Abstract
The Ca2+-permeable cation channel transient receptor potential melastatin 2 (TRPM2) plays a key role in pathogen-evoked phagocyte activation, postischemic neuronal apoptosis, and glucose-evoked insulin secretion, by linking these cellular responses to oxidative stress. TRPM2 channels are coactivated by binding of intracellular ADP ribose and Ca2+ to distinct cytosolically accessible sites on the channels. These ligands likely regulate the activation gate, conserved in the voltage-gated cation channel superfamily, that comprises a helix bundle formed by the intracellular ends of transmembrane helix six of each subunit. For several K+ and TRPM family channels, activation gate opening requires the presence of phosphatidylinositol-bisphosphate (PIP2) in the inner membrane leaflet. Most TRPM family channels inactivate upon prolonged stimulation in inside-out patches; this “rundown” is due to PIP2 depletion. TRPM2 currents also run down within minutes, but the molecular mechanism of this process is unknown. Here we report that high-affinity PIP2 binding regulates Ca2+ sensitivity of TRPM2 activation. Nevertheless, TRPM2 inactivation is not due to PIP2 depletion; rather, it is state dependent, sensitive to permeating ions, and can be completely prevented by mutations in the extracellular selectivity filter. Introduction of two negative charges plus a single-residue insertion, to mimic the filter sequence of TRPM5, results in TRPM2 channels that maintain unabated maximal activity for over 1 h, and display altered permeation properties but intact ADP ribose/Ca2+-dependent gating. Thus, upon prolonged stimulation, the TRPM2 selectivity filter undergoes a conformational change reminiscent of that accompanying C-type inactivation of voltage-gated K+ channels. The noninactivating TRPM2 variant will be invaluable for gating studies.
Keywords: C-type inactivation; cation permeation; PI(4,5)P2; electrostatic effect; gating kinetics
Transient receptor potential melastatin 2 (TRPM2) cation channels belong to the TRP protein family and are expressed in brain neurons, bone marrow, phagocytes, pancreatic β-cells, and cardiomyocytes, where they open under conditions of oxidative stress (1–3). TRPM2 function is required for pathogen-induced migration and chemokine production of phagocytic cells and normal glucose-evoked insulin secretion of pancreatic β-cells; in addition, TRPM2 has been linked to several pathological conditions that lead to apoptosis, including cerebral stroke, myocardial infarction, and neurodegenerative diseases (4).
TRP channels belong to the superfamily of voltage-gated cation channels, and share their overall tetrameric transmembrane (TM) architecture; the ion permeation pathway is built from TM helices 5 and 6, and the intervening pore-loop which forms the selectivity filter (5). The TRPM2 pore does not discriminate between Na+ and K+, and is highly permeable to Ca2+ (1, 2). TRPM2 channels are activated by binding of ADP ribose (ADPR) to the intracellular NUDT9-homology (NUDT9-H) domain unique to TRPM2 and located at its C terminus (1–3). In addition to ADPR, intracellular Ca2+ is an essential coactivator: TRPM2 channels open only in the combined presence of both ligands. The structural elements that form the activating Ca2+ binding sites have not yet been identified; kinetic studies suggest that Ca2+ binds intracellularly from the gate, but in a shielded crevice near the cytoplasmic end of the pore (6).
The biophysical details of TRPM2 gating are largely unknown. A major hurdle in addressing such questions has been the inability to obtain steady-state single-channel recordings, because TRPM2 currents decay rapidly in excised patches, even in the maintained presence of both activating ligands (2, 6). This “rundown” has a time constant of <1 min, reflects a progressive decline in the number of active channels in the patch, and has so far defied attempts to prevent or reverse it (6).
Beyond presenting a technical hurdle, rundown of several classes of ion channels in inside-out patches has highlighted important regulatory mechanisms, including the role of membrane phosphoinositides as key physiological regulators of inwardly rectifying potassium (Kir) channels (7) and several TRPM family members (8). In inside-out patches, rundown of these channels is caused by dephosphorylation of phosphatidylinositol bisphosphate (PIP2); the presence of PIP2 in the inner membrane leaflet is required to open the gate formed by the TM6 helix bundle-crossing (9, 10). Many more mechanisms are associated with channel rundown, including dephosphorylation of the channel protein, dissociation and washout of an essential regulatory subunit, or cysteine oxidation, but for TRPM2 these were at least partly excluded by previous studies (6). Here we considered the possibility that TRPM2 rundown might reflect an irreversible conformational change of the selectivity filter to a state resembling that of C-type inactivated voltage-gated K+ channels (11). This idea was based on previous work on the TRPM4 selectivity filter (12) in which removal of one of two negatively charged side chains or of an uncharged residue, present in TRPM4 and TRPM5 but absent in TRPM2, induced fast irreversible rundown. Here we show that a triple-mutant TRPM2 channel with a TRPM5-like pore (TRPM5-like, T5L) does not run down in inside-out patches over the time course of hours, whereas its regulation of gating by ADPR and intracellular Ca2+ remains very similar to that of wild type (WT). Our results suggest that a mechanism similar to C-type inactivation plays a role in TRPM2 regulation, and might explain its physiologically relevant sensitivity to extracellular protons (13). The T5L mutant provides an excellent background for studying the biophysics of TRPM2 gating at steady state.
Results
Triple Substitution in the TRPM2 Selectivity Filter Eliminates Current Rundown in Inside-Out Patches.
Because pore mutations induced fast rundown in TRPM4 channels (12), we compared the pore sequences of TRPM4 and of closely related TRPM5 with that of TRPM2 (Fig. 1A). The main difference between the pore sequence of TRPM2 and those of TRPM4 and TRPM5 is confined to a stretch of three alignment positions numbered −4′, −3′, and −2′ (Fig. 1A), counting from the invariant aspartate (0′), which is conserved in all TRPM channels and most classes of K+ channels (5). In particular, the TRPM2 selectivity filter lacks two negatively charged side chains at positions −2′ and −3′, and contains a deletion at position −4′. Because in TRPM4, removal of the negatively charged side chain at position −2′ or deletion of the residue at position −4′ both result in rapidly inactivating channels (12), we tested whether in TRPM2 reverse-type mutations could slow current rundown. Because the TRPM5 pore sequence is more similar to that of TRPM2 (Fig. 1A), we chose TRPM5 as a template. Thus, we first introduced negatively charged side chains into positions −3′ and −2′, individually and in combination (G984D, Y985E, and G984E/Y985E). We then also inserted a leucine into position −4′ of the double mutant, creating a pore sequence identical to that of TRPM5 (T5L).
Fig. 1.
TRPM2 pore substitution abolishes rundown. (A) Sequence alignment for TRPM2, TRPM4, and TRPM5, highlighting the pore helix (green) and the selectivity filter (blue). Red, target positions. (B) Inward macroscopic currents of WT TRPM2 and the four pore mutants G984D, Y985E, G984D/Y985E, and T5L at −20 mV, in response to prolonged exposures to 125 μM Ca2+ + 32 μM ADPR (bars). To monitor seal integrity over prolonged periods of recording, mutants channels were periodically shut by brief (∼1-s) removals of Ca2+. Colored lines, single-exponential fits; red boxes, target triplet sequences for each construct. (Inset) Time constants of WT TRPM2 rundown under various ionic conditions (first five bars; blue, control) and using a protocol (Fig. S2C) during which channels spend ∼75% of the time in the closed state (rightmost bar).
Together with WT TRPM2, the four mutant constructs were expressed in Xenopus oocytes and activated in inside-out patches by direct superfusion of the cytosolic face with ADPR + Ca2+ (6). All four mutants expressed well, yielding large macroscopic currents comparable to WT (Fig. 1B). However, whereas WT channel currents decayed rapidly in the maintained presence of saturating concentrations of ADPR and Ca2+ (Fig. 1B, WT graph) (6), the extent and kinetics of this rundown were robustly altered in the mutants. The G984D mutation (negative charge at position −3′) slowed rundown by >10-fold (Fig. 1B, gray single-exponential fit line in G984D graph), yielding an average decay time constant τ = 514 ± 22 s (n = 3) in contrast to τ = 29 ± 5 s (n = 19) typical to WT (Fig. 1B, blue single-exponential fit line in WT graph). Introducing a negative charge into the −2′ position (Y985E) also markedly slowed the decay time course (τ = 156 ± 17 s, n = 3; Fig. 1B, magenta fit line in Y985E graph) and prevented complete inactivation; whereas for WT and G984D, channel currents eventually vanished, for Y985E, a sizeable fraction of the current (Irel = 0.28 ± 0.05, n = 3) survived the rundown and persisted for the duration of our recordings (Fig. 1B, Y985E graph). Double-mutant G984D/Y985E channels behaved qualitatively similarly to Y985E [τ = 208 ± 72 s (n = 4), surviving fractional current Irel = 0.43 ± 0.08 (n = 4); Fig. 1B, green fit line in G984D/Y985E graph], suggesting that the effects of introducing negative charges into positions −3′ and −2′ are not simply additive. Just as for WT (6), time-dependent changes in macroscopic current size for G984D, Y985E, and G984D/Y985E reflected in each case changes in the numbers of open pores, whereas unitary current amplitude remained constant for each construct (Fig. S1). The most dramatic effect on rundown was achieved when we inserted a leucine into the −4′ position of the double-charge mutant, recreating the TRPM5 pore sequence (T5L). In the presence of saturating ADPR and Ca2+ T5L channel current remained essentially constant for as long as the patches could be held, i.e., for more than 1 h (Fig. 1B, T5L graph); a hint of an initial decline could still be observed, but its small fractional amplitude precluded fitting it with an exponential.
TRPM2 Rundown Resembles C-Type Inactivation.
Because the rate of C-type inactivation in K+ channels is sensitive to the species and concentration of ions permeating through the pore (14), we tested whether the same was true for WT TRPM2 rundown. With (symmetrical) Na+ as the permeating ion, the time constant of rundown was significantly (P < 0.01 by ANOVA) correlated with [Na+]: at 39 mM it was slightly shortened, whereas at 564 mM it was ∼twofold prolonged relative to its control value at 144 mM (Fig. 1B, Inset Left and Fig. S2A). Further, substituting symmetrical 144 mM K+ for Na+ accelerated rundown by ∼twofold (Fig. 1B, Inset Center and Fig. S2B); in contrast, elevating extracellular [Ca2+] from 4 μM to 10 mM did not alter its time constant (Fig. 1B, Inset Center).
A further property of C-type inactivation is its strong state dependence: inactivation happens predominantly while the activation gate is open (14). To test whether the rate of WT TRPM2 rundown is affected by the gating state of the channel, we estimated the rundown rate of closed channels by monitoring the decay rate of WT TRPM2 currents evoked by periodic brief applications of Ca2+ in the continued presence of ADPR (Fig. S2C). Under such conditions, the time constant of rundown was robustly prolonged (τ = 91 ± 15 s, n = 7; Fig. 1B, Inset Right), confirming that rundown is slower in the closed state. Accounting for the fact that the channels spend ∼25% of the total time in the open state during this protocol (Fig. S2C), we estimate that the rundown rate of closed channels is ∼10-fold slower (τ ∼ 270 s) than that of open channels.
T5L Pore Substitution Preserves Intact ADPR and Ca2+ Dependence of TRPM2 Gating.
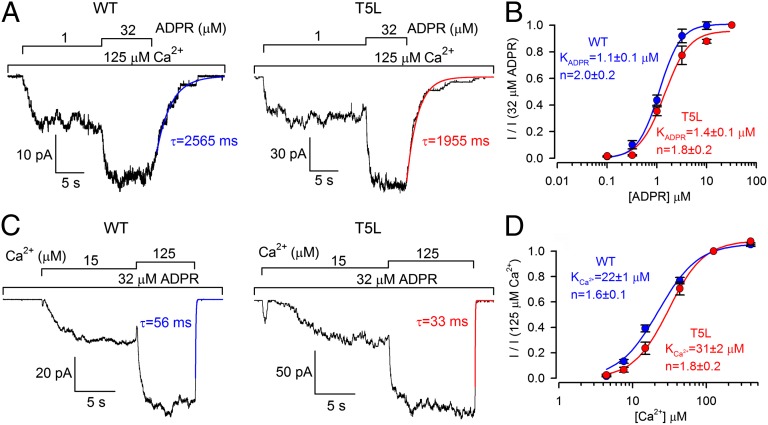
Because a TRPM2 construct that does not inactivate could be invaluable for studying gating, we examined whether the gating properties are altered in T5L TRPM2 (Fig. 2). Fractional currents activated by increasing [ADPR] in the presence of saturating (125 μM) Ca2+ were similar for WT and T5L TRPM2 (Fig. 2A), the resulting dose–response curves (Fig. 2B, blue and red) yielding apparent K1/2 values of 1.1 ± 0.1 μM and 1.4 ± 0.1 μM, respectively. Similarly, comparable fractional currents were observed for WT and T5L TRPM2 upon exposure to increasing [Ca2+] in the presence of saturating (32 μM) ADPR (Fig. 2C), yielding dose–response curves (Fig. 2D, blue and red) with apparent K1/2 values of 22 ± 1 μM and 31 ± 2 μM, respectively (6, 15). Moreover, steady-state gating kinetics of T5L TRPM2 also resembled that reported for WT (6), including inverse dependences on—and differential sensitivities to—intracellular [Ca2+] of single-channel opening and closing rates (Fig. S3).
Fig. 2.
The T5L pore mutation preserves intact ADPR/Ca2+-dependent gating. (A and C) Inward macroscopic currents of WT (Left) and T5L (Right) TRPM2 at −20 mV, elicited by exposures to increasing concentrations of ADPR (bars) in the presence of saturating Ca2+ (A) or increasing concentrations of Ca2+ (bars) in the presence of saturating ADPR (C). Blue and red lines are single exponential fits to the current decay time courses of WT and T5L TRPM2, respectively, upon rapid removal of ADPR in the maintained presence of Ca2+ (A) or upon removal of Ca2+ in the maintained presence of ADPR (C); time constants are indicated. Pipette-free [Ca2+] was ∼4 μM. (B and D) Dose–response curves of fractional current activation (Materials and Methods) by increasing [ADPR] (B) and [Ca2+] (D) in the presence of a saturating concentration of the other ligand, for WT (blue) and T5L TRPM2 (red). Error bars represent SEM; solid lines are fits to the Hill equation.
A hallmark of WT TRPM2 gating is its extremely different response kinetics to removal of its two activating ligands. In the presence of ADPR, sudden removal of intracellular Ca2+ results in rapid current decay (τ ∼ 50 ms; Fig. 2C, blue fit line), suggesting that, even in the open state, Ca2+ bound at the activating sites is readily exchangeable: upon Ca2+ removal, the binding sites instantaneously lose their ligands, unmasking the fast closing rate of unliganded channels (∼20 s−1) (6). In contrast, upon sudden removal of ADPR, in the maintained presence of Ca2+, WT TRPM2 currents decay about two orders of magnitude slower (τ = 2,751 ± 203 ms, n = 10; Fig. 2A, blue fit line). Clearly, at least in the open state, ADPR bound at the NUDT9-H domains is not at rapid equilibrium with the surrounding solution, but rather very tightly bound, or physically occluded. This differential decay rate upon removal of the two ligands is reproduced by the T5L mutant [compare red fit lines in Fig. 2 A and C; τ = 2,073 ± 230 ms (n = 8) following ADPR removal]. Thus, apart from minor quantitative differences, both macroscopic and single-channel gating parameters of the T5L mutant are very similar to those of WT, suggesting an intact gating mechanism.
Increased Apparent Affinities for Permeant Cations but No Change in Pore Diameter Suggest Electrostatic Effect in TRPM2 Pore Mutants.
In contrast to intact gating, unitary conductance of T5L TRPM2 in symmetrical ∼140 mM Na+ was increased by ∼50%, suggesting profoundly altered permeation (Fig. 3A). To address the mechanism of this increased throughput rate, we studied its [Na+] dependence (Fig. 3B, blue and red) by systematically measuring unitary conductances for both WT and T5L TRPM2 in symmetrical [Na+] ranging from ∼40 mM to 1 M, and voltages ranging from −80 to +80 mV (Fig. S4 A and B). Slope conductances for both constructs started to saturate at very high [Na+], and approached each other (Fig. 3 A and B); both dose–response curves were reasonably fit by the Michaelis–Menten equation (Fig. 3B, blue and red fit lines), yielding similar maximal conductances of ∼120 pS, but ∼twofold lower K1/2 for the T5L mutant (101 ± 5 mM vs. 180 ± 17 mM for WT). This difference in apparent affinities for permeating ions was even more pronounced when Ca2+ was the charge carrier (Fig. 3 C and D). Ca2+ throughput rates, quantified as unitary chord conductances at ∼−135 mV with 7–100 mM Ca2+ (Na+-free) in the pipette (extracellular) solution (Fig. S4 C and D), saturated already at ∼20 mM Ca2+ for T5L TRPM2, but for WT failed to saturate even at ∼100 mM (Fig. 3D, red and blue); tentative fits to the Michaelis–Menten equation (Fig. 3D, solid lines) returned K1/2 values of 61 ± 13 mM for WT, but 3.1 ± 0.4 mM for T5L. Similar results were obtained when Mg2+ was the permeant ion (Fig. S5).
Fig. 3.
The T5L mutation increases the apparent affinity for permeating cations. (A and C) Unitary inward currents through single WT (Left) and T5L (Right) TRPM2 channels (A) at ∼−80 mV in symmetrical Na-glutamate solutions of indicated concentrations and (C) at ∼−135 mV with pipette solutions containing indicated concentrations of CaCl2 (but 140 mM Na-gluconate in the bath). (B and D) Dose–response curves of unitary conductance as a function of permeating [Na+] (B) or [Ca2+] (D) for WT (blue) and T5L TRPM2 (red); unitary current-voltage plots in Fig. S4 A–D, respectively, were used to calculate slope conductances for B and inward chord conductances at −135 mV for D. Error bars represent SEM; solid lines in B and D are fits to the Michaelis–Menten equation.
To dissect the individual contributions of the three T5L pore substitutions to the observed changes in permeation properties, we systematically studied unitary conductance parameters of G984D, Y985E, and G984D/Y985E (Fig. S6). Interestingly, [Na+] conductance curves for the three mutants were all very similar to that of T5L (Fig. S6 A and B). Similarly, for all three mutants, conductances in 12 mM Ca2+ were comparable to that of T5L, i.e., ∼threefold higher than for WT (Fig. S6C). Thus, in contrast to the effects on rundown, which were markedly different for G984D, Y985E, and T5L (Fig. 1B), introduction of a single charged side chain at position −3′ or −2′ was sufficient to evoke all of the changes in permeation properties observed for the T5L mutant.
To gauge the pore diameters of WT and T5L TRPM2, we measured inward unitary chord conductances at ∼−130 mV toward extracellular organic cations of increasing sizes (Fig. S7 A and B). Although for each tested cation (at ∼140 mM concentrations), T5L afforded a faster throughput rate than WT (Fig. S7C), plots of conductance vs. cation diameter converged toward zero at similar cutoff values of ∼7 Å (Fig. S7D). Thus, pore diameter seems little affected in the T5L mutant.
Faster Ca2+ Influx Through the T5L Pore Enhances Gating Effects of Extracellular Ca2+.
A previous study showed that the rapid closure observed upon sudden removal of intracellular Ca2+ is dramatically slowed in the presence of millimolar extracellular Ca2+ (compare Fig. 2C with Fig. 4A); the voltage dependence of this effect correlated with the driving force for Ca2+ entry through the pore (6). The results were interpreted to suggest that the activating Ca2+ binding sites, although intracellular, are located in some shielded crevice in immediate vicinity of the pore, where the Ca2+ ions entering from the outside can keep the activating sites saturated despite continuous rapid Ca2+-free rinsing of the channel's cytoplasmic surface (6). This model predicts that the above effect should be more pronounced for the T5L mutant, which conducts Ca2+ in the low-millimolar concentration range far better than WT (Fig. 3D). Congruent with this prediction, when the pipette solution contained 140 mM Na+ + 1 mM Ca2+, the current decay upon removal of cytosolic Ca2+ was markedly slower for T5L TRPM2 than for WT (Fig. 4A) over the entire voltage range tested (Fig. 4B, circles). In contrast, the fast, voltage-independent, decay rate observed in the absence of extracellular Ca2+ (Fig. 2C) was comparable for T5L and WT TRPM2 (Fig. 4B, triangles) (6).
Fig. 4.
Faster Ca2+ influx through the T5L pore enhances gating effects of extracellular Ca2+. (A) Macroscopic WT (Left) and T5L (Right) TRPM2 currents at −60 mV transiently activated by brief exposure to saturating intracellular Ca2+ in the presence of saturating ADPR. Pipette free [Ca2+] was ∼1 mM; note slower current decay compared with Fig. 2C. Solid lines, single-exponential fits. (B) Voltage dependence of WT (blue) and T5L TRPM2 (red) macroscopic closing rates, obtained as inverses of decay time constants, upon sudden removal of cytosolic Ca2+ with 1 mM (circles) or ∼4 μM (triangles) free [Ca2+] in the pipette. Error bars represent SEM.
High-Affinity PIP2 Binding Regulates Sensitivity of TRPM2 for Activation by Intracellular Ca2+.
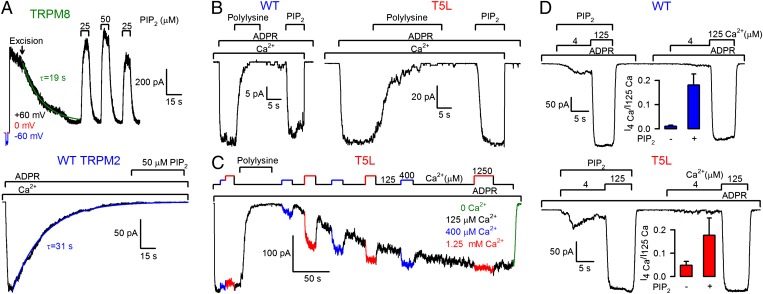
Our results implicated a pore collapse mechanism, rather than PIP2 depletion, as a cause of WT TRPM2 rundown, but it remained a formal possibility that the pore mutations act by decoupling PIP2 effects from the gate. To reliably examine a potential involvement of PIP2 in TRPM2 rundown, we used as a positive control TRPM8 channels, for which PIP2 regulation has been firmly established (16). At 25 °C, with 500 μM menthol in the pipette, TRPM8-expressing oocytes displayed large, outwardly rectifying currents that decayed almost instantaneously following patch excision. Omitting Mg2+ from the bath significantly slowed this rundown; at +60 mV membrane potential the time constant in 0 Mg2+ was 23 ± 4 s (n = 5; Fig. 5A, Upper). Importantly, application of 25 μM dioctanoyl-PI(4,5)P2 (diC8-PIP2) after rundown restored TRPM8 currents to their on-cell values, and 50 μM diC8-PIP2 had little additional effect (Fig. 5A, Upper). In contrast, omitting Mg2+ from the bath did not affect the time course of WT TRPM2 rundown [τ = 29 ± 5 s (n = 9); Fig. 5A, Lower; compare with Fig. 1B]. Moreover, even prolonged exposures to 50 μM diC8-PIP2 did not slow the current decay, or restore currents of deactivated TRPM2 channels (Fig. 5A, Lower), confirming that TRPM2 rundown is a process independent of PIP2 depletion.
Fig. 5.
PIP2 does not prevent rundown, but regulates apparent Ca2+ affinity of TRPM2. (A) Rundown of macroscopic WT TRPM8 (Upper) and TRPM2 (Lower) currents in patches excised into Mg2+-free solutions, and responses to exposure to diC8-PIP2. The TRPM8 (Upper) recording starts in the cell-attached mode with 500 μM menthol in the pipette; membrane voltage was stepped from 0 to −60 and then to +60 mV before excision (arrow). TRPM2 (Lower) was activated by 125 μM Ca2+ + 32 μM ADPR. Solid lines, exponential fits. (B) WT (Left) and T5L (Right) TRPM2 currents activated by 125 μM Ca2+ + 32 μM ADPR are suppressed by 15 μg/mL polylysine and reactivated by 50 μM diC8-PIP2. (C) Suppression and slow recovery of T5L currents in response to brief polylysine (15 μg/mL) treatment; repeated exposures to 400 μM and 1.25 mM Ca2+ (bars) reveal loss and recovery of Ca2+ sensitivity. (D) Fractional activation of WT (Upper) and T5L (Lower) TRPM2 currents by 4 μM Ca2+ (+ 32 μM ADPR) in the presence and absence of 50 μM diC8-PIP2. Colored bars illustrate, for both conditions, average current in 4 μM Ca2+ normalized to that in 125 μM Ca2+.
To further address whether PIP2 plays any role in TRPM2 regulation, we tested the effect on TRPM2 currents of polylysine, a polycation that masks PIP2 headgroups (17). High concentrations (15 μg/mL) of polylysine rapidly abolished WT TRPM2 currents, and this action was indeed due to depletion of free PIP2, because application of 50 μM diC8-PIP2 following polylysine removal restored WT TRPM2 activity (Fig. 5B, Left). Thus, complete depletion of free PIP2 closes the TRPM2 activation gate. Of note, T5L TRPM2 channels, which do not run down, were similarly closed by polylysine and reopened by diC8-PIP2 (Fig. 5B, Right), suggesting that the T5L pore mutation does not decouple PIP2 effects from the gate. Inhibition by polylysine was slowly reversible; the time constant of polylysine washout, measured using T5L-TRPM2, was ∼2–3 min (Fig. 5C). Depletion of free PIP2 acted by decreasing the channels' apparent affinity for activating Ca2+; whereas under control conditions 125 μM Ca2+ supported maximal T5L TRPM2 activity, channels closed by polylysine exposure could be reopened by strongly raising cytosolic Ca2+ (Fig. 5C): shortly after polylysine removal, 1.25 mM Ca2+ supported approximately half-maximal activity, and gradual current restoration was accompanied by a restoration of Ca2+ sensitivity (Fig. 5C). Moreover, for both WT and T5L TRPM2, fractional activity in 4 μM cytosolic Ca2+ (plus 32 μM ADPR) was significantly (P < 0.02) higher in the presence of 50 μM diC8-PIP2 (Fig. 5D) compared with control conditions, consistent with a slightly higher apparent Ca2+ affinity in the presence of high concentrations of PIP2.
Discussion
Introduction of two negative charges (per subunit) plus a single-residue insertion into the TRPM2 selectivity filter abolished the rapid rundown of WT TRPM2 channels. Kinetically, WT TRPM2 rundown is approximated by an all-or-none transition of single channels from a high-open probability gating mode to an irreversibly shut state (6), reminiscent of the strong cooperativity between the four subunits of a K+-channel undergoing C-type inactivation (18, 19). Here we found that the rundown is state-dependent, and sensitive to the species and concentration of the permeating ion (Fig. 1B, Inset and Fig. S2), as expected for a C-type mechanism (14). Together, these findings suggest that TRPM2 rundown reflects a conformational rearrangement of the selectivity filter like that during C-type inactivation of voltage-gated K+ channels. Thus, in addition to the ADPR/Ca2+-operated activation gate, which, based on the intracellular location of the ligand binding sites, is most likely formed by the TM6 bundle-crossing, an independent inactivation gate in TRPM2 is associated with the selectivity filter.
Consistent with this picture, we found that TRPM2 rundown is unrelated to the depletion of membrane PIP2 (Fig. 5A), which is known to affect the intracellular gate (9, 10). However, because many TRPM family channels are regulated by PIP2, it seemed likely that PIP2 should have some effect on TRPM2. Indeed, we found that PIP2 regulates the apparent Ca2+ affinity of the channels, as has been described for TRPM4 and TRPM5 (20, 21). In our inside-out patches, under control conditions, the apparent K1/2 for Ca2+ activation of TRPM2 is ∼20 μM (Fig. 2D), whereas complete depletion of free PIP2 by a large concentration of polylysine shifts the K1/2 to >1 mM (Fig. 5C). Interestingly, even exposure to very high levels of PIP2 only modestly increased the apparent Ca2+ affinity of TRPM2 (Fig. 5D; estimated K1/2 for Ca2+ is ∼10 μM in the presence of 50 μM diC8-PIP2), although membrane PIP2 concentrations must be already very low at the beginning of our experiments, as our bath solutions contain 2 mM Mg2+ and our recordings start >40 s following patch excision (compare with Fig. 5A, Upper). Thus, TRPM2 channels must bind PIP2 with very high affinity, much tighter than the closely related TRPM8 channels, such that even trace amounts of membrane PIP2 are sufficient to support close-to-maximal TRPM2 activity. It will be interesting to elucidate the structural underpinnings of this differential PIP2 sensitivity of TRPM family channels.
Recent studies have provided a hint for the physiological relevance of TRPM2 inactivation. TRPM2 channels in the plasma membrane play important roles at sites of inflammation and ischemia (1–4), and are also found in lysosomal membranes (22), suggesting exposure of the extracellular channel surface to low pH in the body. Extracellular protons both reversibly inhibit (23, 24) and irreversibly inactivate (13) TRPM2; irreversible inactivation was shown to involve extracellular proton interaction sites near the selectivity filter. Possibly, extracellular acidity limits Ca2+ influx through TRPM2 by driving the channels into a conformation similar to the C-type inactivated state identified here. In addition, by affecting the membrane potential, TRPM2 inactivation should also modulate Ca2+ entry through CRAC channels, the other major Ca2+ influx pathway in immune cells.
Introduction of negative charges into the selectivity filter stabilized the TRPM2 pore, which suggests that TRPM2 pore collapse is impeded by charge repulsion across the pore. Consistent with this interpretation, the effects of the two charge insertions were not additive (Fig. 1B), indicating that the two neighboring residues do not act independently. In addition to charge, conformational effects also play an important role, because a single-residue insertion at position −4′ of the double-charge mutant caused further strong stabilization (Fig. 1B). These results are congruent with a study on TRPM4 in which “inverse” mutations, such as a single-residue deletion at position −4′ (ΔQ980) or charge removal at position −2′ (D982A), yielded rapidly inactivating channels (12). Although in the absence of a TRPM2 crystal structure we cannot provide a precise explanation for the stabilizing effect of the leucine insertion at position −4′ in T5L TRPM2, high-resolution crystal structures of both K+ channels (5) and nonselective cation channels (25) show complex networks of side-chain interactions important for filter stabilization. For instance, a conserved hydrogen bond between the invariant aspartate at position 0′ of the filter (Fig. 1A) and a tyrosine at position −13′ in the pore helix of the same subunit is essential for selectivity and pore stability in K+ channels (25). It is noteworthy that in WT TRPM2 a tyrosine is found at position −12′; readjustment of this position to −13′, due to the insertion in T5L, is likely associated with a large change in side chain orientation of this tyrosine.
Somewhat surprisingly, the T5L substitution did not reproduce the exclusive monovalent selectivity characteristic of the TRPM5 pore, but instead increased conductivity toward divalent cations (Fig. 3 C and D and Fig. S5), highlighting the importance of the protein context for determining functional properties of the filter. Nevertheless, the correlation between the increased Ca2+ flux rate through the T5L pore (Fig. 3D) and the enhanced gating effects of extracellular Ca2+ in this mutant (Fig. 4) is consistent with the previous assignment of the activating Ca2+ binding sites as intracellular but near the pore (6).
Unitary conductances in various [Na+], [Ca2+], and [Mg2+] revealed increased apparent affinities for permeating cations in all our pore mutants (Fig. 3 and Figs. S5 and S6). Interestingly, whereas the individual substitutions had distinct effects on the kinetics of rundown (Fig. 1B), introduction of a negative charge at position −2′ or −3′ equivalently affected permeation properties (Fig. S6). The effects of the two charge mutations were not additive, and the leucine insertion at position −4′ had no additional effect (Fig. 3 and Fig. S6). The increase in apparent affinities of the pore mutants was much larger toward divalent cations and was not accompanied by a change in maximal conductances (Fig. 3 and Fig. S6), consistent with increased local cation concentrations near the mouth of the pore due to an electrostatic effect; pore diameter remained ∼7 Å in T5L, as in WT (Fig. S7). The observed symmetry with respect to the direction of current flow of these electrostatic effects in the mutants (Fig. S4 A and B), which contrasts with the asymmetric effects of native or engineered negative charges in the cytosolic vestibule of K+ channels (26, 27), might be explained by the central location of these charges in the TRPM2 selectivity filter and/or its larger diameter, and resembles the effects of perturbations in the central ring of charges of the nicotinic acetylcholine receptor (28).
Beyond the identification of the inactivation gate in TRPM2, the practical impact of this study is the generation of a noninactivating TRPM2 mutant. Because T5L TRPM2 preserves intact gating (Fig. 2 and Fig. S3), it will be an excellent model for studying steady-state gating properties of TRPM2.
Materials and Methods
Molecular Biology.
TRPM2 mutations were made using QuikChange (Stratagene); pGEMHE-TRPM2 and pGEMSH-TRPM8 cDNA were linearized by Nhe1 and transcribed in vitro with T7 polymerase (Ambion); cRNA was stored at −80 °C.
Isolation and Injection of Xenopus Oocytes.
Oocytes were isolated from anaesthetized adult female Xenopus laevis and injected with cRNA as described (6). Single-channel and macroscopic recordings were made 2–3 d after injection of 1–10 ng of cRNA.
Excised Inside-Out Patch Recording.
Recordings shown in Figs. 1, 2, and 5 were done as described (6) in symmetrical Na-gluconate–based solutions, which eliminate endogenous Ca2+-activated Cl− currents. The tip of the patch pipette was filled to ∼1 cm height with 140 mM Na-gluconate, 2 mM Mg-gluconate2, 10 mM Hepes (pH 7.4 with NaOH; free [Ca2+] ∼4 μM; 8 mM Ca(gluconate)2 was added to obtain a free [Ca2+] of ∼1 mM in Fig. 5). The pipette electrode was placed into a 140-mM NaCl-based solution carefully layered on top. Bath solution contained 140 mM Na-gluconate, 2 mM Mg-gluconate2, 10 mM Hepes (pH 7.1 with NaOH), and either 1 mM EGTA [to obtain “zero” (∼8 nM) Ca2+] or 32 μM to 10 mM Ca-gluconate2 (to obtain 8–1,250 μM free [Ca2+]). For Fig. S7 the conditions were identical, except that Na-gluconate in the pipette solution was systematically exchanged for the glutamate salt of mono- (MA+), di- (M2A+), tri- (M3A+), and tetramethylamine (M4A+), or N-methyl-d-glucamine (NMDG+). For Fig. 3 A and B and Figs. S4 A and B and S6 A and B, Na-gluconate was replaced by 35–1,000 mM Na-glutamate in both pipette and bath solutions, the pipette electrode layer contained isosmotic NaCl; 0.2–2 mM Ca-glutamate2 was added to the bath to obtain ∼125 μM free Ca2+. For Fig. 3 C and D and Figs. S4 C and D and S6C, pipette solution contained 5–100 mM CaCl2, 0.5 mM MgCl2, and 10 mM Hepes [pH 7.4 with Ca(OH)2], and patches were superfused with standard Na-gluconate bath solution. Under such conditions only small endogenous chloride currents were recorded at very negative voltages, but even unitary outward TRPM2 currents could be clearly resolved on top of a smooth outward chloride current upon cytosolic addition/removal of 32 μM Na2-ADPR (Sigma) in the maintained presence of 125 μM Ca2+. TRPM8 currents were recorded in symmetrical Na-gluconate, with 500 μM menthol in the pipette solution. Dioctanoyl-PI(4,5)P2 (Cayman Chemical) was added to the bath from a 2.5-mM aqueous stock solution. The continuously flowing 25 °C bath solution was exchanged using computer-driven electronic valves; solution exchange time constant was <100 ms. Currents were digitized at 10 kHz, filtered at 2 kHz, and recorded to disk.
Data Analysis.
Macroscopic current decay time courses were fit by single-exponential functions using nonlinear least-squares methods. Fractional activation by test concentrations of Ca2+ or ADPR were calculated by dividing steady current in the test segment with that in 125 μM Ca2+ + 32 μM ADPR in the same patch; for WT, correction for rundown was done as described (15). Unitary conductances were estimated from fits of Gaussian functions to all-points histograms; measured junction potentials were corrected offline. Single-channel gating parameters (Fig. S3) were extracted by maximum-likelihood fitting of dwell-time histograms, as described (6).
Supplementary Material
Acknowledgments
We thank Dorottya Mayer for oocyte isolation and injection; Tibor Rohács for the TRPM8 clone, for PIP2, and for much valuable advice; and David Gadsby for discussions. L.C. is a Bolyai Research Fellow of the Hungarian Academy of Sciences. Support for this work was provided by Országos Tudományos Kutatási Alapprogramok Grant F 68143 (to L.C.) and an International Early Career Scientist grant from the Howard Hughes Medical Institute (to L.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204702109/-/DCSupplemental.
References
- 1.Perraud AL, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 2.Sano Y, et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- 3.Hara Y, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi N, Kozai D, Kobayashi R, Ebert M, Mori Y. Roles of TRPM2 in oxidative stress. Cell Calcium. 2011;50:279–287. doi: 10.1016/j.ceca.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 6.Csanády L, Törocsik B. Four Ca2+ ions activate TRPM2 channels by binding in deep crevices near the pore but intracellularly of the gate. J Gen Physiol. 2009;133:189–203. doi: 10.1085/jgp.200810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang CL, Feng SY, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 8.Rohacs T, Nilius B. Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflugers Arch. 2007;455:157–168. doi: 10.1007/s00424-007-0275-6. [DOI] [PubMed] [Google Scholar]
- 9.Hansen SB, Tao X, MacKinnon R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature. 2011;477:495–498. doi: 10.1038/nature10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell. 2011;147:199–208. doi: 10.1016/j.cell.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuello LG, Jogini V, Cortes DM, Perozo E. Structural mechanism of C-type inactivation in K(+) channels. Nature. 2010;466:203–208. doi: 10.1038/nature09153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilius B, et al. The selectivity filter of the cation channel TRPM4. J Biol Chem. 2005;280:22899–22906. doi: 10.1074/jbc.M501686200. [DOI] [PubMed] [Google Scholar]
- 13.Yang W, et al. State-dependent inhibition of TRPM2 channel by acidic pH. J Biol Chem. 2010;285:30411–30418. doi: 10.1074/jbc.M110.139774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: A tale of two inactivation mechanisms. Neuron. 1995;15:951–960. doi: 10.1016/0896-6273(95)90185-x. [DOI] [PubMed] [Google Scholar]
- 15.Tóth B, Csanády L. Identification of direct and indirect effectors of the transient receptor potential melastatin 2 (TRPM2) cation channel. J Biol Chem. 2010;285:30091–30102. doi: 10.1074/jbc.M109.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohács T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 17.Suh BC, Hille B. Electrostatic interaction of internal Mg2+ with membrane PIP2 Seen with KCNQ K+ channels. J Gen Physiol. 2007;130:241–256. doi: 10.1085/jgp.200709821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogielska EM, et al. Cooperative subunit interactions in C-type inactivation of K channels. Biophys J. 1995;69:2449–2457. doi: 10.1016/S0006-3495(95)80114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panyi G, Sheng ZF, Deutsch C, Deutsch C. C-type inactivation of a voltage-gated K+ channel occurs by a cooperative mechanism. Biophys J. 1995;69:896–903. doi: 10.1016/S0006-3495(95)79963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilius B, et al. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 2006;25:467–478. doi: 10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange I, et al. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal. 2009;2:ra23. doi: 10.1126/scisignal.2000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du J, Xie J, Yue L. Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity. J Gen Physiol. 2009;134:471–488. doi: 10.1085/jgp.200910254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starkus JG, Fleig A, Penner R. The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular acidification. J Physiol. 2010;588:1227–1240. doi: 10.1113/jphysiol.2010.187476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauer DB, Zeng WZ, Raghunathan S, Jiang YX. Protein interactions central to stabilizing the K+ channel selectivity filter in a four-sited configuration for selective K+ permeation. Proc Natl Acad Sci USA. 2011;108:16634–16639. doi: 10.1073/pnas.1111688108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nimigean CM, Chappie JS, Miller C. Electrostatic tuning of ion conductance in potassium channels. Biochemistry. 2003;42:9263–9268. doi: 10.1021/bi0348720. [DOI] [PubMed] [Google Scholar]
- 27.Brelidze TI, Niu XW, Magleby KL. A ring of eight conserved negatively charged amino acids doubles the conductance of BK channels and prevents inward rectification. Proc Natl Acad Sci USA. 2003;100:9017–9022. doi: 10.1073/pnas.1532257100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imoto K, et al. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988;335:645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.