The ErbB/HER network is composed of four receptors and 11 ligands (1). The receptors are known to exist in at least two different conformations (2). In the absence of ligand, EGFR, ErbB3, and ErbB4 exist in a closed or tethered conformation that buries their dimerization arm and prevents unregulated signaling (3). In the ligand-bound state, the receptors are open and expose their dimerization arm, which in turn allows for homo- and hetero- associations. To date, crystal structures for complexes are reported only for EGFR and ErbB4 homodimers. Strikingly, receptor dimerization is mediated exclusively by receptor contacts specifically in domain II. In contrast, ligand is sandwiched between domains I and III, and is spatially removed from the dimerization interface.
In contrast to its three siblings, ErbB2 lacks the ability to bind any known ligand, but rather participates as a coreceptor with other ligand-bound ErbB receptors. Structural studies of ErbB2 provide an explanation why it does not (4, 5). ErbB2’s vestigial ligand binding domains form an interface with each other and therefore cannot accommodate ligand binding. As a result, ErbB2 exists in a conformation that permanently exposes its dimerization arm allowing it to partner with other ligand-bound ErbBs. Thus, ErbB2 functions as a common coreceptor for all other ErbB–ligand complexes. The association of ErbB2 with ErbB3 is particularly intriguing because ErbB3 cannot transduce a signal by itself (6). It contains several amino acids within its tyrosine kinase domain that render it an inactive or a very, very poor kinase (7, 8). However, the heterocomplex between ErbB3 and ErbB2 is observed to be the most potent signaling combination of all ErbB receptors and robustly activates PI3K and MAPK pathways.
In PNAS, Zhang et al. (9) provide a possible answer to a longstanding, unanswered questioned that has annoyed the ErbB field: How does ErbB2 become activated in a simple heterodimeric model if ErbB3 is a dead or an impaired kinase? Several years ago, the same laboratory developed an RNA aptamer by using systematic evolution of ligands by exponential enrichment that selectively binds to ErbB3. Binding of the aptamer, termed A30, does not interfere with ligand binding, heterodimerization, or downstream signaling to PI3K-AKT (10). Rather, A30 is very effective in blocking heregulin-mediated activation of ErbB2 and subsequent activation of the MAPK pathway. The lack of ErbB2 activation observed in the presence of A30 led the authors to hypothesize that ErbB2 phosphorylates itself in trans and that a single copy of ErbB2 in the heterocomplex was insufficient for complete activation.
Previous work published by the same group (11) demonstrated that a minimal aptamer could be cross-linked to ErbB3 through the introduction of a photoactive group. In the new study of Zhang et al. (9), the authors use an insect cell-expressed version of ErbB3 ECD to map A30 binding to a segment near the junction of domains III and IV. This junction is accessible to A30 in the open and tethered conformations. Because aptamers are notorious for their nonspecific binding properties, a series of mutagenesis experiments were performed to further demonstrate the specificity of A30 binding. A pair of arginines at positions 471 and 472, when mutated to glutamates, resulted in the complete loss of A30 binding. Interestingly, this mutant still retains WT responsiveness to ligand, both in terms of ErbB2 phosphorylation and activation of MAPK.
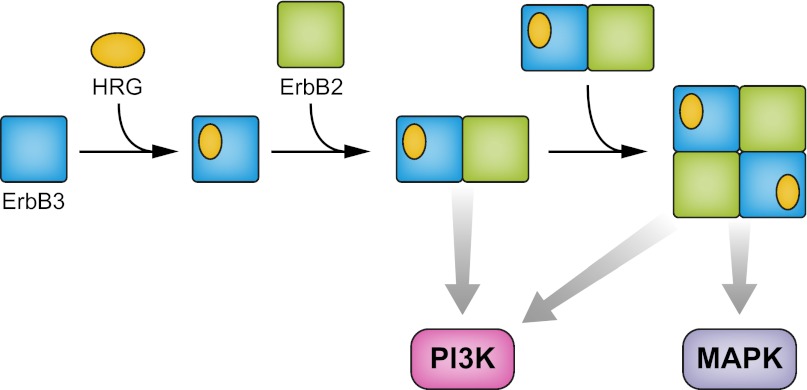
Because the aptamer binding site is located far from the ligand binding areas or the dimerization arm, the authors hypothesize that this region of ErbB3 must be involved in a new interface with ErbB2. To explain this observation, the authors propose a side-by-side arrangement of heterodimers; in other words, a dimer of dimers (Fig. 1). Evidence for the tetrameric state was provided by coimmunoprecipitation experiments with two differently tagged species of ErbB2. Although the overall recovery of coimmunoprecipitated ErbB2 was very low, data are presented that the two-tagged species do exist in a ligand-dependent complex. As predicted, A30 suppresses the observed coimmunoprecipitation.
Fig. 1.
Ligand-activated ErbB2–ErbB3–heregulin (HRG) complex is proposed to be a side-by-side dimer of dimers. MAPK activation occurs primarily in the tetrameric state as a result of ErbB2 transactivation. The heterodimeric state is sufficient for PI3K activation.
Several limitations to the study of Zhang et al. (9) should also be mentioned. First, no structural data are available to support the existence of the proposed tetrameric state. Second, although mutants of ErbB3 were generated that lack A30 binding, these mutants still couple to ErbB2. One would expect that additional mutations at this interface could be generated that decouple ErbB2 activation. Third, at present, the existence of the tetramer relies solely on the properties of the A30 aptamer to probe this unique interface. The generation of other reagents that also recognize this region would help strengthen the model. Fourth, more extensive studies are required to demonstrate that A30 antagonizes an ErbB2-ErbB3 biological response. Last, the model predicts that a similar surface also exists on the ErbB2 side of the interface. Obviously, it would be satisfying if this region were also identified.
Nevertheless, the conclusions of this study are especially interesting in light of the recent Food and Drug Administration approval of Perjeta (pertuzumab) for the treatment of HER2-positive breast cancer. Pertuzumab is an antibody that binds to the dimerization arm of ErbB2 and prevents its function as a coreceptor with the other ErbBs, most notably ErbB3 (12, 13). The phase III trial that supported the approval of pertuzumab (14) was conducted in 808 patients with metastatic breast cancer receiving first-line treatment. The individuals in the treatment group who received the combination of pertuzumab, trastuzumab (Herceptin), and docetaxel showed in a significant reduction in the risk of progression or death and an absolute increase in progression-free survival of 6.1 mo (14). These findings suggest that targeting ErbB2 with two monoclonal antibodies that have complementary mechanisms of action results in more comprehensive blockade of ErbB2 signaling. Zhang et al. (9) also used pertuzumab in the aforementioned study in PNAS. As predicted, pertuzumab is more effective than A30 in suppressing ligand-activated ErbB3 activation. Moreover, the combination of A30 with pertuzumab is synergistic. Similar experimental synergistic findings are also reported for pertuzumab and trastuzumab (15). A key difference, however, is that trastuzumab does not block ErbB2 activation but rather disrupts noncanonical ErbB2–ErbB3 interactions that result in constitutive ErbB3 activation (16). Importantly, the aforementioned clinical results with pertuzumab and trastuzumab validate these laboratory findings.
In conclusion, the side-by-side model put forth by Zhang et al. (9) provides insights on an enigma related to the mechanism of ErbB2-ErbB3 activation. Taken at face value, the report argues that higher-order complexes not only exist, but also are crucial for normal signaling. Strategies designed to further dissect and examine this model should be readily attainable and may provide for a generation of unique antagonists with potential clinical utility.
Footnotes
Conflict of interest statement: M.X.S. is an employee of Genentech, a member of the Roche Group; and a shareholder of Roche.
See companion article on page 13237.
References
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Burgess AW, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 3.Liu P, et al. A single ligand is sufficient to activate EGFR dimers. Proc Natl Acad Sci USA. 2012;109:10861–10866. doi: 10.1073/pnas.1201114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho HS, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 5.Garrett TP, et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11:495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 6.Tzahar E, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5267–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL., 3rd Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci USA. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA. 2010;107:7692–7697. doi: 10.1073/pnas.1002753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Park E, Kani K, Landgraf R. Functional isolation of activated and unilaterally phosphorylated heterodimers of ERBB2 and ERBB3 as scaffolds in ligand-dependent signaling. Proc Natl Acad Sci USA. 2012;109:13237–13242. doi: 10.1073/pnas.1200105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CH, Chernis GA, Hoang VQ, Landgraf R. Inhibition of heregulin signaling by an aptamer that preferentially binds to the oligomeric form of human epidermal growth factor receptor-3. Proc Natl Acad Sci USA. 2003;100:9226–9231. doi: 10.1073/pnas.1332660100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park E, Baron R, Landgraf R. Higher-order association states of cellular ERBB3 probed with photo-cross-linkable aptamers. Biochemistry. 2008;47:11992–12005. doi: 10.1021/bi8004208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin MC, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 13.Agus DB, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J, et al. CLEOPATRA Study Group Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheuer W, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 16.Junttila TT, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]