Abstract
Interest in algae has significantly accelerated with the increasing recognition of their potentially unique role in medical, materials, energy, bioremediation, and synthetic biological research. However, the introduction of tools to study, control, or expand the inner-workings of algae has lagged behind. Here we describe a general molecular method based on guanidinium-rich molecular transporters (GR-MoTrs) for bringing small and large cargos into algal cells. Significantly, this method is shown to work in wild-type algae that have an intact cell wall. Developed using Chlamydomonas reinhardtii, this method is also successful with less studied algae including Neochloris oleoabundans and Scenedesmus dimorphus thus providing a new and versatile tool for algal research.
Keywords: cell penetrating peptides, imaging, protein delivery
Algae represent a potentially inexpensive, scalable, CO2-fixing, solar-powered source of diverse chemical products including biofuels, synthetic building blocks, nanomaterials, recombinant proteins, vaccines, antibodies, medicinal leads, and food additives (1–5). They are also promising organisms for drug discovery and screening and have recognized value for bioremediation and as biosensors (6–8). However, as encountered in the delivery of agents (e.g., siRNA and biologics) into mammalian cells, efforts to study or control the inner-workings of algal cells, as required for numerous research and commercial applications, are severely limited by problems encountered in the delivery of probes, genes, and biomacromolecules across algal cell wall and membrane barriers. The delivery of chemical and biological agents into algal cells has been limited to physical and mechanical techniques (e.g. glass bead transfection, microinjection, electroporation, sonication, and biolistic methods) that are primarily used with cell wall-deficient mutants (9–11). While effective for many applications, these delivery methods are not scalable, show high variability within a given cell population, and can produce cellular damage and contamination (for instance, biolistic gold or tungsten particles). A molecular method to deliver, on variable scale, small molecules, probes, and biomacromolecules across the cell wall and membrane of wild-type algae, as required to probe and manipulate intracellular pathways in intact algae, would enable new opportunities in algal research and in the use of algae as photoautotrophic tools for synthetic biology. At the same time, such studies would serve to advance our understanding of biological barriers, a goal of central significance in the life sciences and agricultural and medical research.
We have shown previously that the ability of guanidinium-rich molecular transporters (GR-MoTrs), including guanidinium-rich cell-penetrating peptides and nonpeptidic agents, to enter mammalian cells is related to the number and spatial array of their guanidinium groups (12). Subsequent studies by us and others have shown that GR-MoTrs enable or enhance the delivery of a variety of cargos—including small molecules, metals, imaging agents, iron particles, and proteins—into a variety of mammalian cell types (13–16). GR-MoTr-drug conjugates have also advanced to clinical trials for various indications including stroke, psoriasis, and ischemic damage (17). Despite this progress with mammalian cells, little is known about the ability of GR-MoTrs to enter nonmammalian cells, especially those organisms of research and commercial significance that possess a cell wall. Only a few studies of GR-MoTrs with plant cells have been reported (18–22) and, to our knowledge, there is only a single investigation of GR-MoTrs with algae (23). In the latter study, Chlorella vulgaris, a species of green algae with a cellulosic cell wall, was found to be impermeable to a GFP-nona-(L)-arginine fusion protein.
The importance of algal delivery methods, the lack of studies in this area, and the large variation in cell wall and plasma membrane composition of different algal species prompted our interest in determining whether GR-MoTr-mediated uptake could be achieved in any algal species. We initially chose to study GR-MoTr uptake with Chlamydomonas reinhardtii because molecular and genetic techniques are well established for this organism, there are a wide variety of characterized mutants—including those exhibiting altered metabolite production and photosynthesis—and the genome is fully sequenced and annotated allowing delivery methods to be exploited to probe gene and pathway function (10, 24). We also studied several other algae from the same class, Chlorophyceae. We show for the first time that GR-MoTr-mediated uptake can be achieved in C. reinhardtii and other algal species, providing fundamental insights on differing algal barriers and a new tool for molecular manipulation or imaging of algae as required for research and commercial development.
Results and Discussion
Delivery of Small Molecule Probes to Chlamydomonas reinhardtii.
To investigate GR-MoTr uptake in algae, we elected to covalently attach a GR-MoTr to an optical probe, fluorescein. Fluorescein was selected for this purpose because, in our studies, it did not enter algal cells and its fluorescence can be visualized with 488 nm laser light thereby minimizing interference from autofluorescence. The photosynthetic machinery of green algae autofluoresces across the visible spectrum but with lower levels of autofluorescence in the green to orange range (Fig. S1). The inherent autofluorescence of the algal chloroplast was used to track the cells by irradiating with 633 nm laser light. For this study, covalently linked fluorescein-oligo-(D)-arginine conjugates of 4, 8, and 10 arginines (Fl-r4, Fl-r8, Fl-r10, respectively) were synthesized following our previously reported procedure (Fig. S2) (12).
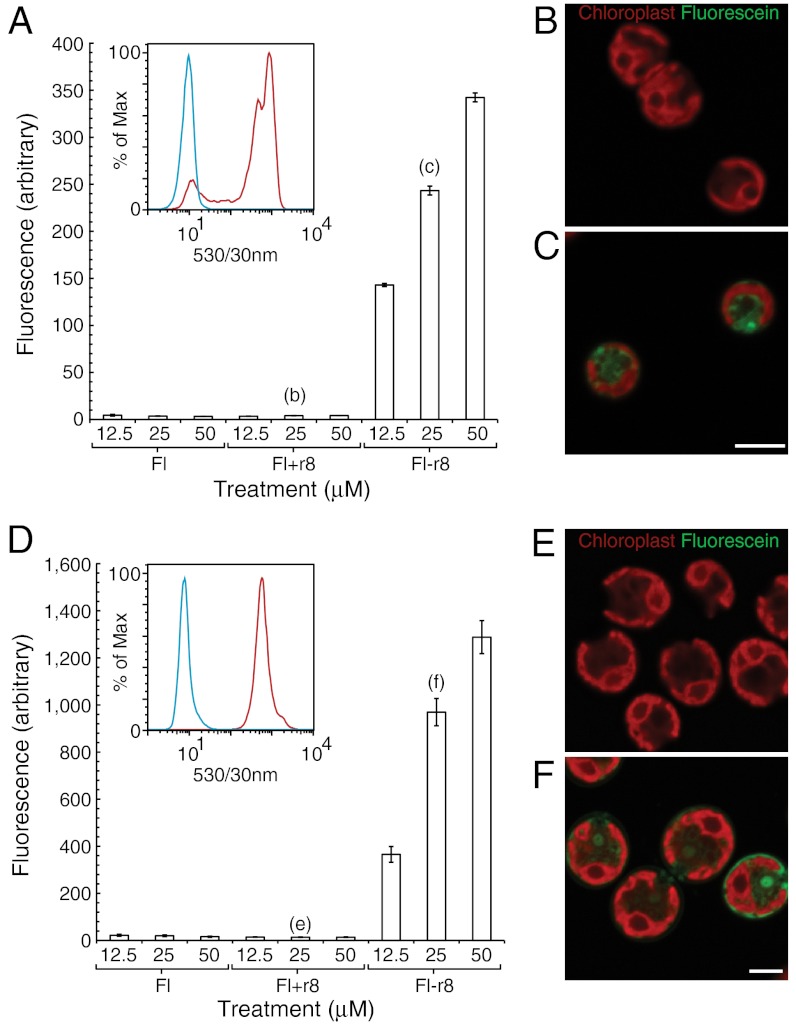
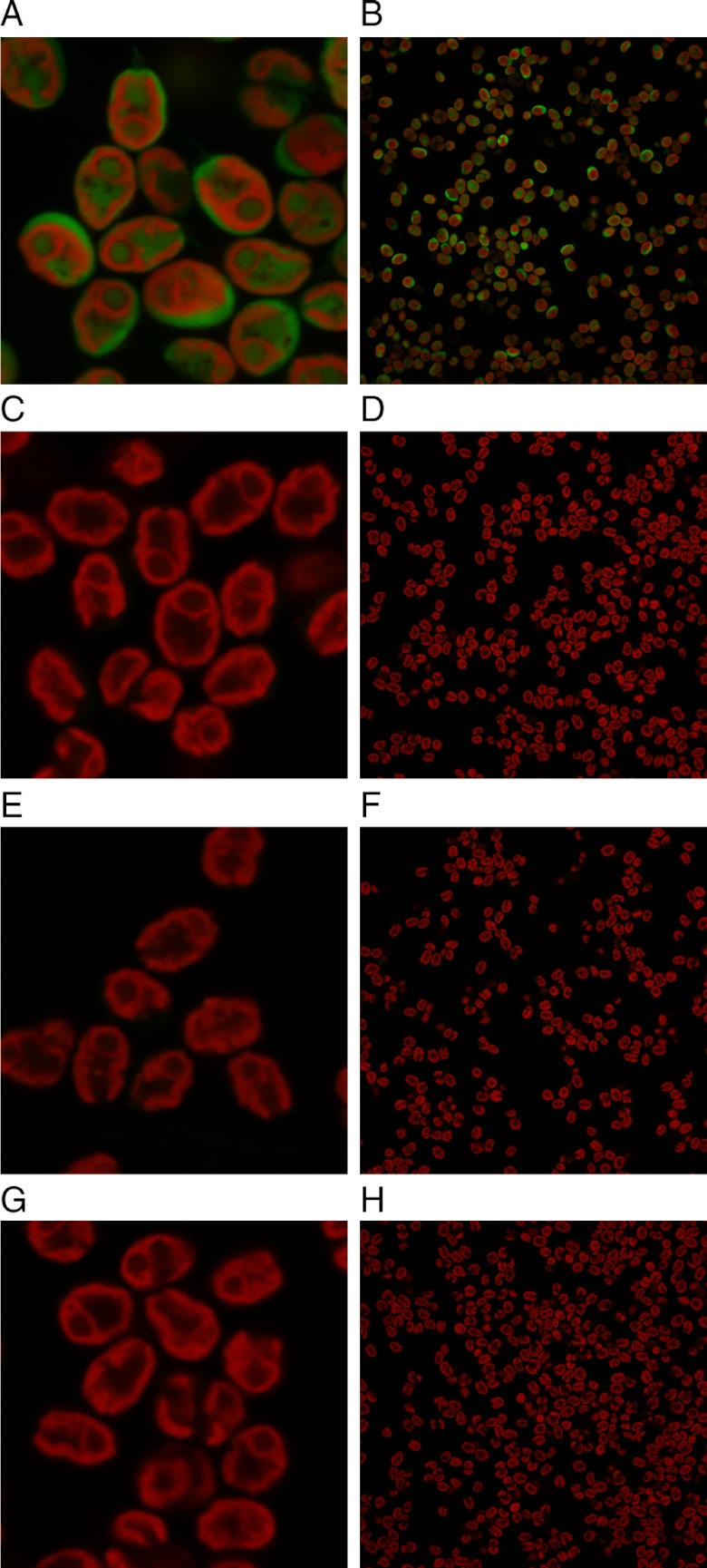
Access to the intracellular space and organelles of algae requires translocation across two barriers: the cell wall and cell membrane. To address passage across the latter, a C. reinhardtii mutant that is deficient in cell wall production (mutant cc-4350, a derivative of cw-15) was examined first. This cell wall-deficient mutant also provides a more straightforward comparison with the robust uptake of GR-MoTrs in mammalian systems (13). C. reinhardtii mutant cc-4350 was treated with Fl-r4, Fl-r8, and Fl-r10 at concentrations of 12.5, 25 and 50 μM. Fluorescein alone (Fl) and noncovalent 1∶1 mixtures of fluorescein and r8 (Fl+r8) at the same concentrations were used as controls. The cells were then analyzed by flow cytometry to determine levels of fluorescence. As had been observed in mammalian systems, Fl itself and the noncovalent mixture of Fl and r8 (Fl+r8) did not show any uptake. In striking contrast, the Fl-r8 covalent conjugate showed robust concentration-dependent uptake, behaving much as it does in the previously studied mammalian systems (12) (Fig. 1A, Fl-r4, and Fl-r10 data can be seen in Fig. S3). To determine whether the fluorescent compounds had been internalized or simply deposited on the cell surface, high-resolution confocal Z-stack fluorescent images were taken of cell wall-deficient cc-4350 cells after treatment with the noncovalent Fl+r8 control or the Fl-r8 conjugate (Fig. 1 B and C). The Z-stack images indicated that Fl-r8 was indeed internalized; whereas, in setting-matched images of cells treated with Fl+r8 controls there was no apparent internalization.
Fig. 1.
Flow cytometry and fluorescence microscopy of cell wall-deficient or wild-type C. reinhardtii treated with Fl, noncovalent mixture Fl+r8, or Fl-r8 conjugate. Graphs of the mean fluorescence from flow cytometry of (A) cell wall-deficient cc-4350 or (D) wild-type C. reinhardtii. Inset in each graph is a representative histogram from a single condition in the flow cytometry data where blue is the noncovalent Fl+r8 control and red is Fl-r8 conjugate, both at 25 μM. Confocal Z-layers of (B) cell wall-deficient and (E) wild-type cells treated with the noncovalent Fl+r8 control have no apparent internalization. Confocal Z-layers of (C) cell wall-deficient and (F) wild-type cells treated with Fl-r8 conjugate show internalization and not surface staining. The letter over the bar graph indicates the treatment conditions used for the corresponding image. (C, F) Scale bar equals 5 μm.
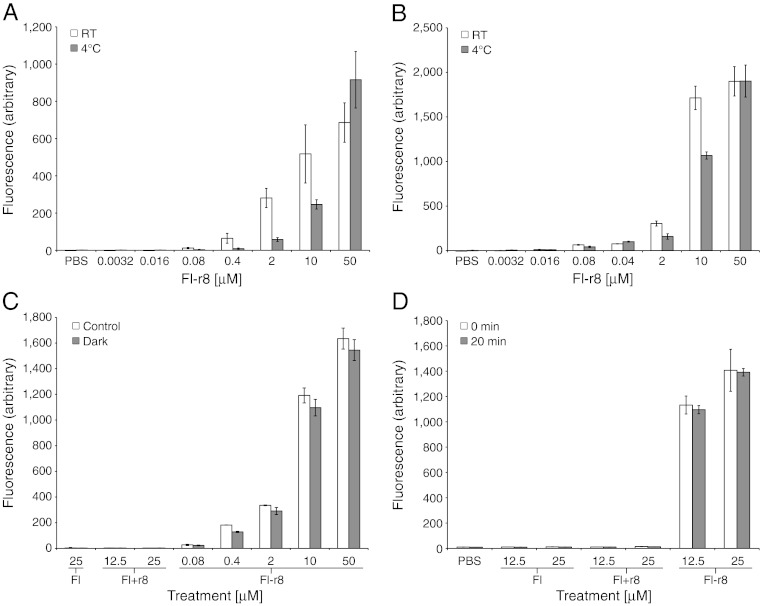
To determine next whether these GR-MoTrs are capable of penetrating the cell wall, wild-type C. reinhardtii were treated with the Fl-oligoarginine conjugates. Uptake of the conjugates was analyzed by flow cytometry (Fig. 1D, Fl-r4, and Fl-r10 can be seen in Fig. S3). Significantly, the wild-type algae showed robust uptake of the Fl-r8 conjugate but no uptake of Fl itself or the noncovalent mixture of Fl+r8. The corresponding confocal Z-stack images of the treated cells revealed that Fl-r8 had been internalized indicating that GR-MoTrs can cross the cell wall and the cell membrane of C. reinhardtii (Fig. 1 E and F). Having established that GR-MoTrs can enter wild-type cells, we sought to investigate whether biochemical or physical changes affect delivery. Because C. reinhardtii behavior and cellular biochemistry are affected by the presence, direction, and intensity of light (25), treatment of wild-type C. reinhardtii with Fl-r8 in the dark was examined and found not to affect uptake relative to a light-on control (Fig. 2C). When C. reinhardtii cells were preincubated and treated at 4 °C (a condition that slows or shuts down endosomal uptake pathways as well as many enzymatic functions) there was a weak, dose-dependent effect on Fl-r8 uptake (Fig. 2 A and B), which differs from the more dramatic reduction in uptake seen in mammalian cells (12).
Fig. 2.
Flow cytometry data of C. reinhardtii treated at 4 °C, in the dark and after acid-induced deflagellation. In all graphs the mean fluorescence is plotted, error is SD. (A) Cell wall-deficient cc-4350 and (B) wild-type treated at room temperature vs. 4 °C. (C) Treatment of wild-type in the dark vs. ambient light. (D) Wild-type cells were deflagellated and one sample was immediately treated while a second sample was allowed to recover for 20 min and then treated.
Wild-type C. reinhardtii have two flagella enclosed in a membrane but not a cell wall. To determine if uptake into the wild-type cells was occurring primarily or solely through the flagella, deflagellated C. reinhardtii were prepared using acid shock (26) and tested for uptake of the Fl-r8 covalent conjugate in a time-dependent manner. There was no apparent decrease in the amount of uptake or in the percentage of cells taking in Fl-r8 in the deflagellated cells (Fig. 2D). Studies on the mechanism of GR-MoTr-mediated uptake in C. reinhardtii and other algae are in progress.
Delivery to Other Species of Algae.
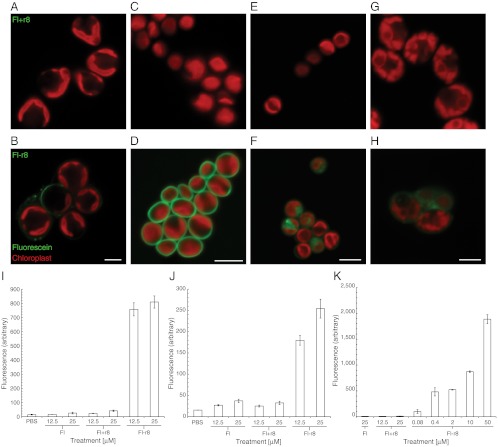
Although C. reinhardtii is arguably the most studied and commonly used model algae in academic research, there are many species of algae that are of academic as well as industrial interest for which few molecular tools exist. In addition, it is well known that different species of algae have widely varying cell wall compositions (27) and, as such, provide a unique opportunity to investigate how barrier type affects GR-MoTr entry, an additional major motivation for this study. Several green algae species in the class Chlorophyceae including Neochloris oleoabundans, Scenedesmus dimorphus, Chlorella protothecoides and Botryococcus braunii were tested. Dramatic species-specific differences in uptake of Fl-r8 were observed. Fluorescent images of the species reveal that some internalize Fl-r8 similarly to C. reinhardtii (N. oleoabundans) and others become coated with Fl-r8 (C. protothecoides) (Fig. 3 A–H). Still other species, such as S. dimorphus and B. braunii, display complex behavior within a single population of cells with some cells showing internalization of Fl-r8, some showing cell surface staining with Fl-r8, and some remaining unstained. These differences might be explained by the morphological heterogeneity of S. dimorphus (28) and by the complex extracellular matrix of B. braunii (29). Flow cytometry was performed with those species that showed internalization or cell surface staining to determine if the uptake pattern was similar to that of C. reinhardtii (Fig. 3 I–K). The species-specific differences in uptake of the GR-MoTrs could be utilized for algal cell differentiation in mixtures of species by fluorescence microscopy or flow cytometry (Fig. S4). These species-specific differences also present intriguing questions about the evolution of barrier function and at the same time opportunities for the selective manipulation of species.
Fig. 3.
Fluorescence microscopy and flow cytometry of various algal species treated with Fl, noncovalent Fl+r8, or Fl-r8 conjugate. Cells were treated with noncovalent Fl+r8 or Fl-r8 conjugate at 25 μM for all fluorescence images, scale bar equals 5 μm. Botryococcus braunii treated with (A) the control mixture Fl+r8 shows no uptake and with (B) Fl-r8 conjugate shows sporadic surface labeling. Chlorella protothecoides treated with (C) Fl+r8 shows no labeling and with (D) Fl-r8 conjugate shows surface staining. Neochloris oleoabundans treated with (E) Fl+r8 shows no uptake and with (F) Fl-r8 conjugate shows uptake. Scenedesmus dimorphus treated with (G) Fl+r8 shows no uptake and with (H) Fl-r8 conjugate shows a heterogeneous profile within a given sample with some cells showing uptake of Fl-r8, some showing surface labeling, and others having no apparent labeling. Graphs of the mean fluorescence as determined by flow cytometry of (I) S. dimorphus, (J) N. oleoabundans, and (K) C. protothecoides treated with conjugates and controls.
Delivery of Protein Cargo.
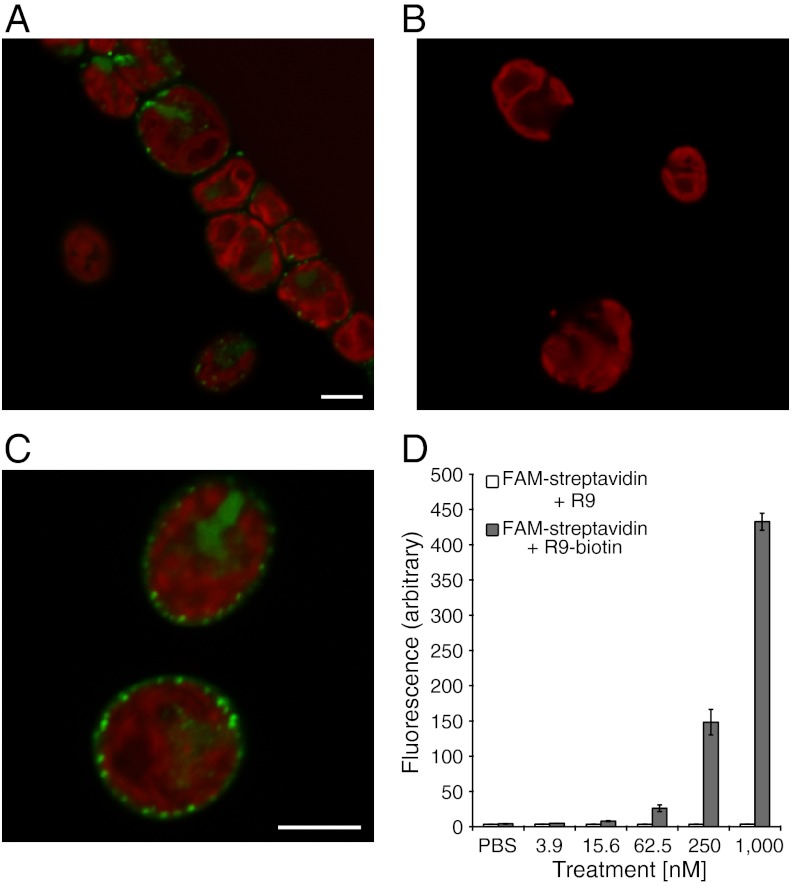
The efficient delivery of small molecules and probes, such as fluorescein, into algal cells creates new avenues by which algae can be imaged and manipulated. For other applications, however, delivery of larger cargos (e.g., biomacromolecules, quantum dots, and nanoparticles) is required. To address this significant challenge, a biotin-conjugated arginine 9-mer (biotin-R9) noncovalently complexed to FAM-labeled streptavidin (FAM-streptavidin) was incubated with wild-type C. reinhardtii. As controls, cells were treated with FAM-streptavidin alone or a mixture of FAM-streptavidin and nona-arginine (i.e., nona-arginine with no biotin conjugation) at the same concentrations used with the FAM-streptavidin:biotin-R9 complex. No uptake was observed with either of the controls. In striking contrast, remarkably effective uptake was observed with the approximately 60 kDa GR-MoTr-protein complex (Fig. 4, additional images in Fig. S5).
Fig. 4.
Fluorescence microscopy and flow cytometry of wild-type C. reinhardtii treated with FAM-streptavidin, FAM-streptavidin and nonaarginine (with no biotin), or FAM-streptavidin:biotin-R9 complex. (A, C) Cells treated with a FAM-streptavidin:biotin-R9 complex show internalization as well as cell wall binding. (B) Cells treated with the control FAM-streptavidin at the same concentration show no indication of binding or uptake. (D) Graph of the mean fluorescence from flow cytometry indicates the level of uptake for the noncovalent FAM-streptavidin and nonaarginine mixture vs. FAM-streptavidin:biotin-R9 complex. (A, C) Scale bar equals 5 μm.
To confirm that proteins delivered via molecular transporters maintain their activity after delivery, wild-type C. reinhardtii and cell wall mutant cc-4350 were treated with a horseradish peroxidase-streptavidin conjugate (HRP-streptavidin) complexed to biotin-R9. As controls, cells were treated with HRP-streptavidin alone, HRP-streptavidin and nona-arginine, or biotin-R9 alone. Following treatment of the live cells, the cells were adhered to a slide, fixed, and then treated with either HRP substrate (enhanced chemiluminescent substrate) or PBS. Confocal microscopy of the algal cells revealed robust signal from cells treated with all the necessary components for delivery (HRP-streptavidin complexed with biotin-R9) indicating that the molecular transporter delivered the approximately 100 kDa HRP-streptavidin protein conjugate successfully through the cell wall and cell membrane of live cells and that the HRP protein remained catalytically competent following this delivery (Fig 5, additional images and cc-4350 data in Fig. S6). The controls showed little to no signal when treated with HRP substrate. To the best of our knowledge, this is the first demonstration of protein uptake into algal cells using molecular transporters and the first demonstration that a protein can remain catalytically competent after this delivery through the cell wall and cell membrane.
Fig. 5.
Fluorescence microscopy of wild-type C. reinhardtii showing delivery of an active enzyme. Wild-type cells were treated with (A, B) HRP-streptavidin:biotin-R9 complex, (C, D) HRP-streptavidin and nonaarginine mixture, and (E, F) HRP-streptavidin alone and incubated with a chemical detection reagent for HRP (HRP substrate) that is converted to a fluorescent product after turnover by the enzyme. Only cells treated with HRP-streptavidin:biotin-R9 complex followed by the HRP substrate show any fluorescent signal. (G, H) Wild-type cells treated with HRP-streptavidin:biotin-R9 complex and not treated with the HRP substrate showed no fluorescence. Microscope settings were the same for all high-resolution images and all low-resolution images, respectively.
Conclusion
Biological barriers are critical to cellular life but at the same time severely limit, due to size, log P, charge, and other physical properties, the universe of tools and methods that can be used for the study and manipulation of cells. This limitation is exacerbated in organisms like algae that have a cell wall and membrane barrier yet represent a potentially bountiful source of molecules for research and industrial and clinical applications. Using the model organism C. reinhardtii as well as other algal species, we have developed a unique, effective, and general molecular method for delivering small and large cargo into algae. This is the first example of GR-MoTr-mediated transport into any species of algae and notably involves efficient passage across cell wall and membrane barriers. Importantly, this method was shown to work with protein cargo, including a catalytically competent enzyme, in C. reinhardtii. This study opens a range of opportunities in algal research that had previously been limited to cell wall-free systems including the delivery of small molecule probes (30), peptides and proteins (30–33), genetic material (30, 31, 34–36), and radioactive tracers (37). Species-specific and selective targeting with additional vectors and control of intracellular cargo release are also possible (38). Given the breadth of our findings, GR-MoTrs could prove useful with many other industrially significant or academically interesting species for which there are currently no established molecular manipulation or transformation techniques. Ongoing studies are directed at defining the range and type of cargos that GR-MoTrs can deliver into algae and at further delineating the mechanisms of entry and subcellular distribution. Our research provides a versatile new tool to study and manipulate an underutilized sustainable resource, photosynthetic single-celled algae.
Materials and Methods
Materials.
All Chlamydomonas strains were purchased from the Chlamydomonas Resource Center (Chlamy.org); other species of algae were purchased from the culture collection at the University of Texas at Austin (UTEX). Fluorescein sodium salt was purchased from Sigma-Aldrich (St. Louis, MO) and used as purchased. Octa-(D)-arginine was obtained from UCB bioproducts. Fluorescein-oligo-(D)-arginine conjugates were synthesized via a peptide synthesizer as previously reported (12). Nona-(L)-arginine-biotin conjugate (#64708) and FAM-Streptavidin (#60664) were obtained from Anaspec (Fremont, CA. 94555). Streptavidin-conjugated HRP was obtained from Life Technologies (S911, Grand Island, NY). Concanavalin A was obtained from Sigma-Aldrich (C7275, St. Louis, MO).
Growth and Maintenance of Strains.
Chlamydomonas reinhardtii strains were grown in tris-acetate-phosphate (TAP) medium or TAP medium supplemented with 2 mM arginine (as necessary) under a 16∶8 light:dark cycle. Cells were grown in 50 or 100 mL suspension culture in Erlenmeyer flasks and used generally at a concentration of 3 × 106 cells/mL. Wild-type strain cc-124 was used as was cell wall mutant cc-4350.
Other species were grown generally in the same manner with variations in media as appropriate: Botryococcus braunii was grown in Waris+soil extract, Chlorella protothecoides in TAP media, Neochloris oleoabundans in TAP+Arg, and Scenedesmus dimorphus in TAP media. All cell lines were kept on solid and in liquid culture, and new liquid cultures were started periodically off of plates.
Flow Cytometry Experiments.
Cells were plated at a concentration of 6 × 105 cells/well in conical-bottomed 96-well plates, supernatant was removed, and 200 μL of treatment (12.5, 25 or 50 μM of fluorescein alone (Fl), noncovalent fluorescein, and r8 (Fl+r8), Fl-r4, Fl-r8, or Fl-r10) was applied for 5 min. All conditions were in triplicate. Cells were spun at 1,500 rpm for 5 min in an Eppendorf 3810 centrifuge equipped with a plate rotor; the supernatant was removed and replaced with 200 μL PBS. Plates were spun again, PBS was removed and replaced with 200 μL PBS, and wells were analyzed using a FACScaliber (BD Biosciences) equipped with a 96-well sampler attachment running Platemanager and Cellquest. All analysis was performed using FlowJo (Tree Star software).
Experiments for testing the effect of cold temperatures on cellular uptake were performed with the same conditions except as follows: cells were cooled to 4 °C for 5 min previous to start, and treatments and washes were precooled to 4 °C. All procedures were carried out on ice, and centrifugation was performed at 4 °C.
Experiments testing the effects of a transfer to dark conditions on uptake were performed in a darkroom for all steps followed by covering plates with aluminum foil for centrifugation.
Experiments using mixtures of algal species were performed as above, but mixtures were plated such that their total cell count was 6 × 105 cells/well. Mixtures shown were a 1∶1∶1 ratio containing equal numbers of wild-type C. reinhardtii, S. dimorphus, and N. oleoabundans.
Deflagellation.
Cells at 3 × 106 cells/mL were acidified to pH 4.5 with 0.5 M acetic acid for 60 s, then brought rapidly back to approximately pH 7.2. Cells were immediately centrifuged and then treated, or left without shaking and samples taken at 0 and 20 min.
FAM-Streptavidin Uptake Experiments.
Protein delivery experiments were performed using a nona-(L)-arginine-biotin conjugate and a FAM-streptavidin conjugate (Anaspec, Fremont, CA). Control experiments also utilized nona-arginine (R9, i.e., not conjugated to biotin). Reagents were prepared according to the manufacturer. Reagents were mixed at a 4∶1 (biotin-R9 to FAM-streptavidin) ratio in PBS. Final concentrations for FAM-Streptavidin and biotin-R9 were 200 nM and 800 nM, respectively. The mixture was allowed to sit for 30 min. For the control, reagents were mixed at a 4∶1 (R9 to FAM-streptavidin) ratio in PBS. Final concentrations were the same as above and the mixture was allowed to sit for 30 min. Wild-type C. reinhardtii cells (6 × 105) were pelleted at 1,500 rpm for 5 min and then mixed with the FAM-streptavidin:biotin-R9 complex, R9 and FAM-streptavidin mixture, or FAM-streptavidin alone and allowed to sit for 30 min. Cells were then pelleted at 1,500 rpm for 5 min and washed with 200 μL PBS. Cells were pelleted again, supernatant was removed, and 5 μL PBS was added before placing on slides, or 200 μL PBS was added for FACS experiments.
Microscopy Experiments.
All microscopy was performed with a Zeiss LSM710 confocal microscope using 488 nm and 663 nm laser lines. Images for Fl-r8 and controls were taken with matched settings for each pair of images (x-y-z resolution, bit-depth, averaging, pixel dwell-time, digital zoom, laser energy, pinhole, and exposure). Cells were prepared as for FACS experiments, however after the final wash cells were brought up in 5 μL PBS. Slides were prepared with a thin layer of 1% agarose, and cells were placed on the agarose and then covered with a cover slip. Care was taken to ensure that cells were disturbed as little as possible, and small holes were made in the agarose to allow some bubbles of liquid to form on each slide allowing comparison between samples that were free swimming and samples that were pinned between agarose and the cover slip. Samples prepared in this manner and not disrupted were found to be viable for several days.
HPR-Streptavidin Uptake Experiments.
Protein delivery experiments were performed using a nona-(L)-arginine-biotin conjugate (Anaspec, Fremont, CA) and a horseradish peroxidase-streptavidin conjugate (HRP-streptavidin) (Life Sciences, Grand Island, NY). Control experiments also utilized nona-arginine (R9, i.e., not conjugated to biotin). Reagents were prepared according to the manufacturer. Reagents were mixed at a 4∶1 (biotin-R9 to HRP-streptavidin) ratio in PBS. Final concentrations for HRP-streptavidin and biotin-R9 were 1 μM and 4 μM, respectively. The mixture was allowed to sit for 10 min. For the control, reagents were mixed at a 4∶1 (R9 to HRP-streptavidin) ratio in PBS. Final concentrations were the same as above and the mixture was allowed to sit for 10 min. Wild-type C. reinhardtii or mutant cc-4350 cells (2 × 106) wwere pelleted at 1,500 rpm for 5 min then mixed with the HRP-streptavidin:biotin-R9 complex, R9 and HRP-streptavidin mixture, HRP-streptavidin alone, or biotin-R9 alone and allowed to sit for 20 min. Cells were then pelleted at 1,500 rpm for 5 min and washed with 400 μL PBS. Cells were pelleted again, the supernatant was removed, and 80 μL PBS was added. Forty microliters of this cell solution was added to two different wells in a labtek eight-well multiwell coverslip. Multiwell coverslips were prepared by adding 200 μL of 0.1 μg/mL concanavalin A per well, incubating for 20 min, removing the liquid, and allowing it to dry completely. Once cells had adhered to the multiwell coverslips, 360 μL of 4% paraformaldehyde (PFA) was added, and cells were fixed for 15 min. PFA was removed and 300 mL of ECL reagent (Supersignal West Femto, Pierce Biotechnology, Rockford IL) or PBS was added to each well. After 30 min, supernatant was removed from each well, and 100 μL Prolong Gold mounting media (Life Sciences, Grand Island, NY) was added to each well and images were taken using a Zeiss LSM710 as for other experiments. All images were taken with the same settings for direct comparison.
Supplementary Material
ACKNOWLEDGMENTS.
We would like to acknowledge Christopher Lipski for providing a sample of Fl-r4, Michelle Scott for help with flow cytometry and microscopy, and Mina Lee for maintenance of the algal strains. This work was supported through grant DE-AC02-05CH11231 from the Director, Office of Science, Office of Biological and Environmental Research, Radiochemistry and Imaging Instrumentation of the US Department of Energy, and through grants NIH-CA31841 and NIH-CA31845 from the National Institutes of Health. Support of this work through fellowships from the National Science Foundation (EIG, BMT) and the Stanford Graduate Fellowship (EIG, BMT) are gratefully acknowledged.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202509109/-/DCSupplemental.
References
- 1.Specht E, Miyake-Stoner S, Mayfield S. Microalgae come of age as a platform for recombinant protein production. Biotechnol Lett. 2010;32:1373–1383. doi: 10.1007/s10529-010-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christenson L, Sims R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol Adv. 2011;29:686–702. doi: 10.1016/j.biotechadv.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Radakovits R, Jinkerson RE, Darzins A, Posewitz MC. Genetic engineering of algae for enhanced biofuel production. Eukaryotic Cell. 2010;9:486–501. doi: 10.1128/EC.00364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JB, Craggs RJ, Shilton AN. Wastewater treatment high rate algal ponds for biofuel production. Bioresour Technol. 2011;102:35–42. doi: 10.1016/j.biortech.2010.06.158. [DOI] [PubMed] [Google Scholar]
- 5.Mayfield SP, et al. Chlamydomonas reinhardtii chloroplasts as protein factories. Curr Opin Biotechnol. 2007;18:126–133. doi: 10.1016/j.copbio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Davis TA, Volesky B, Mucci A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003;37:4311–4330. doi: 10.1016/S0043-1354(03)00293-8. [DOI] [PubMed] [Google Scholar]
- 7.Marshall WF. Quantitative high-throughput assays for flagella-based motility in Chlamydomonas using plate-well image analysis and transmission correlation spectroscopy. J Biomol Screening. 2009;14:133–141. doi: 10.1177/1087057108328131. [DOI] [PubMed] [Google Scholar]
- 8.Nagle DG, Zhou YD. Marine natural products as inhibitors of hypoxic signaling in tumors. Phytochem Rev. 2009;8:415–429. doi: 10.1007/s11101-009-9120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azencott HR, Peter GF, Prausnitz MR. Influence of the cell wall on intracellular delivery to algal cells by electroporation and sonication. Ultrasound Med Biol. 2007;33:1805–1817. doi: 10.1016/j.ultrasmedbio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris EH. 2nd Ed. Vol 1. Kidlington, Oxford: Elsevier; 2009. The Chlamydomonas Sourcebook; pp. 293–302. [Google Scholar]
- 11.Kilian O, Benemann CS, Niyogi KK, Vick B. High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc Natl Acad Sci USA . 2011;108:21265–21269. doi: 10.1073/pnas.1105861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wender PA, et al. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc Natl Acad Sci USA. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wender PA, Galliher WC, Goun EA, Jones LR, Pillow TH. The design of guanidinium-rich transporters and their internalization mechanisms. Adv Drug Delivery Rev. 2008;60:452–472. doi: 10.1016/j.addr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wender PA, Cooley CB, Geihe EG. Beyond cell penetrating peptides: Designed molecular transporters. Drug Discov Today Technol. 2011;9:e49–e55. doi: 10.1016/j.ddtec.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tung CH, Weissleder R. Arginine-containing peptides as delivery vectors. Adv Drug Delivery Rev. 2003;55:281–294. doi: 10.1016/s0169-409x(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 16.Torchilin VP. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv Drug Delivery Rev. 2008;60:548–558. doi: 10.1016/j.addr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RM, Harrison SD, Maclean D. Cell-Penetrating Peptides: Methods and Protocols. New York: Humana Press; 2011. pp. 535–551. [DOI] [PubMed] [Google Scholar]
- 18.Chang M, Chou J-C, Lee H-J. Cellular internalization of fluorescent proteins via arginine-rich intracellular delivery peptide in plant cells. Plant Cell Physiol. 2005;46:482–488. doi: 10.1093/pcp/pci046. [DOI] [PubMed] [Google Scholar]
- 19.Chugh A, Eudes F. Cellular uptake of cell-penetrating peptides pVEC and transportan in plants. J Pept Sci. 2007;14:477–481. doi: 10.1002/psc.937. [DOI] [PubMed] [Google Scholar]
- 20.Eggenberger K, Birtalan E, Schroder T, Brase S, Nick P. Passage of trojan peptoids into plant cells. ChemBioChem. 2009;10:2504–2512. doi: 10.1002/cbic.200900331. [DOI] [PubMed] [Google Scholar]
- 21.Chugh A, Amundsen E, Eudes F. Translocation of cell-penetrating peptides and delivery of their cargoes in triticale microspores. Plant Cell Rep. 2009;28:801–810. doi: 10.1007/s00299-009-0692-4. [DOI] [PubMed] [Google Scholar]
- 22.Unnamalai N, Kang BG, Lee WS. Cationic oligopeptide-mediated delivery of dsRNA for post-transcriptional gene silencing in plant cells. FEBS Lett. 2004;566:307–310. doi: 10.1016/j.febslet.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Liu BR, Chou J-C, Lee H-J. Cell membrane diversity in noncovalent protein transduction. J Membr Biol. 2008;222:1–15. doi: 10.1007/s00232-008-9096-6. [DOI] [PubMed] [Google Scholar]
- 24.Merchant SS, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreimer G. The green algal eyespot apparatus: A primordial visual system and more? Curr Genet. 2009;55:19–43. doi: 10.1007/s00294-008-0224-8. [DOI] [PubMed] [Google Scholar]
- 26.Hartzell LB, Hartzell HC, Quarmby LM. Mechanisms of flagellar excision 1. The role of intracellular acidification. Exp Cell Res. 1993;208:148–153. doi: 10.1006/excr.1993.1232. [DOI] [PubMed] [Google Scholar]
- 27.Abo-Shady AM, Mohamed YA, Lasheen T. Chemical composition of the cell wall in some green algae species. Biol Plant. 1993;35:629–632. [Google Scholar]
- 28.Cepák V, Pribyl P, Kvíderová J, Lukavsky J. Comparative study of zooid and non-zooid forming strains of Scenedesmus obliquus. Physiology and cytomorphology. Folia Microbiol. 2006;51:349–356. doi: 10.1007/BF02931829. [DOI] [PubMed] [Google Scholar]
- 29.Casadevalli E, Dif D, Largeau C, Gudin C, Chaumont D, Desanti O. Studies on batch and continuous cultures of Botryococcus braunii: Hydrocarbon production in relation to physiological state, cell ultrastructure, and phosphate nutrition. Biotechnol Bioeng. 1985;27:286–295. doi: 10.1002/bit.260270312. [DOI] [PubMed] [Google Scholar]
- 30.Stewart KM, Horton KL, Kelley SO. Cell-penetrating peptides as delivery vehicles for biology and medicine. Org Biomol Chem. 2008;6:2242–2255. doi: 10.1039/b719950c. [DOI] [PubMed] [Google Scholar]
- 31.Deshayes S, Konate K, Aldrian G, Crombez L, Heitz F, Divita G. Structural polymorphism of non-covalent peptide-based delivery systems: Highway to cellular uptake. Biochim Biophys Acta. 2010;1798:2304–2314. doi: 10.1016/j.bbamem.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 33.Zhou H, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Won YW, Kim HA, Lee M, Kim YH. Reducible poly(oligo-D-arginine) for enhanced gene expression in mouse lung by intratracheal injection. Mol Ther. 2010;18:734–742. doi: 10.1038/mt.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehto T, et al. Delivery of nucleic acids with stearylated (RxR)4 peptide using a non-covalent co-incubation strategy. J Controlled Release. 2010;141:42–51. doi: 10.1016/j.jconrel.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 36.Siprashvili Z, et al. Gene transfer via reversible plasmid condensation with cysteine-flanked, internally spaced arginine-rich peptides. Hum Gene Ther. 2003;14:1225–1233. doi: 10.1089/104303403767740768. [DOI] [PubMed] [Google Scholar]
- 37.Constantini DL, Hu M, Reilly RM. Peptide motifs for insertion of radiolabeled biomolecules into cells and routing to the nucleus for cancer imaging or radiotherapeutic applications. Cancer Biother Radiopharm. 2008;23:3–24. doi: 10.1089/cbr.2007.0430. [DOI] [PubMed] [Google Scholar]
- 38.Jones LR, et al. Releasable luciferin-transporter conjugates: Tools for the real-time analysis of cellular uptake and release. J Am Chem Soc. 2006;128:6526–6527. doi: 10.1021/ja0586283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.