Abstract
γ-Hydroxybutyric acid (GHB) binding to brain-specific high-affinity sites is well-established and proposed to explain both physiological and pharmacological actions. However, the mechanistic links between these lines of data are unknown. To identify molecular targets for specific GHB high-affinity binding, we undertook photolinking studies combined with proteomic analyses and identified several GABAA receptor subunits as possible candidates. A subsequent functional screening of various recombinant GABAA receptors in Xenopus laevis oocytes using the two-electrode voltage clamp technique showed GHB to be a partial agonist at αβδ- but not αβγ-receptors, proving that the δ-subunit is essential for potency and efficacy. GHB showed preference for α4 over α(1,2,6)-subunits and preferably activated α4β1δ (EC50 = 140 nM) over α4β(2/3)δ (EC50 = 8.41/1.03 mM). Introduction of a mutation, α4F71L, in α4β1(δ)-receptors completely abolished GHB but not GABA function, indicating nonidentical binding sites. Radioligand binding studies using the specific GHB radioligand [3H](E,RS)-(6,7,8,9-tetrahydro-5-hydroxy-5H-benzocyclohept-6-ylidene)acetic acid showed a 39% reduction (P = 0.0056) in the number of binding sites in α4 KO brain tissue compared with WT controls, corroborating the direct involvement of the α4-subunit in high-affinity GHB binding. Our data link specific GHB forebrain binding sites with α4-containing GABAA receptors and postulate a role for extrasynaptic α4δ-containing GABAA receptors in GHB pharmacology and physiology. This finding will aid in elucidating the molecular mechanisms behind the proposed function of GHB as a neurotransmitter and its unique therapeutic effects in narcolepsy and alcoholism.
Keywords: γ-hydroxybutyric acid receptor, γ-hydroxybutyric acid high-affinity binding sites, α4-subunit knockout, photoaffinity ligand
The GABA metabolite γ-Hydroxybutyric acid (GHB) is present in micromolar concentrations in the mammalian brain, where it has been proposed to act as a neurotransmitter (1). Additionally, GHB is a drug of abuse (Fantasy) and a registered drug for treating narcolepsy (2) and alcoholism (3). GHB binds to at least two distinct populations of low- and high-affinity binding sites in the brain (4). When GHB is ingested in high doses and reaches millimolar concentrations in the brain, it induces behavioral effects such as sedation, motor incoordination and hypothermia (3). These actions are largely mediated by metabotropic GABAB receptors, because effects are prevented by GABAB receptor antagonist pretreatment (5) and completely abolished in GABAB(1) KO mice (6). In addition to the validated GABAB receptor effects and other suggested receptors (7), GHB binds with nanomolar to micromolar affinity to a remarkably abundant protein of distinct spatial distribution and ontogenesis (4), representing an additional functional target. Interestingly, this high-affinity binding protein is preserved in GABAB(1) KO mice (6) and can be specifically probed with [3H](E,RS)-(6,7,8,9-tetrahydro-5-hydroxy-5H-benzocyclohept-6-ylidene)acetic acid ([3H]NCS-382) (6) and [125I]4-hydroxy-4-[4-(2-iodobenzyloxy)phenyl]butanoate ([125I]BnOPh-GHB) (8). Furthermore, several reports point to GHB-induced effects that cannot be consequences of GABAB receptor activation alone: Fos expression studies with GHB indicate a unique pattern of neuronal activation, which in several ways, is different from the pattern produced by the GABAB receptor agonist baclofen (9). Numerous effects induced by GHB, including sedation, catalepsy (10), increased striatal dopamine release, changes in EEG pattern (11), discriminative stimulus properties (12), and reinforcing effects (13), are dose-dependently decreased by pretreatment with the GHB receptor-specific ligand NCS-382. Additionally, ataxia seems to be mediated through the high-affinity GHB sites (14). The reported euphoric effect of GHB and its therapeutic effect in narcolepsy cannot be mimicked by baclofen (3) and thus, might involve other targets. Drug discrimination studies also show that rats are able to distinguish between GHB and baclofen, further supporting that the effects and mechanisms of the two drugs are different (15). Taken together, these findings strongly suggest that GHB acts at targets in addition to the GABAB receptor.
Structurally and behaviorally, GHB, in many ways, resembles its endogenous precursor GABA. Thus, in addition to effects at GABAB receptors, ionotropic GABAA receptors have also been studied as possible GHB targets. The role of GABAA receptors in mediating effects of GHB has been controversial, in part because of the heterogeneity of this receptor class and the lack of recombinant functional studies performed; thus, the large number of subtype combinations that can be formed from the numerous known subunits [α(1–6), β(1–3), γ(1–3), δ, ε, θ, π, and ρ(1–3)] (16) have not been investigated.
Depending on composition, GABAA receptors can be found at both synaptic and extrasynaptic locations and mediate phasic and tonic inhibition, respectively (17). The majority of GABAA receptors contain a γ-subunit, and these receptors can be found at both synaptic and extrasynaptic locations, whereas the δ-subunit predominates on peri- and extrasynaptic locations (18, 19), most commonly accompanied by α(4/6)-subunits.
In 1987, the work by Snead and Nichols (20) reported evidence for coupling of the GHB binding site to a GABA-gated chloride channel; whereas effects of GHB on the major GABAA (synaptic) receptors have been refuted (21, 22), effects at extrasynaptic receptors have, until now, not been systematically investigated. In fact, several studies infer a role for GHB at extrasynaptic GABAA receptors, such as a correlation between elevated GHB levels and increased tonic extrasynaptic inhibition through GABAA receptors (23). More specifically, effects involved receptor subtypes containing α4- and δ-subunits (24–26).
In this study, we have exploited an in-house–developed, high-affinity, and selective GHB photoligand (8, 27, 28) to cross-link and partially purify the high-affinity GHB binding protein from rat brain cortex, with several GABAA subunits emerging as candidate proteins. Functional studies in Xenopus laevis oocytes and radioligand binding studies in KO mouse brain tissue verified α4βδ-receptors as high-affinity targets for GHB. Thus, we present direct molecular evidence for a GHB–GABAA receptor interaction in both recombinant and native systems.
Results
Proteomics Identify GABAA Receptor Subunits as Candidates for High-Affinity GHB Binding Sites.
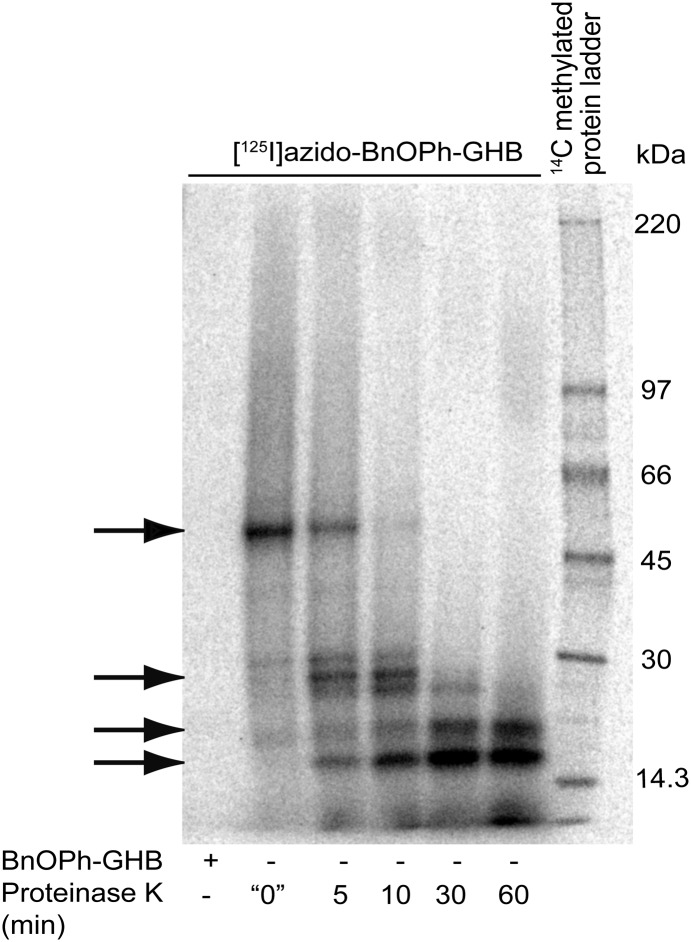
Using an engineered GHB photoaffinity ligand (28), we previously determined the high-affinity GHB binding protein to be ∼50 kDa (8). In attempt to provide better validity in the identifications made by the proteomics analysis, we also subjected samples to limited proteolysis by time-dependent treatment with proteinase K, which resulted in minor bands of ∼28, ∼21, and ∼18 kDa (Fig. 1). High-resolution orbitrap mass spectrometric analysis by nanoscale liquid chromatography–tandem MS spectra of each of these bands identified several GABAA receptor subunits: α1, α2, α3, α5, β1, β2, β3, and γ1 (Table S1 and Dataset S1).
Fig. 1.
Photoaffinity labeling of high-affinity GHB binding sites from rat brain and isolation of target proteins. SDS/PAGE separations of [125I]azido-BnOPh-GHB radiophotoaffinity-labeled partially degraded binding proteins using time-dependent Proteinase K limited proteolysis. Arrows indicate the four protein bands isolated for proteomics analysis (top to bottom: ∼50, ∼28, ∼21, and ∼18 kDa).
GHB Is a Partial Agonist at Particular Subtypes of Recombinant GABAA Receptors.
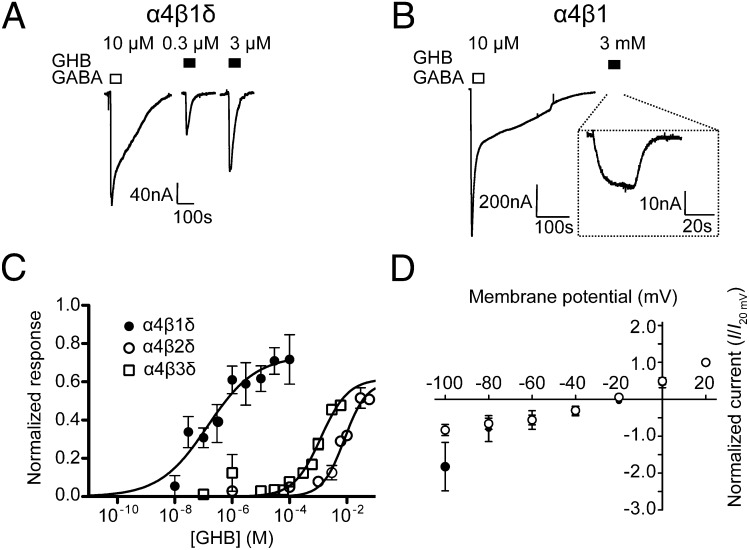
Prompted by the proteomics results, we systematically investigated the effects of GHB at different human recombinant GABAA receptor subtypes expressed in X. laevis oocytes. At various synaptic and extrasynaptic receptor combinations (α1β2γ2L, α5β3γ2L, α2β1δ, α1β3δ, and ρ1), GHB at concentrations of 1 mM and higher was without effect. However, when oocytes were injected with the combination of α4-, β(1–3)-, and δ-subunits, GHB induced inward currents, exhibiting both an intriguing dependence on α4/δ for efficacy and β1 for potency (Table 1). GHB activated α4β1δ receptors with high nanomolar potency [EC50 = 140 nM (30–660)], inducing a maximum current of 74 ± 10% relative to GABA (Fig. 2 A and C). At α4β1 and α4β1γ2L, 3 mM GHB elicited a small response (3 ± 0.2%, P = 0.01 and 2 ± 2%, P = 0.31, Z test compared with control, respectively) (Fig. 2B and Table 1). In oocytes injected with RNA for neither α4 and β1 nor α4, β1, and δ did we find any indication that GHB could antagonize the GABA response (Fig. S1). Substitution of the β1-subunit with β2 or β3 led to a slight reduction in the relative agonist efficacy (53–76%) but a >7,000-fold reduction in potency [EC50 = 8.4 mM (4.0–17) and 1.0 mM (0.6–2.8), respectively] (Fig. 2C). Construction of a current–voltage (I-V) curve in X. laevis oocytes expressing α4β1δ-receptors confirmed that GHB activated a chloride channel (Fig. 2D). Effects of GHB were undetectable in α4β3-, α4β3γ2L-, and α4β2γ2L-receptors (Table 1). By contrast, when coexpressing α1-, α2-, or α6-subunits with β1δ, currents induced by GHB were dramatically reduced (α1 and α6) compared with α4 (Table 1) or completely absent (α2).
Table 1.
Two-electrode voltage clamp recordings of GHB-elicited currents from X. laevis oocytes expressing different α(1,4,6)- and α4F71L-containing GABAA receptors
| I/IGABA, max ± SEM | EC50 (95% CI; M) | nH (95% CI) | n | |
| α4β1δ | 0.74 ± 0.1 | 1.4 × 10−7 (0.3–6.6 × 10−7) | 0.52 (0.09–0.95) | 5 |
| α4β1γ2L | 0.02 ± 0.02* | † | † | 4 |
| α4β1 | 0.03 ± 0.002* | † | † | 4 |
| α4β2δ | 0.53 ± 0.06 | 8.4 × 10−3 (4.0–17 × 10−3) | 1.6 (0.52–1.8) | 4 |
| α4β2γ2L | † | † | † | 5 |
| α4β2 | ‡ | ‡ | ‡ | 22 |
| α4β3δ | 0.76 ± 0.08 | 1.0 × 10−3 (0.6–2.8 × 10−3) | 1.3 (0.6–1.2) | 6 |
| α4β3γ2L | † | † | † | 5 |
| α4β3 | † | † | † | 5 |
| α1β1δ | 0.02 ± 0.01§ | ¶ | ¶ | 8 |
| α6β1δ | 0.06 ± 0.02§ | ¶ | ¶ | 10 |
| Mutant data | ||||
| α4F71Lβ1 GHB | † | † | † | 6 |
| α4F71Lβ1δ GHB | † | † | † | 10 |
| α4F71Lβ1 GABA | § | 7.2 × 10−7 (5.5–9.3 × 10−7) | 0.89 (0.7–1.1) | 4 |
| α4F71Lβ1δ GABA | § | 1.8 × 10−7 (1.3–2.6 × 10−7) | 1.03 (0.7–1.4) | 6 |
*Normalized current at 3 mM GHB.
†Because of high estimated EC50 value, no curve could be generated.
‡GABA-induced currents were not high enough to estimate GHB responses.
§GABA concentrations used to determine maximum current for each subunit were, respectively, 10 μM, 1 mM, 10 μM, 1 μM, 1 mM, 10 μM, 10 μM, 1 mM, 10 μM, 10 mM, and 1 mM.
¶I/IGABA not applicable.
Fig. 2.
Pharmacological characterization of GHB at recombinant αβδ-receptors in X. laevis oocytes. Representative GHB current traces at (A) α4β1δ and (B) α4β1 GABAA receptors. (C) Concentration response curve at α4β1–3δ-receptors normalized to GABAmax (means ± SEM; n = 4–6). (D) GHB (closed circles) and GABA (open circles) I-V relationships at α4β1δ-receptors (n = 7). Reversal potential was not significantly different for GABA and GHB currents (Vrev = −25.2 ± 3.9 and −27.8 ± 4.8, respectively; P = 0.68, Student t test).
Molecular Pharmacology of the GHB α4β1δ-Receptor Interaction.
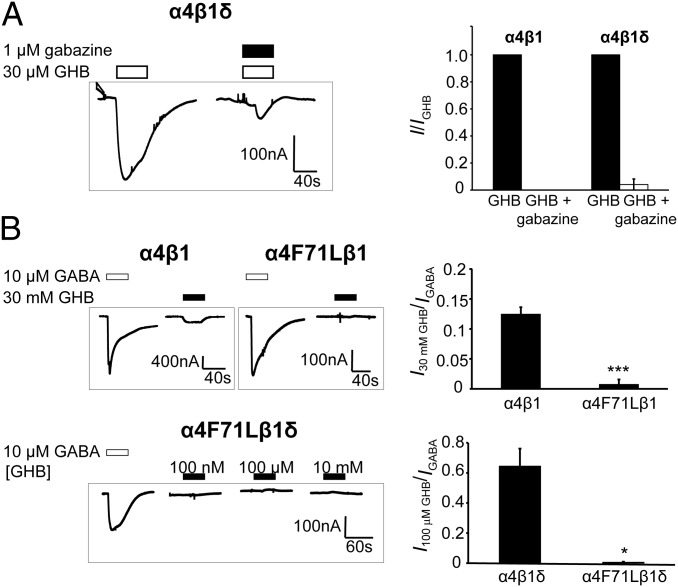
The pharmacology of the α4β1δ subtype in relation to GHB was further investigated. The response induced by 30 μM GHB was completely blocked by coapplication of the GABAA receptor antagonist gabazine (1 μM) (Fig. 3A). In attempts to delineate the GHB binding site, we investigated the role of a conserved α-subunit Phe residue (F64 in α1 and F71 in α4). This residue lines the GABA binding site of αβγ-receptors, and when mutated to Leu, it gives a marked (>200-fold) increase in GABA EC50 at α1β2γ2 GABAA receptors (29). Interestingly, introduction of a mutated α4F71L-subunit completely abolished GHB activity at α4β1 and α4β1δ compared with WT receptors (Fig. 3B). By contrast, GABA EC50 values were only increased about fivefold for α4(F71L)β1 (P = 0.058) and α4(F71L)β1δ (P = 0.001) (Table 1).
Fig. 3.
Abolishment of GHB response by gabazine coapplication and α4F71L point mutation. (A, Left) Representative gabazine inactivation trace of GHB currents at α4β1δ. (A, Right) Summarized data displaying fraction of GHB current at α4β1/δ (means ± SEM; n = 3). (B, Left) Representative traces of GABA and GHB-elicited currents from α4β1, α4(F71L)β1 (Upper), and α4(F71L)β1δ (Lower). (B Right) Summary (means ± SEM) of 30 mM GHB effects at α4β1 vs. α4(F71L)β1 (***P = 6.3 × 10−5) and 100 μM GHB at α4β1δ vs. α4(F71L)β1δ (*P = 0.011).
Radioligand Binding Studies to Link GHB Binding and Function.
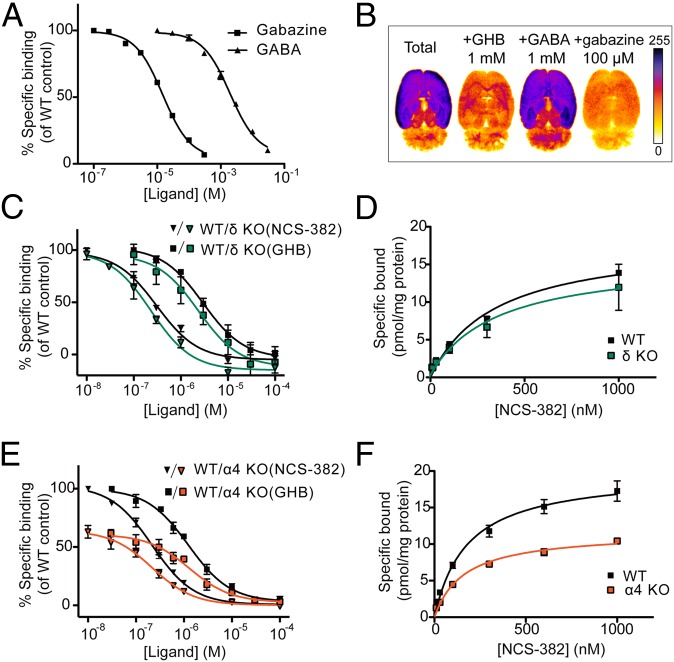
To link GHB function at α4βδ-receptors with the GHB high-affinity binding site, we probed numerous GABAA ligands for their ability to inhibit high-affinity GHB binding ([3H]NCS-382 binding) (Table S2). Only one of these ligands, gabazine, inhibited binding with an IC50 in the mid micromolar range, whereas the IC50 for GABA was in the low millimolar range (Fig. 4A). Additionally, by autoradiography, we found that gabazine inhibited [125I]BnOPh-GHB binding in a regionally specific manner similar to GHB (Fig. 4B) (8). Next, we investigated [3H]NCS-382 binding in brain tissue from α4 and δ KO mice and WT littermates. Whereas [3H]NCS-382 binding did not differ statistically between δ KO and WT mice (Fig. 4 C and D), a significant reduction in Bmax in α4 KO membranes compared with WT was observed (P < 0.01) (Fig. 4 E and F and Table 2, mean ± SEM).
Fig. 4.
GHB high-affinity radioligand binding to rat and KO mouse brain preparations. (A) Gabazine and GABA inhibition of [3H]NCS-382 (16 nM) binding to rat brain homogenate (pKi 4.7 ± 0.11 and 2.7 ± 0.021, respectively; n = 3). (B) Autoradiograms of [125I]BnOPh-GHB (100 pM) binding to horizontal brain sections (n = 2). (C and E) Inhibition by GHB and NCS-382 and (D and F) saturation of [3H]NCS-382 binding (16 nM) to membrane preparations from α4- and δ-subunit KO mouse brains, respectively (means ± SEM), showing significantly lower binding in α4 KO vs. WT (P = 0.0056).
Table 2.
[3H]NCS-382 binding data from α4 and δ KO brain tissue
| GHB IC50 (pIC50 ± SEM; M)* | NCS-382 IC50 (pIC50 ± SEM; M)† | Bmax (pmol/mg protein) | |
| δ−/− | 2.12 ⋅ 10−6 (5.68 ± 0.06) | 2.24 ⋅ 10−7 (6.70 ± 0.11) | 10.1 ± 3.01 |
| δ+/+ | 3.12 ⋅ 10−6 (5.51 ± 0.05) | 2.65 ⋅ 10−7 (6.62 ± 0.10) | 14.3 ± 2.93 |
| α4−/− | 1.22 ⋅ 10−6 (5.97 ± 0.16) | 2.16 ⋅ 10−7 (6.67 ± 0.03) | 12.2 ± 0.89‡ |
| α4+/+ | 1.19 ⋅ 10−6 (5.94 ± 0.09) | 2.20 ⋅ 10−7 (6.67 ± 0.06) | 20.1 ± 1.90 |
*n = 3.
†n = 5.
‡P = 0.0056, unpaired Student t test.
Discussion
The present study identifies α4βδ-receptors, particularly α4β1δ-receptors, as high-affinity targets for GHB, which are likely to represent the elusive GHB receptor. The dependence of α4- and δ-subunits for eliciting a GHB-induced response and the remarkable selectivity for the β1-subunit serve as a base for hypotheses regarding the unique properties of GHB. Our finding is supported by several studies, indicating a possible role for GHB at extrasynaptic α4-containing GABAA receptors. A study using metabolic fingerprinting recently reported that the actions of GHB are similar to several ligands acting at extrasynaptic GABAA receptors (25). A link between GHB and α4 has also been indicated by the ability of GHB to antagonize increases in α4 mRNA levels induced by ethanol withdrawal (24) and the association of chronically elevated GHB levels seen in succinic semialdehyde dehydrogenase KO mice with increased tonic inhibition at extrasynaptic GABAA receptors (23).
Similar to other δ-subunit–preferring GABAA agonists, such as 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP or gaboxadol) (30), we find that the δ-subunit plays a clear potentiating role for GHB function at recombinant α4βδ-receptors. It is tempting to speculate that this finding could be physiologically relevant. Indeed, in δ KO mice, systemic administration of GHB fails to induce spike-and-wave discharges and epileptic absence seizures (26), supporting that the δ-subunit is also required for GHB-induced responses in vivo. In contrast to its essential role for function, the δ-subunit seems not to be crucial for binding, because no significant difference in binding levels was found between WT and δ KO mice. By contrast, the α4-subunit seems to be an important determinant for [3H]NCS-382 binding, which was indicated by a 39% reduction of Bmax in α4 KO compared with WT tissue. The residual [3H]NCS-382 binding may be explained by compensatory mechanisms such as up-regulation of other GABAA receptor subunits (31) or the ability of [3H]NCS-382 to bind to additional GABAA interfaces. This latter point is supported by the ability of GHB to activate not only α4β1(δ) but also α1β1δ- and α6β1δ-receptors. Although a compensatory mechanism in δ KO mice could possibly mask an involvement of the δ-subunit in ligand binding, our proteomics data do not support this hypothesis.
The finding that α4F71 is a key residue in mediating GHB activity at α4β1δ-receptors holds interesting implications for additional dissection of the GHB binding site. The only fivefold reduction in GABA EC50 inflicted by the mutation indicates that GABA interacts differently in δ-containing compared with γ-containing receptors, which was recently reported (32), and it suggests that GHB and GABA could have distinct but overlapping binding sites. This finding would also explain why GHB displays partial agonism at α4β1δ-receptors compared with GABA but does not antagonize the GABA response. Alternatively, the maximal response for GABA in oocytes injected with RNA for α4, β1 and δ could well be the result of a mixture of currents evoked from individual α4β1- and α4β1δ-receptors (32), which would underestimate GHB efficacy. It is also possible that posttranslational modifications occur or variant stoichiometric forms of the α4β1δ-receptors are expressed that have different pharmacological properties. This finding would result in relatively larger errors in the efficacy of compounds for α4β1δ-receptors compared with α4β1-receptors, which seems to be the case for GHB.
From the current data, we propose that NCS-382/GHB binds to the β–α interface in a site also recognized by GABA and gabazine but notably, in a way alternative to their high-affinity binding modes (33). Similar to effects of THIP (30), the δ-subunit plausibly increases GHB sensitivity by inducing allosteric effects in relation to either receptor binding or gating. The remarkable abundance of [3H]NCS-382 binding sites in fore- and midbrain regions (20–30 pmol/mg; present study) (6) may, thus, be explained by binding to a number of β–α interfaces that seem functionally silent in the absence of the δ-subunit, an observation also supported by the variety of α- and β-subunits in our proteomics analyses.
The markedly higher GHB sensitivity at β1-containing vs. β(2/3)-containing α4δ GABAA receptors makes analogs designed specifically for the high-affinity GHB binding site (27, 34) interesting as pharmacological tools for studying β1-containing receptors. To date, only few such compounds have been reported (35, 36). The preference for β1 may, in part, explain the sleep-mediating effects of GHB, because the endogenous pathway of sleep has been shown to depend mainly on β1-containing GABAA receptors, whereas anesthesia and hypnosis are mediated through β3-containing receptors (37, 38). β1 is associated with extrasynaptic receptors (39) and abundantly found in the hippocampus and cortex (40), where high-affinity GHB binding sites are predominantly located (6). Although less abundant, α4 and δ are also present in hippocampus and cortex (40). β1 (and to a lesser degree, α4 and δ) is present in several brain regions important for modulation of sleep and facilitation of EEG synchronization such as the reticular thalamic nucleus (37), which may underlie the unique therapeutic effect of GHB in narcolepsy. Furthermore, a correlation has been shown between activity at β1-subunit–containing GABAA receptors and ataxia, which is a proposed GHB receptor-mediated effect, because it is induced by GHB receptor-specific ligands and not antagonized by GABAB antagonists (14). The α4β1δ-subtype has been identified as an important target for endogenous neurosteroids, and receptor expression is modulated by fluctuating levels of these subtypes throughout the estrus cycle, potentially causing great sex differences in response to α4β1δ-receptor ligands (41, 42). Furthermore, α4β1δ up-regulation has been associated with increased anxiety and hyperalgesia because of disinhibition of GABAergic output neurons (43). Whether GHB effects are affected by neurosteroids, age, and sex remains to be investigated.
Overall, we have provided correlation between GHB high-affinity binding sites and α4β1δ-receptors in vitro. Extensive elaborate studies are now needed to clarify the contribution of extrasynaptic α4β1δ-receptors to the in vivo pharmacological effects of GHB.
Materials and Methods
Chemical Compounds.
GHB and GABA were purchased from Sigma. NCS-382 and gabazine (SR 95531) hydrobromide were from Tocris Bioscience. Lithium BnOPh-GHB was prepared in-house as described previously (27). [3H]NCS-382 (20 Ci/mmol) was purchased from Biotrend. The radioligands [125I]BnOPh-GHB and [125I]azido-BnOPh-GHB were prepared as previously described (28, 44).
Proteomics.
The photoaffinity radiolabeling with [125I]azido-BnOPh-GHB of rat membrane was carried out exactly as previously described (8, 28) and treated with proteinase K (Sigma-Aldrich) and denaturing gel electrophoresis. The isolated gel bands were prepared for liquid chromatography–MS. Additional details in SI Materials and Methods.
Electrophysiology.
Expression of recombinant GABAA receptors in X. laevis oocytes.
Human cDNAs containing the α1-, α2-, α4-, α5-, α6-, β1-, β2-, β3-, δ-, and γ2L-subunits were subcloned into vectors suitable for mRNA transcription and injected into oocytes to express the desired GABAA subtypes as previously described (45). Stages V–VI oocytes were microinjected with 0.5–5 ng mRNA using the following ratios of mRNA: α4β1δ (1:1:5); α4β(2/3)δ (5:1:5); α4β(1–3)γ2L (1:1:10); and α(1, 2)β1δ (5:1:5). After injection, oocytes were maintained at 18 °C in the ND96 wash solution [96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM Hepes (hemisodium salt) augmented with 50 mg/mL gentamycin and tetracycline]. The α4F71L mutant was generated by QuikChange mutagenesis (Stratagene) according to the manufacturer’s instructions. The correct identity of the mutant construct was confirmed by DNA sequencing.
Two-electrode voltage clamp recordings.
Whole-cell currents were measured 3–5 d after injection of cRNA by two-electrode voltage clamp (Digidata 1200, Geneclamp 500B amplifier) together with a Powerlab/200 (AD Instruments) and Chart version 3.5. Oocytes were voltage-clamped at −60 mV. The recording microelectrodes were filled with 3 M KCl and had resistance between 0.2 and 1 MΩ. Increasing concentrations of GABA were applied until a plateau in the current was reached. GABAAR subtypes were evaluated for GHB activation by the application of GHB up to 3–10 mM. Gabazine was dissolved in 10 mM stock concentration in H2O. I-V curves were generated by holding the oocyte at varying membrane potentials and applying either 10 mM GABA or 30 μM GHB. The peak currents on agonist application were measured, and only oocytes that had full I-V curves measured for both GABA and GHB were included in the analysis.
Radioligand Binding Studies.
Tissue and membrane preparation.
Membranes were prepared as described previously (46). δ KO and WT brain tissue (cerebral cortex) was from adult male and female mice (2–4 mo; C57BL/6J × 129Sv/SvJ) (47). Tissue from four KO mice (one female and three males) and four WT males was pooled for membrane preparations. α4 KO and WT brain tissue (midbrain + cerebral cortex) was from adult male mice (aged 4–6 mo) of a mixed strain 129 × 1/S1 × C57BL/6J genetic background (48). Two individual pools of α4 KO membranes prepared from three and two male KO mice were used.
[3H]NCS-382 homogenate binding assay and [125I]BnOPh-GHB autoradiography.
The [3H]NCS-382 binding assay was performed in 96-well plate format modified from the original report (49, 50) using 35–50 μg protein (well-washed membranes), 16 nM [3H]NCS-382, and test compound in 200 μL total incubation buffer per well (triplicates). Nonspecific binding was determined with 1 mM GHB. The reaction was terminated by rapid filtration through GF/C unifilters, and radioactivity was measured on a Packard TopCount NXT Microplate Scintillation Counter (PerkinElmer). Autoradiography was performed as previously reported (8), except that the incubation buffer was 50 mM Tris⋅HCl buffer (pH 7.4). In brief, horizontal brain slices (20 μm; bregma = 4.5–4.8 mm) (51) from male Sprague–Dawley rats (∼250 g; Charles River) were preincubated for 30 min in buffer and then incubated at room temperature for 30 min with 100 pM [125I]BnOPh-GHB in the absence (total binding) or presence of competing compound. Sections were briefly washed, dried for 1 h at room temperature, and fixed in paraformaldehyde. Dried sections were exposed to a BAS-2040 phosphor imaging plate for 1.5 h at room temperature and scanned on a BAS-2500 bioimaging analyzer.
Data Analysis.
Pharmacological data and statistical analyses were carried out using GraphPad Prism 5.0 (GraphPad Software Inc). Autoradiograms were analyzed using ImageJ V.1.43s (http://rsbweb.nih.gov/ij). The amplitude of each current response to GABA (I) was normalized to the amplitude of the maximum current response to GABA (Imax). Normalized dose–response curves were analyzed by nonlinear regression. Mean parameters of each curve were derived from at least three oocytes.
I-V curves were analyzed according to Eq. 1,
where I0 is the current at 0 mV and Vrev is the reversal potential. To control for rectification, Vrev was determined using data from −40 to +20 mV. Curves were normalized to each agonist rather than both to GABA to correct for changes in GHB efficacy resulting from the differing proportion of receptors without δ-subunit incorporation expressed in individual oocytes [i.e., for GHB, I(30 μM GHB)/I(30 μM GHB, +20 mV); for GABA, I(10 μM GABA)/I(10 μM GABA, +20 mV)]. The reversal potential between GHB and GABA was compared with a Student t test.
Inhibition curves and homologous displacement binding curves were analyzed by nonlinear regression. Bmax values were estimated from homologous (NCS-382) competition curves exactly as described (52). For visual interpretation, saturation curves were constructed and fitted using the equation for one-site saturation. Calculations were based on the equation, where added ligand is the sum of [[3H]NCS-382] and [NCS-382], and specific disintegrations per minute (DPM) was calculated from specific counts per minute (CPM) values and a known counting efficiency of 0.375 (TopCount NXT). KO and WT data are presented as mean values ± SEM (n) and compared by unpaired, two-tailed Student t test.
Supplementary Material
Acknowledgments
We thank the Department of Pharmacology, University of Sydney, for managing and maintaining the X. laevis colony. We also thank Drs. D. Belelli and E. Korpi for the gifts of α4 and δ KO tissue, respectively. We are thankful to Andrew Kobryn for excellent technical assistance and Dr. Bernhard Bettler for fruitful discussions. N.A., H.B.-O., B.F., R.P.C., M.C., and P.W. are supported by Australian National Health and Medical Research Council Grant #1003619. L.F.E., T.B., and M.C. acknowledge support from the Drug Research Academy. J.V.O. and P.W. are supported by the Novo Nordisk Foundation. M.C. is supported by the Australian Academy of Science European Travel Award. P.W. acknowledges support from the Alfred Benzon Foundation, the For Women in Science prize sponsored by L'Oréal, United Nations Educational, Scientific, and Cultural Organization, and The Royal Danish Academy of Science and Letters, and the A. P. Møller Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204376109/-/DCSupplemental.
See Commentary on page 13142.
References
- 1.Bernasconi R, Mathivet P, Bischoff S, Marescaux C. Gamma-hydroxybutyric acid: An endogenous neuromodulator with abuse potential? Trends Pharmacol Sci. 1999;20:135–141. doi: 10.1016/s0165-6147(99)01341-3. [DOI] [PubMed] [Google Scholar]
- 2.Drasbek KR, Christensen J, Jensen K. Gamma-hydroxybutyrate—a drug of abuse. Acta Neurol Scand. 2006;114:145–156. doi: 10.1111/j.1600-0404.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 3.Carter LP, Koek W, France CP. Behavioral analyses of GHB: Receptor mechanisms. Pharmacol Ther. 2009;121:100–114. doi: 10.1016/j.pharmthera.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benavides J, et al. High affinity binding sites for γ-hydroxybutyric acid in rat brain. Life Sci. 1982;30:953–961. doi: 10.1016/0024-3205(82)90624-5. [DOI] [PubMed] [Google Scholar]
- 5.Carai MAM, et al. γ-aminobutyric acidB (GABAB)-receptor mediation of different in vivo effects of γ-butyrolactone. J Pharmacol Sci. 2008;106:199–207. doi: 10.1254/jphs.fp0071487. [DOI] [PubMed] [Google Scholar]
- 6.Kaupmann K, et al. Specific γ-hydroxybutyrate-binding sites but loss of pharmacological effects of γ-hydroxybutyrate in GABA(B)(1)-deficient mice. Eur J Neurosci. 2003;18:2722–2730. doi: 10.1111/j.1460-9568.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- 7.Andriamampandry C, et al. Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator γ-hydroxybutyrate (GHB) FASEB J. 2003;17:1691–1693. doi: 10.1096/fj.02-0846fje. [DOI] [PubMed] [Google Scholar]
- 8.Wellendorph P, et al. Novel radioiodinated γ-hydroxybutyric acid analogues for radiolabeling and Photolinking of high-affinity γ-hydroxybutyric acid binding sites. J Pharmacol Exp Ther. 2010;335:458–464. doi: 10.1124/jpet.110.170670. [DOI] [PubMed] [Google Scholar]
- 9.van Nieuwenhuijzen PS, McGregor IS, Hunt GE. The distribution of γ-hydroxybutyrate-induced Fos expression in rat brain: Comparison with baclofen. Neuroscience. 2009;158:441–455. doi: 10.1016/j.neuroscience.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt C, et al. Anti-sedative and anti-cataleptic properties of NCS-382, a γ-hydroxybutyrate receptor antagonist. Eur J Pharmacol. 1991;203:393–397. doi: 10.1016/0014-2999(91)90896-x. [DOI] [PubMed] [Google Scholar]
- 11.Maitre M, et al. A specific γ-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties. J Pharmacol Exp Ther. 1990;255:657–663. [PubMed] [Google Scholar]
- 12.Colombo G, et al. Blockade of the discriminative stimulus effects of γ-hydroxybutyric acid (GHB) by the GHB receptor antagonist NCS-382. Physiol Behav. 1995;58:587–590. doi: 10.1016/0031-9384(95)00086-x. [DOI] [PubMed] [Google Scholar]
- 13.Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. Intravenous self-administration of gamma-hydroxybutyric acid in drug-naive mice. Eur Neuropsychopharmacol. 1998;8:293–296. doi: 10.1016/s0924-977x(97)00087-4. [DOI] [PubMed] [Google Scholar]
- 14.Carter LP, et al. Novel γ-hydroxybutyric acid (GHB) analogs share some, but not all, of the behavioral effects of GHB and GABAB receptor agonists. J Pharmacol Exp Ther. 2005;313:1314–1323. doi: 10.1124/jpet.104.077578. [DOI] [PubMed] [Google Scholar]
- 15.Koek W, et al. Discriminative stimulus effects of γ-hydroxybutyrate (GHB) in rats discriminating GHB from baclofen and diazepam. J Pharmacol Exp Ther. 2005;314:170–179. doi: 10.1124/jpet.105.083394. [DOI] [PubMed] [Google Scholar]
- 16.Whiting PJ, et al. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- 17.Belelli D, et al. Extrasynaptic GABAA receptors: Form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snead OC, 3rd, Nichols AC. γ-Hydroxybutyric acid binding sites: Evidence for coupling to a chloride anion channel. Neuropharmacology. 1987;26:1519–1523. doi: 10.1016/0028-3908(87)90173-0. [DOI] [PubMed] [Google Scholar]
- 21.Serra M, Sanna E, Foddi C, Concas A, Biggio G. Failure of γ-hydroxybutyrate to alter the function of the GABAA receptor complex in the rat cerebral cortex. Psychopharmacology (Berl) 1991;104:351–355. doi: 10.1007/BF02246035. [DOI] [PubMed] [Google Scholar]
- 22.Xie X, Smart TG. γ-hydroxybutyrate hyperpolarizes hippocampal neurones by activating GABAB receptors. Eur J Pharmacol. 1992;212:291–294. doi: 10.1016/0014-2999(92)90347-7. [DOI] [PubMed] [Google Scholar]
- 23.Dósa Z, Nieto-Gonzalez JL, Korshoej AR, Gibson KM, Jensen K. Effect of gene dosage on single-cell hippocampal electrophysiology in a murine model of SSADH deficiency (γ-hydroxybutyric aciduria) Epilepsy Res. 2010;90:39–46. doi: 10.1016/j.eplepsyres.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Follesa P, et al. γ-hydroxybutyric acid and diazepam antagonize a rapid increase in GABA(A) receptors α(4) subunit mRNA abundance induced by ethanol withdrawal in cerebellar granule cells. Mol Pharmacol. 2003;63:896–907. doi: 10.1124/mol.63.4.896. [DOI] [PubMed] [Google Scholar]
- 25.Nasrallah FA, Maher AD, Hanrahan JR, Balcar VJ, Rae CD. γ-Hydroxybutyrate and the GABAergic footprint: A metabolomic approach to unpicking the actions of GHB. J Neurochem. 2010;115:58–67. doi: 10.1111/j.1471-4159.2010.06901.x. [DOI] [PubMed] [Google Scholar]
- 26.Cope DW, et al. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med. 2009;15:1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Høg S, et al. Novel high-affinity and selective biaromatic 4-substituted γ-hydroxybutyric acid (GHB) analogues as GHB ligands: Design, synthesis, and binding studies. J Med Chem. 2008;51:8088–8095. doi: 10.1021/jm801112u. [DOI] [PubMed] [Google Scholar]
- 28.Sabbatini P, et al. Design, synthesis, and in vitro pharmacology of new radiolabeled γ-hydroxybutyric acid analogues including photolabile analogues with irreversible binding to the high-affinity γ-hydroxybutyric acid binding sites. J Med Chem. 2010;53:6506–6510. doi: 10.1021/jm1006325. [DOI] [PubMed] [Google Scholar]
- 29.Sigel E, Baur R, Kellenberger S, Malherbe P. Point mutations affecting antagonist affinity and agonist dependent gating of GABAA receptor channels. EMBO J. 1992;11:2017–2023. doi: 10.1002/j.1460-2075.1992.tb05258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA(A) receptors. J Neurophysiol. 2011;106:2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suryanarayanan A, et al. Subunit compensation and plasticity of synaptic GABAA receptors induced by ethanol in α4 subunit knockout mice. Front Neurosci. 2011;5:1–7. doi: 10.3389/fnins.2011.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karim N, et al. Low nanomolar GABA effects at extrasynaptic α4β1/β3δ GABA(A) receptor subtypes indicate a different binding mode for GABA at these receptors. Biochem Pharmacol. 2012 doi: 10.1016/j.bcp.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Boileau AJ, Newell JG, Czajkowski C. GABA(A) receptor β 2 Tyr97 and Leu99 line the GABA-binding site. Insights into mechanisms of agonist and antagonist actions. J Biol Chem. 2002;277:2931–2937. doi: 10.1074/jbc.M109334200. [DOI] [PubMed] [Google Scholar]
- 34.Wellendorph P, et al. Novel cyclic γ-hydroxybutyrate (GHB) analogs with high affinity and stereoselectivity of binding to GHB sites in rat brain. J Pharmacol Exp Ther. 2005;315:346–351. doi: 10.1124/jpet.105.090472. [DOI] [PubMed] [Google Scholar]
- 35.Thompson SA, et al. Salicylidene salicylhydrazide, a selective inhibitor of β 1-containing GABAA receptors. Br J Pharmacol. 2004;142:97–106. doi: 10.1038/sj.bjp.0705689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sergeeva OA, et al. Fragrant dioxane derivatives identify β1-subunit-containing GABAA receptors. J Biol Chem. 2010;285:23985–23993. doi: 10.1074/jbc.M110.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gee KW, et al. Limiting activity at β1-subunit-containing GABAA receptor subtypes reduces ataxia. J Pharmacol Exp Ther. 2010;332:1040–1053. doi: 10.1124/jpet.109.161885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanovsky Y, et al. GABAA receptors involved in sleep and anaesthesia: β1- versus β3-containing assemblies. Pflugers Arch. 2012;463:187–199. doi: 10.1007/s00424-011-0988-4. [DOI] [PubMed] [Google Scholar]
- 39.Harrison NL. Mechanisms of sleep induction by GABA(A) receptor agonists. J Clin Psychiatry. 2007;68(Suppl 5):6–12. [PubMed] [Google Scholar]
- 40.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 41.Lovick TA, Griffiths JL, Dunn SMJ, Martin IL. Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131:397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Griffiths J, Lovick T. Withdrawal from progesterone increases expression of α4, β1, and δ GABA(A) receptor subunits in neurons in the periaqueductal gray matter in female Wistar rats. J Comp Neurol. 2005;486:89–97. doi: 10.1002/cne.20540. [DOI] [PubMed] [Google Scholar]
- 43.Lovick TA, Devall AJ. Progesterone withdrawal-evoked plasticity of neural function in the female periaqueductal grey matter. Neural Plast. 2009;2009:730902. doi: 10.1155/2009/730902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen M, Høg S, Wellendorph P, Martiny L. Radioiododestannyllation of novel GHB neuroreceptor ligands. J Labelled Comp Radiopharm. 2010;53:318–320. [Google Scholar]
- 45.Karim N, et al. 3-Hydroxy-2′-methoxy-6-methylflavone: A potent anxiolytic with a unique selectivity profile at GABA(A) receptor subtypes. Biochem Pharmacol. 2011;82:1971–1983. doi: 10.1016/j.bcp.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Ransom RW, Stec NL. Cooperative modulation of [3H]MK-801 binding to the N-methyl-D-aspartate receptor-ion channel complex by L-glutamate, glycine, and polyamines. J Neurochem. 1988;51:830–836. doi: 10.1111/j.1471-4159.1988.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 47.Mihalek RM, et al. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chandra D, et al. GABAA receptor α 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta AK, Muschaweck NM, Maeda DY, Coop A, Ticku MK. Binding characteristics of the γ-hydroxybutyric acid receptor antagonist [(3)H](2E)-(5-hydroxy-5,7,8,9-tetrahydro-6H-benzo[a][7]annulen-6-ylidene) ethanoic acid in the rat brain. J Pharmacol Exp Ther. 2001;299:1148–1153. [PubMed] [Google Scholar]
- 50.Wellendorph P, Høg S, Skonberg C, Bräuner-Osborne H. Phenylacetic acids and the structurally related non-steroidal anti-inflammatory drug diclofenac bind to specific γ-hydroxybutyric acid sites in rat brain. Fundam Clin Pharmacol. 2009;23:207–213. doi: 10.1111/j.1472-8206.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- 51.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- 52.Motulsky H, Neubig R. Analyzing radioligand binding data. In: Crawley J, et al., editors. Current Protocols in Neuroscience. New York: Wiley; 1997. pp. 7.5.1–7.5.56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.