28.Gladwin MT, et al. Relative role of heme nitrosylation and beta-cysteine 93 nitrosation in the transport and metabolism of nitric oxide by hemoglobin in the human circulation. Proc Natl Acad Sci USA. 2000;97:9943–9948. doi: 10.1073/pnas.180155397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modin A, et al. Nitrite-derived nitric oxide: A possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol Scand. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 30.Dejam A, Hunter CJ, Gladwin MT. Effects of dietary nitrate on blood pressure [letter] N Engl J Med. 2007;356:1590–author reply 1590. doi: 10.1056/NEJMc070163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.