Abstract
The cell nucleus is a major site for polyglutamine (polyQ) toxicity, but the underlying mechanisms involved have yet been fully elucidated. Here, we report that mutant RNAs that carry an expanded CAG repeat (expanded CAG RNAs) induce apoptosis by activating the nucleolar stress pathway in both polyQ patients and transgenic animal disease models. We showed that expanded CAG RNAs interacted directly with nucleolin (NCL), a protein that regulates rRNA transcription. Such RNA–protein interaction deprived NCL of binding to upstream control element (UCE) of the rRNA promoter, which resulted in UCE DNA hypermethylation and subsequently perturbation of rRNA transcription. The down-regulation of rRNA transcription induced nucleolar stress and provoked apoptosis by promoting physical interaction between ribosomal proteins and MDM2. Consequently, p53 protein was found to be stabilized in cells and became concentrated in the mitochondria. Finally, we showed that mitochondrial p53 disrupted the interaction between the antiapoptotic protein, Bcl-xL, and the proapoptotic protein, Bak, which then caused cytochrome c release and caspase activation. Our work provides in vivo evidence that expanded CAG RNAs trigger nucleolar stress and induce apoptosis via p53 and describes a polyQ pathogenic mechanism that involves the nucleolus.
Keywords: Drosophila, Machado-Joseph disease, RNA toxicity, spinocerebellar ataxia
The nucleolus is the site for ribosome biogenesis including ribosomal RNA (rRNA) transcription, and dysregulation of nucleolar function has been shown to cause diseases (1). Upstream binding factor (UBF) is a nucleolar-specific, high-mobility group-box–containing protein (2) that binds to upstream control element (UCE) of the rRNA promoter to trigger the formation of preinitiation complex for RNA polymerase I-mediated rRNA transcription (3). It has been reported that inhibition of rRNA transcription results in apoptosis in neurons (4), and perturbation of rRNA transcription has recently been observed in patients with Alzheimer’s disease (5). However, the cause of rRNA transcription down-regulation in neurodegeneration and how such dysregulation is related to neurodegeneration still remain largely undefined. “Nucleolar stress” is a term used to describe a stress response pathway through which the nucleolus signals to the cytosol to elicit apoptosis. When rRNA transcription is halted, the unassembled free ribosomal proteins, such as RpL5 (6), RpL11 (7), and RpL23 (8), accumulate in cells and associate with the MDM2 E3 ubiquitin ligase. The ribosomal protein/MDM2 interaction down-regulates MDM2-mediated ubiquitination of p53, which subsequently causes accumulation of p53 and activation of the downstream nucleolar stress signaling cascade (9), including mitochondrial cytochrome c release and caspase activation (10). This process is an effective mechanism to eliminate cells that are incapable of performing protein synthesis efficiently due to ribosome biogenesis defects. It has recently been reported that p53 mediates neuronal death upon nucleolar disruption in an in vivo Parkinson disease model (11).
Polyglutamine (polyQ) toxicity is attributed to the toxic gain-of-function nature of the disease proteins that harbor the expanded polyQ domain (12). The contribution of expanded CAG RNA toxicity to the pathogenesis of polyQ diseases, including Machado–Joseph disease (MJD), which is a dominant form of spinocerebellar ataxia (13), has recently been described. When the expanded CAG repeat sequence was isolated from the MJD disease gene and expressed as an untranslated RNA in vivo (ensuring that no expanded polyQ domain would be translated), progressive neural degeneration was observed (13). This finding clearly indicates that expanded CAG RNAs per se are neurotoxic (14). In recent years, increasing attention has been directed toward the understanding of pathogenic mechanisms exploited by expanded CAG RNAs (14–20). In this study, we provide evidence that expanded CAG RNAs induce nucleolus stress and trigger apoptosis via p53 stabilization in both polyQ patient cell and transgenic animal disease models. We demonstrated that expanded CAG RNAs deprive the nucleolar protein nucleolin (NCL) of binding onto ribosomal RNA (rRNA) promoter, which then down-regulates rRNA transcription. In addition, we revealed a role of the p53-mitochondrial nucleolar stress pathway in expanded CAG RNA-mediated toxicity of polyQ degeneration.
Results and Discussion
Expanded CAG RNAs Perturbed Ribosomal RNA Transcription.
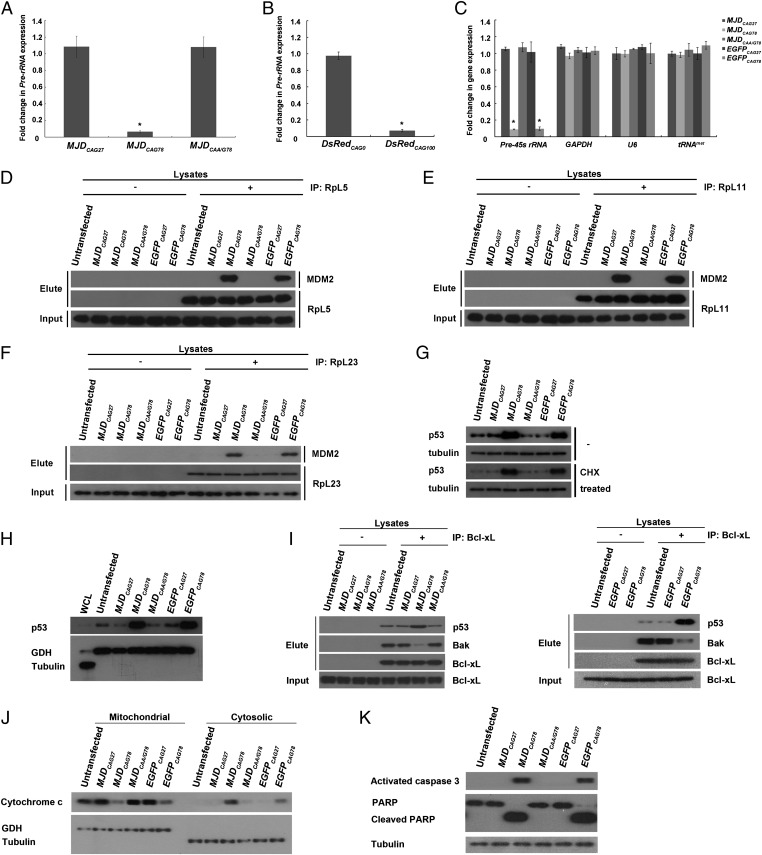
We previously reported that the cell nucleus is a toxic site for polyQ toxicity (17, 21). From a gene expression study, we observed that the pre-rRNA expression level was down-regulated in our Drosophila polyQ disease models (Fig. 1). Compared with the unexpanded MJD control transgenic flies (MJDCAG27), a reduction of pre-rRNA levels was observed in degenerative expanded MJDCAG78 transgenic flies (Fig. 1A and Fig. S1A) (17). We also detected a similar reduction in a mouse model of polyQ disease (22). A down-regulation of pre-45s rRNA level was observed in 12-wk-old symptomatic R6/2 polyQ transgenic mice, but not in the 2-wk-old asymptomatic ones (Fig. S1B). Both cellular pathologies (17, 21, 22) and neuropathological symptoms (22) have been reported in the 12-wk-old transgenic mouse brains. Further, a reduction of pre-45s rRNA level was consistently observed in cell lines of patients with MJD (Fig. S1C), which indicates that the down-regulation of rRNA transcription we observed in the transgenic animal models (Fig. 1A and Fig. S1B) was not due to transgene overexpression. In addition, no transcription defect was observed in genes mediated by RNA polymerases II and III (Fig. S1C). Our data thus indicate that expanded CAG RNAs particularly perturb rRNA transcription.
Fig. 1.
Expanded CAG RNAs perturbed rRNA transcription and induced nucleolar stress. (A–C) Real-time PCR analysis of pre-rRNA expression in Drosophila (A and B) and pre-45s rRNA, GAPDH, U6, and tRNAmet expression in cell (C) models expressing CAG constructs. Error bars represent ±SD. This experiment was repeated three times. (D–F) Coimmunoprecipitation of ribosomal proteins [RpL5 (D), RpL11 (E), and RpL23 (F)] and MDM2 E3 ubiquitin ligase in CAG RNA-expressing cells. (G) Effect of expanded CAG RNA expression on p53 protein stability. CHX represents cycloheximide and was used to inhibit de novo protein synthesis. Tubulin was used as a loading control. (H) Effect of expanded CAG RNA expression on mitochondrial accumulation of p53. Tubulin and glutamate dehydrogenase (GDH) were used, respectively, as cytosolic and mitochondrial fractionation controls. (I) Coimmunoprecipitation of Bcl-xL with p53 and Bak. (J) Cytosolic and mitochondrial fractionations of cytochrome c in CAG RNA-expressing cells. Tubulin and GDH were used as cytosolic and mitochondrial fractionation controls, respectively. (K) Effect of expanded CAG RNA expression on cleavage of caspase 3 and poly(ADP ribose) polymerase. Tubulin was used as a loading control. “+” indicates that antibody was present in the immunoprecipitation reactions and “−” indicates that no antibody was included in the reactions. All experiments were repeated three times, and representative blots or gels are shown.
As mutant RNA and protein species both contribute to polyQ toxicity in the cell nucleus (17, 21), we next investigated whether both of these species contributed to the rRNA transcription dysregulation in our models. In contrast to that in the expanded MJDCAG78 transgenic flies, expression of an expanded but discontinuous CAG repeat construct MJDCAA/G78 did not cause any perturbation of pre-rRNA gene expression (Fig. 1A) (13, 17). As both MJDCAG78 and MJDCAA/G78 transgenic flies expressed the MJD disease protein at a similar level (13), our data thus indicate that polyQ protein does not have a significant contribution to perturbation of rRNA transcription. The only difference between the MJDCAG78 and MJDCAA/G78 transgenes lies in the CAG repeat region, where the CAG continuity of the MJDCAA/G78 transgene is disrupted by another glutamine-coding triplet CAA (13); this result indicates that the continuity of CAG repeat is crucial for the pathogenic RNA to initiate an inhibitory effect on rRNA transcription. A similar perturbation effect was further observed in an independent untranslated CAG RNA fly model, DsRedCAG (13); this finding thus consolidates the deleterious effect of expanded CAG RNAs on rRNA transcription (Fig. 1A).
We next investigated the effect of expanded CAG RNAs on rRNA transcription in mammalian cell models (Fig. S2). In contrast to the controls, including cells transfected with unexpanded CAG (MJDCAG27 and EGFPCAG27) and interrupted expanded CAG (MJDCAA/G78) constructs, a lower level of pre-45s rRNA was observed in cells that expressed MJDCAG78 or EGFPCAG78 RNA (Fig. 1C). We also performed fluorescence in situ hybridization to determine the subcellular localization of expanded MJDCAG and EGFPCAG RNAs and found that both expanded MJDCAG78 and EGFPCAG78 RNAs formed microscopic foci in the nucleolar region (23, 24) (Fig. S3). As rRNA transcription takes place in the nucleolus (1), our fluorescence in situ hybridization data are thus in support of a rRNA transcription inhibitory property of expanded CAG RNAs.
Expanded CAG RNAs Induced Stabilization and Mitochondrial Accumulation of p53.
Inhibition of pre-rRNA gene expression has been reported to elevate p53 protein level (25) and trigger neuronal apoptosis (4) via the ribosomal protein–MDM2-p53 nucleolar stress pathway (26). We thus investigated this pathway in cells expressing MJDCAG78 or EGFPCAG78 RNA. In addition to the observation of a reduction in rRNA transcription (Fig. 1 A–C and Fig. S1 B and C), we detected an intense physical interaction between ribosomal proteins (RpL5, RpL11, and RpL23) and E3 ubiquitin ligase MDM2 (Fig. 1 D–F). In contrast, no such protein–protein interaction was observed in the controls. One cellular function of MDM2 is to mediate proteasomal degradation of p53 (9). We further showed that the ribosomal protein–MDM2 interaction compromised cellular p53 degradation, and as a result an elevated level of cellular p53 protein was detected in expanded CAG RNA-expressing cells (Fig. 1G). We further found that the stabilized cellular p53 protein was enriched in the mitochondrial fraction of these cells (Fig. 1H).
Expanded CAG RNAs Induced Mitochondrial Cytochrome c Release and Caspase Activation.
Cellular accumulation of p53 has been reported to disrupt the interactions between pro- and antiapoptotic Bcl-2 family proteins (10, 27). Under nonstress conditions, the antiapoptotic protein Bcl-xL interacts with the proapoptotic protein Bak. This interaction prevents the oligomerization of Bak. When p53 accumulates in the mitochondria, it interacts with Bcl-xL and allows Bak to oligomerize on mitochondrial outer membrane to form pores. Such pore formation subsequently mediates cytochrome c release from the mitochondria (27). We observed an enhanced association between p53 and Bcl-xL in cells that expressed MJDCAG78 and EGFPCAG78 RNAs and a concomitant reduction in the interaction between Bcl-xL and Bak (Fig. 1I). Further, the detection of cytochrome c in the cytoplasm in these cells (Fig. 1J) indicates a leakage of cytochrome c from the mitochondria. Cytochrome c release from mitochondria is a universal inducer of caspase cleavage. As predicted, we observed prominent cleavage of caspase 3 in cells expressing MJDCAG78 and EGFPCAG78 RNAs (Fig. 1K). In addition, cleavage of poly(ADP ribose) polymerase (PARP), a caspase 3 substrate, was detected (Fig. 1K), which confirms the functionality of the cleaved caspase 3 in expanded CAG RNA-expressing cells. Altogether, our findings indicate that expanded CAG RNAs induce cell death by activating the ribosomal protein–MDM2-p53 nucleolar stress pathway. We also detected a physical interaction between RpL5, L11 and L23 and MDM2 (Fig. S4 A–C), and p53 protein accumulation (Fig. S4D) in 12-wk-old symptomatic R6/2 transgenic mice, but not in the 2-wk-old asymptomatic ones and the nontransgenic control. This result further confirms that the ribosomal protein–MDM2-p53 nucleolar stress pathway is involved in polyQ neurotoxicity in vivo.
Expanded CAG RNAs Interfered with RNA Polymerase I Preinitiation Complex Formation.
After confirming the role of nucleolar stress in polyQ degeneration, we next carried out chromatin immunoprecipitation (ChIP) to determine whether the rRNA transcription machinery was perturbed by expanded CAG RNAs. We detected reduced binding of the largest subunit of RNA polymerase I (RPA194) (28) to the rRNA promoter in both MJDCAG78 and EGFPCAG78 RNA-expressing cells (Fig. 2A and Fig. S5A); this result suggests that the activity of the rRNA promoter is compromised in these cells. UBF is a transcription regulatory protein that binds to UCE of the rRNA promoter and triggers the formation of preinitiation complex (PIC) for RNA polymerase I-mediated rRNA transcription (3); we thus investigated whether UBF–UCE interaction was affected in cells expressing expanded CAG RNAs. It was found that less UBF was able to bind to UCE in both MJDCAG78- and EGFPCAG78-expressing cells compared with the MJDCAG27, EGFPCAG27, and MJDCAA/G78 controls (Fig. 2B and Fig. S5B). Our data clearly demonstrate that expanded CAG RNAs inhibit rRNA transcription by interfering with the binding of UBF to UCE, which consequently compromises the association between RNA polymerase I and rRNA promoter.
Fig. 2.
Expanded CAG RNAs interfered with the formation of rRNA transcription preinitiation complex and promoted hypermethylation of rRNA promoter. (A and B) Chromatin immunoprecipitation of the largest subunit of RNA polymerase I (RPA194) and rRNA promoter (A) and upstream binding factor (UBF) and upstream control element (UCE) (B) in CAG RNA-expressing cells. “+” indicates that antibody was present in the immunoprecipitation reactions and “−” indicates that antibody was not included in the reactions. (C) The effect of DNA methyltransferase inhibitor 5-azacytidine on expanded CAG RNA-induced UCE CpG DNA hypermethylation. Real-time PCR analysis of UCE DNA methylation status is shown. Error bars represent ±SD. All experiments were repeated three times.
Expanded CAG RNAs Induced Hypermethylation of rRNA Promoter.
Cytosine methylation at CpG dinucleotide sites on UCE has been shown to affect rRNA transcription (29); we therefore performed the HpaII-PCR assay to investigate the overall methylation status of UCE (30, 31) in MJDCAG78 and EGFPCAG78 RNA-expressing cells. Compared with the controls, UCEs extracted from cells that expressed MJDCAG78 and EGFPCAG78 RNAs were found to be hypermethylated (Fig. 2C). In contrast, we did not observe such a hypermethylation-inducing effect on non-rRNA promoters (32–34) (Fig. S6). This result illustrates that expanded CAG RNA specifically modifies the methylation status of UCE. When we treated cells that expressed MJDCAG78 and EGFPCAG78 RNAs with a DNA methyltransferase inhibitor, 5-azacytidine (30), UCE hypermethylation was restored to control levels (Fig. 2C). This result indicates the involvement of DNA methyltransferase activity in expanded CAG RNA-induced UCE hypermethylation.
Expanded CAG RNAs Interacted with Nucleolin.
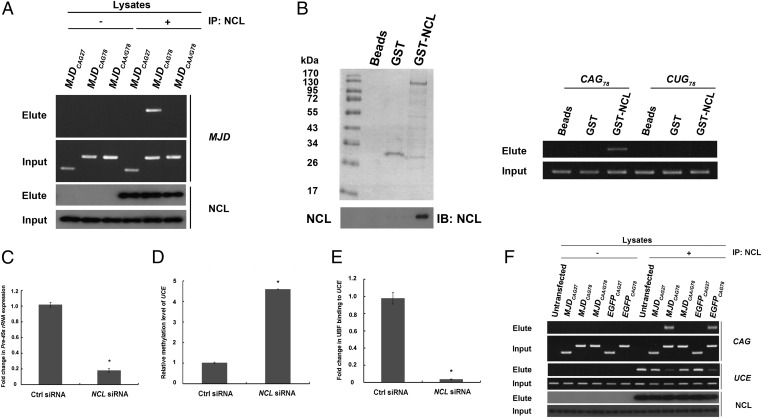
An affinity pull-down experiment using in vitro transcribed S1 aptamer-tagged (35) MJDCAG78 RNAs (S1-MJDCAG78) was performed with an aim to identify expanded CAG RNA-interacting proteins. We found that the nucleolar protein, NCL (36), specifically associated with expanded CAG RNAs (Fig. S7). We further showed that endogenous NCL interacted specifically with MJDCAG78 RNA, but not with the unexpanded CAG and discontinuous expanded CAG control RNAs in our cell models (Fig. 3A). This result indicates that CAG continuity is necessary to mediate the interaction between expanded CAG RNAs and NCL. By means of deletion mapping, we found that both the RRM2 and RRM3 domains of NCL are involved in the expanded CAG RNAs interaction (Fig. S8). To determine whether NCL interacts directly with expanded CAG RNAs, we incubated purified glutathione (GST)-NCL fusion protein with in vitro transcribed RNAs that contain either an expanded CAG78 or a CUG78 trinucleotide repeat. We found that GST-NCL interacted with CAG78 but not with CUG78 RNA (Fig. 3B). This result clearly shows that NCL interacts directly and specifically with expanded CAG RNAs.
Fig. 3.
Physical interaction between expanded CAG RNAs and nucleolin compromised the binding of nucleolin to upstream control element. (A) Pull-down of MJDCAG RNA by means of NCL immunoprecipitation. “+” indicates that antibody was present in the immunoprecipitation reactions and “−” indicates that no antibody was included in the reactions. This experiment was repeated three times, and a representative gel is shown. (B) Direct physical interaction between NCL and expanded CAG RNA. Purified GST-NCL protein and in vitro transcribed RNAs (CAG78 and CUG78) were used in the binding reactions. Nonfusion GST protein was used as a negative control. Western blotting was performed to confirm the expression of the GST-NCL protein. This experiment was repeated four times, and a representative blot is shown. (C) Real-time PCR analysis of pre-45s rRNA expression levels in NCL siRNA-treated cells. Error bars represent ±SD. This experiment was repeated three times. (D) Real-time PCR analysis of UCE DNA methylation status in NCL siRNA-treated cells. Error bars represent ±SD. This experiment was repeated three times. (E) Coimmunoprecipitation of upstream binding factor (UBF) and upstream control element (UCE) in NCL siRNA-treated cells. Real-time PCR analysis was performed to determine UBF–UCE interaction. Error bars represent ±SD. This experiment was repeated three times. (F) Chromatin immunoprecipitation of NCL with expanded CAG RNAs and UCE in cells. “+” indicates that antibody was present in the immunoprecipitation reactions and “−” indicates that no antibody was included in the reactions. This experiment was repeated three times, and a representative gel is shown.
Expanded CAG RNAs Reduced the Binding of Nucleolin on rRNA Promoter.
Nucleolin has previously been reported to regulate RNA polymerase I-mediated transcription (37); we thus further examined its role in rRNA transcription in MJDCAG78 and EGFPCAG78 RNA-expressing cells. As previously reported (37), we detected a reduction in pre-45s rRNA levels when NCL expression was knocked down in normal cells (Fig. 3C and Fig. S9) and also observed that UCE in the rRNA promoter was hypermethylated in these cells (Fig. 3D). The above results indicate the involvement of NCL in regulating UCE methylation status. Further, we demonstrated that knockdown of NCL expression reduced the binding of UBF to UCE (Fig. 3E and Fig. S5C). Taken together, our data indicate that NCL regulates rRNA transcription via modulating the methylation status of UCE (Fig. 3D) and UBF–UCE binding (Fig. 3E). We next investigated whether expanded CAG RNAs interfere with NCL function in cells that expressed MJDCAG78 and EGFPCAG78 RNAs and found that the expression of expanded CAG RNAs compromised NCL/UCE interaction (Fig. 3F). Intriguingly, the reduced level of binding of NCL to UCE was accompanied by an enhanced association between NCL and expanded CAG RNAs. Our findings indicate that expanded CAG RNAs prevent NCL from interacting with UCE by directly binding to NCL, and the RNA–protein interaction between expanded CAG RNAs and NCL consequently causes down-regulation of rRNA transcription.
Overexpression of Nucleolin Rescued rRNA Transcription and Suppressed Nucleolar Stress Caused by Expanded CAG RNAs.
Our study showed that expanded CAG RNA expression impairs nucleolus function by depriving cellular NCL of binding onto UCE (Fig. 3F), and such an effect is reminiscent of NCL knockdown (Fig. 3 C–E). We next determined whether overexpression of NCL could rescue expanded CAG RNA-mediated dysregulation of rRNA transcription. Our results showed that NCL overexpression alleviated UCE hypermethylation in cells that expressed MJDCAG78 or EGFPCAG78 RNA (Fig. 4A) in a dose-dependent manner (Fig. S10). We further observed that NCL overexpression restored UBF–UCE interaction (Fig. 4 B and C) and rescued rRNA transcription in expanded CAG RNA-expressing cells (Fig. 4D). Intriguingly, NCL overexpression also suppressed the expanded CAG RNA-mediated nucleolar stress response, including cellular p53 accumulation, caspase 3 activation, and cleavage of PARP (Fig. 4E). To further investigate the relationship between UCE hypermethylation and p53 accumulation, we treated expanded CAG RNA-expressing cells with 5-azacytidine and found that inhibition of UCE DNA methylation reduced cellular accumulation of p53 in MJDCAG78 and EGFPCAG78 RNA-expressing cells (Fig. S11). This finding indicates an involvement of DNA hypermethylation in the induction of expanded CAG RNA-mediated nucleolar stress. Moreover, we showed that exogenous NCL did not alter the expression levels of expanded CAG RNAs (Fig. S12), illustrating that the suppressive effect of NCL overexpression we observed was not due to a reduction of expanded CAG transgene expression. In summary, our findings show that NCL binds to UCE of rRNA promoter and regulates rRNA transcription. We further performed coimmunoprecipitation to determine whether NCL interacts with expanded polyQ protein and failed to detect any interaction (soluble or insoluble) between the two proteins (Fig. S13). This result indicates that expanded polyQ protein does not affect the cellular availability of NCL, which is therefore in line with our results generated from the “RNA-only” models (Fig. 1 B and C) and again illustrates that expression of expanded CAG RNAs per se perturbs rRNA transcription. Activation of the p53 pathway has previously been reported in polyQ degeneration, including MJD (38). Although we demonstrated that expanded CAG RNAs trigger nucleolar stress by perturbing rRNA transcription and activating the p53 pathway in polyQ toxicity, our findings do not exclude any contribution of toxic polyQ protein species to the induction of nucleolar stress via other pathways. For instance, it has been reported that expanded polyQ protein induces apoptosis by promoting phosphorylation of p53 on Ser46 (39), and p53 may trigger cell death by inducing the expression of apoptosis-inducing genes (40).
Fig. 4.
Nucleolin overexpression restored rRNA transcription and suppressed nucleolar stress. (A) Real-time PCR analysis of upstream control element (UCE) DNA methylation status in cells coexpressing CAG RNAs and NCL. Error bars represent ±SD. This experiment was repeated three times. (B and C) Chromatin immunoprecipitation of upstream binding factor (UBF) and UCE in CAG RNA-expressing cells with or without NCL coexpression. Real-time PCR analysis was performed to determine UBF–UCE interaction. Error bars represent ±SD. This experiment was repeated three times. (D) Real-time PCR analysis of pre-45s rRNA expression levels in cells cotransfected with CAG and NCL constructs. Error bars represent ±SD. This experiment was repeated three times. (E) The effect of NCL overexpression on p53 protein stabilization and cleavage of caspase 3 and poly(ADP ribose) polymerase in expanded CAG RNA-expressing cells. “+” indicates NCL overexpression and “−” indicates no NCL overexpression. This experiment was repeated three times, and a representative blot is shown.
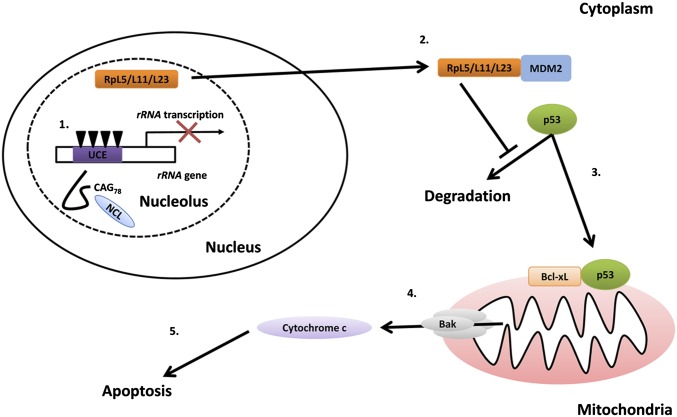
Inhibition of pre-rRNA synthesis has been reported to cause neurodegeneration in vivo (25), and dysregulation of CpG methylation of the rRNA promoter has been observed in patients with neurodegenerative disease, such as Alzheimer’s disease (5). We recently reported that the cell nucleus is a toxic subcellular compartment for expanded CAG RNA to mediate toxicity in polyQ degeneration (17). In this study, we showed that CAG repeat expansion confers a pathogenic property to the mutant RNA molecules that enables them to directly interact with the nucleolar protein NCL. Such RNA–protein interaction causes DNA hypermethylation in the rRNA promoter and results in a reduction in rRNA transcription. Consequently, the nucleolar stress pathway is activated followed by apoptosis induction (Fig. 5). Our study unequivocally demonstrates that the nucleolus is a major site for expanded CAG RNA toxicity in polyQ diseases. In addition to the induction of DNA hypermethylation in rRNA promoter, dysregulation of UBF1 acetylation has also been shown to cause impairment of rRNA transcription in R6/2 polyQ transgenic mice (41). These observations suggest that perturbation of any component of the rRNA transcription machinery would lead to neuronal cell dysfunction or death. In this study, we report that the pathogenic biomolecule expanded CAG RNA is capable of inducing a nucleolar stress response in neurodegeneration. Our findings thus deliver mechanistic insights into the RNA-mediated toxicity of polyQ pathogenesis and may help develop alternative therapeutic directions. The identification of an additional biomolecular inducer(s) that causes rRNA transcriptional dysregulation in individual neurodegenerative diseases is key to our further understanding of the role of the nucleolar stress pathway in neurodegeneration.
Fig. 5.
A proposed model for the activation of nucleolar stress signaling by expanded CAG RNAs. The expression of expanded CAG RNA interacts directly with nucleolin (NCL, in light blue). The expanded CAG-specific RNA–protein interaction causes CpG hypermethylation (inverted triangles) of the upstream control element (UCE, in purple) in the rRNA promoter and results in reduction of rRNA transcription. A functional ribosome consists of both rRNAs and ribosomal proteins. A reduced level of rRNAs causes accumulation of free ribosomal proteins (such as RpL5, RpL11, and RpL23, in orange). The interaction between free ribosomal proteins and E3 ubiquitin ligase MDM2 (in blue) leads to mitochondrial accumulation of p53. The interaction between p53 and antiapoptotic proteins (Bcl-xL, in orange) causes oligomerization of proapoptotic proteins (Bak, in gray) on the mitochondrial membrane and results in cytochrome c (in light purple) release. Cytosolic cytochrome c in turn activates the caspase cascade and induces apoptosis.
Materials and Methods
Real-time PCR Taqman gene expression assays were performed on an ABI 7500 Real-time PCR system. In situ hybridization and immunostaining were performed according to refs. 42 and 21 respectively with minor modifications. Mitochondrial fractionation was performed according to ref. 43. Other materials and methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank past and present members of the Laboratory of Drosophila Research for insightful comments and discussion and Dr. T. C. Cheng for technical support. We thank Profs. Nancy Bonini and Michael Kastan for reagents. This work was supported by Hong Kong Research Grants Council, The Chinese University of Hong Kong Biochemistry Collaborative Research Fund, and Hong Kong Spinocerebellar Ataxia Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204089109/-/DCSupplemental.
References
- 1.Kressler D, Hurt E, Bassler J. Driving ribosome assembly. Biochim Biophys Acta. 2010;1803:673–683. doi: 10.1016/j.bbamcr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Bell SP, Learned RM, Jantzen HM, Tjian R. Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science. 1988;241:1192–1197. doi: 10.1126/science.3413483. [DOI] [PubMed] [Google Scholar]
- 3.Sanij E, Hannan RD. The role of UBF in regulating the structure and dynamics of transcriptionally active rDNA chromatin. Epigenetics. 2009;4:374–382. doi: 10.4161/epi.4.6.9449. [DOI] [PubMed] [Google Scholar]
- 4.Kalita K, Makonchuk D, Gomes C, Zheng JJ, Hetman M. Inhibition of nucleolar transcription as a trigger for neuronal apoptosis. J Neurochem. 2008;105:2286–2299. doi: 10.1111/j.1471-4159.2008.05316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietrzak M, Rempala G, Nelson PT, Zheng JJ, Hetman M. Epigenetic silencing of nucleolar rRNA genes in Alzheimer’s disease. PLoS ONE. 2011;6:e22585. doi: 10.1371/journal.pone.0022585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai MS, Lu H. Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J Biol Chem. 2004;279:44475–44482. doi: 10.1074/jbc.M403722200. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, et al. Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway. Mol Cell Biol. 2003;23:8902–8912. doi: 10.1128/MCB.23.23.8902-8912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin A, Itahana K, O’Keefe K, Zhang Y. Inhibition of HDM2 and activation of p53 by ribosomal protein L23. Mol Cell Biol. 2004;24:7669–7680. doi: 10.1128/MCB.24.17.7669-7680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Lu H. Signaling to p53: Ribosomal proteins find their way. Cancer Cell. 2009;16:369–377. doi: 10.1016/j.ccr.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaseva AV, Moll UM. The mitochondrial p53 pathway. Biochim Biophys Acta. 2009;1787:414–420. doi: 10.1016/j.bbabio.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rieker C, et al. Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J Neurosci. 2011;31:453–460. doi: 10.1523/JNEUROSCI.0590-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 13.Li LB, Yu Z, Teng X, Bonini NM. RNA toxicity is a component of ataxin-3 degeneration in Drosophila. Nature. 2008;453:1107–1111. doi: 10.1038/nature06909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wojciechowska M, Krzyzosiak WJ. Cellular toxicity of expanded RNA repeats: Focus on RNA foci. Hum Mol Genet. 2011;20:3811–3821. doi: 10.1093/hmg/ddr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LC, et al. Muscleblind participates in RNA toxicity of expanded CAG and CUG repeats in Caenorhabditis elegans. Cell Mol Life Sci. 2011;68:1255–1267. doi: 10.1007/s00018-010-0522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Eyk CL, et al. Perturbation of the Akt/Gsk3-β signalling pathway is common to Drosophila expressing expanded untranslated CAG, CUG and AUUCU repeat RNAs. Hum Mol Genet. 2011;20:2783–2794. doi: 10.1093/hmg/ddr177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsoi H, Lau CK, Lau KF, Chan HY. Perturbation of U2AF65/NXF1-mediated RNA nuclear export enhances RNA toxicity in polyQ diseases. Hum Mol Genet. 2011;20:3787–3797. doi: 10.1093/hmg/ddr297. [DOI] [PubMed] [Google Scholar]
- 18.Mykowska A, Sobczak K, Wojciechowska M, Kozlowski P, Krzyzosiak WJ. CAG repeats mimic CUG repeats in the misregulation of alternative splicing. Nucleic Acids Res. 2011;39:8938–8951. doi: 10.1093/nar/gkr608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu RJ, et al. Long tract of untranslated CAG repeats is deleterious in transgenic mice. PLoS ONE. 2011;6:e16417. doi: 10.1371/journal.pone.0016417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Mezer M, Wojciechowska M, Napierala M, Sobczak K, Krzyzosiak WJ. Mutant CAG repeats of Huntingtin transcript fold into hairpins, form nuclear foci and are targets for RNA interference. Nucleic Acids Res. 2011;39:3852–3863. doi: 10.1093/nar/gkq1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan WM, et al. Expanded polyglutamine domain possesses nuclear export activity which modulates subcellular localization and toxicity of polyQ disease protein via exportin-1. Hum Mol Genet. 2011;20:1738–1750. doi: 10.1093/hmg/ddr049. [DOI] [PubMed] [Google Scholar]
- 22.Davies SW, et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 23.Ochs RL, Lischwe MA, Spohn WH, Busch H. Fibrillarin: A new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54:123–133. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 24.Boisvert FM, van Koningsbruggen S, Navascués J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol. 2007;8:574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- 25.Parlato R, et al. Activation of an endogenous suicide response after perturbation of rRNA synthesis leads to neurodegeneration in mice. J Neurosci. 2008;28:12759–12764. doi: 10.1523/JNEUROSCI.2439-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulon S, Westman BJ, Hutten S, Boisvert FM, Lamond AI. The nucleolus under stress. Mol Cell. 2010;40:216–227. doi: 10.1016/j.molcel.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mihara M, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 28.Seither P, Coy JF, Pouska A, Grummt I. Molecular cloning and characterization of the cDNA encoding the largest subunit of mouse RNA polymerase I. Mol Gen Genet. 1997;255:180–186. doi: 10.1007/s004380050487. [DOI] [PubMed] [Google Scholar]
- 29.Santoro R, Grummt I. Molecular mechanisms mediating methylation-dependent silencing of ribosomal gene transcription. Mol Cell. 2001;8:719–725. doi: 10.1016/s1097-2765(01)00317-3. [DOI] [PubMed] [Google Scholar]
- 30.Santoro R, Grummt I. Epigenetic mechanism of rRNA gene silencing: Temporal order of NoRC-mediated histone modification, chromatin remodeling, and DNA methylation. Mol Cell Biol. 2005;25:2539–2546. doi: 10.1128/MCB.25.7.2539-2546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singer-Sam J, et al. Use of a HpaII-polymerase chain reaction assay to study DNA methylation in the Pgk-1 CpG island of mouse embryos at the time of X-chromosome inactivation. Mol Cell Biol. 1990;10:4987–4989. doi: 10.1128/mcb.10.9.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei M, et al. BRCA1 promoter methylation in sporadic breast cancer is associated with reduced BRCA1 copy number and chromosome 17 aneusomy. Cancer Res. 2005;65:10692–10699. doi: 10.1158/0008-5472.CAN-05-1277. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalo V, et al. Gastrointestinal Oncology Group of the Spanish Gastroenterological Association Aberrant gene promoter methylation associated with sporadic multiple colorectal cancer. PLoS ONE. 2010;5:e8777. doi: 10.1371/journal.pone.0008777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson ED, Kavalali ET, Monteggia LM. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci. 2008;28:395–406. doi: 10.1523/JNEUROSCI.3796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srisawat C, Engelke DR. Streptavidin aptamers: Affinity tags for the study of RNAs and ribonucleoproteins. RNA. 2001;7:632–641. doi: 10.1017/s135583820100245x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mongelard F, Bouvet P. Nucleolin: A multiFACeTed protein. Trends Cell Biol. 2007;17:80–86. doi: 10.1016/j.tcb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Rickards B, Flint SJ, Cole MD, LeRoy G. Nucleolin is required for RNA polymerase I transcription in vivo. Mol Cell Biol. 2007;27:937–948. doi: 10.1128/MCB.01584-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou AH, et al. p53 activation mediates polyglutamine-expanded ataxin-3 upregulation of Bax expression in cerebellar and pontine nuclei neurons. Neurochem Int. 2011;58:145–152. doi: 10.1016/j.neuint.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Grison A, et al. Ser46 phosphorylation and prolyl-isomerase Pin1-mediated isomerization of p53 are key events in p53-dependent apoptosis induced by mutant huntingtin. Proc Natl Acad Sci USA. 2011;108:17979–17984. doi: 10.1073/pnas.1106198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bieging KT, Attardi LD. Deconstructing p53 transcriptional networks in tumor suppression. Trends Cell Biol. 2012;22:97–106. doi: 10.1016/j.tcb.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J, et al. Dysregulation of upstream binding factor-1 acetylation at K352 is linked to impaired ribosomal DNA transcription in Huntington’s disease. Cell Death Differ. 2011;18:1726–1735. doi: 10.1038/cdd.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinaud R, Mello CV, Velho TA, Wynne RD, Tremere LA. Detection of two mRNA species at single-cell resolution by double-fluorescence in situ hybridization. Nat Protoc. 2008;3:1370–1379. doi: 10.1038/nprot.2008.115. [DOI] [PubMed] [Google Scholar]
- 43.Chan CM, et al. The ion channel activity of the SARS-coronavirus 3a protein is linked to its pro-apoptotic function. Int J Biochem Cell Biol. 2009;41:2232–2239. doi: 10.1016/j.biocel.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.