Abstract
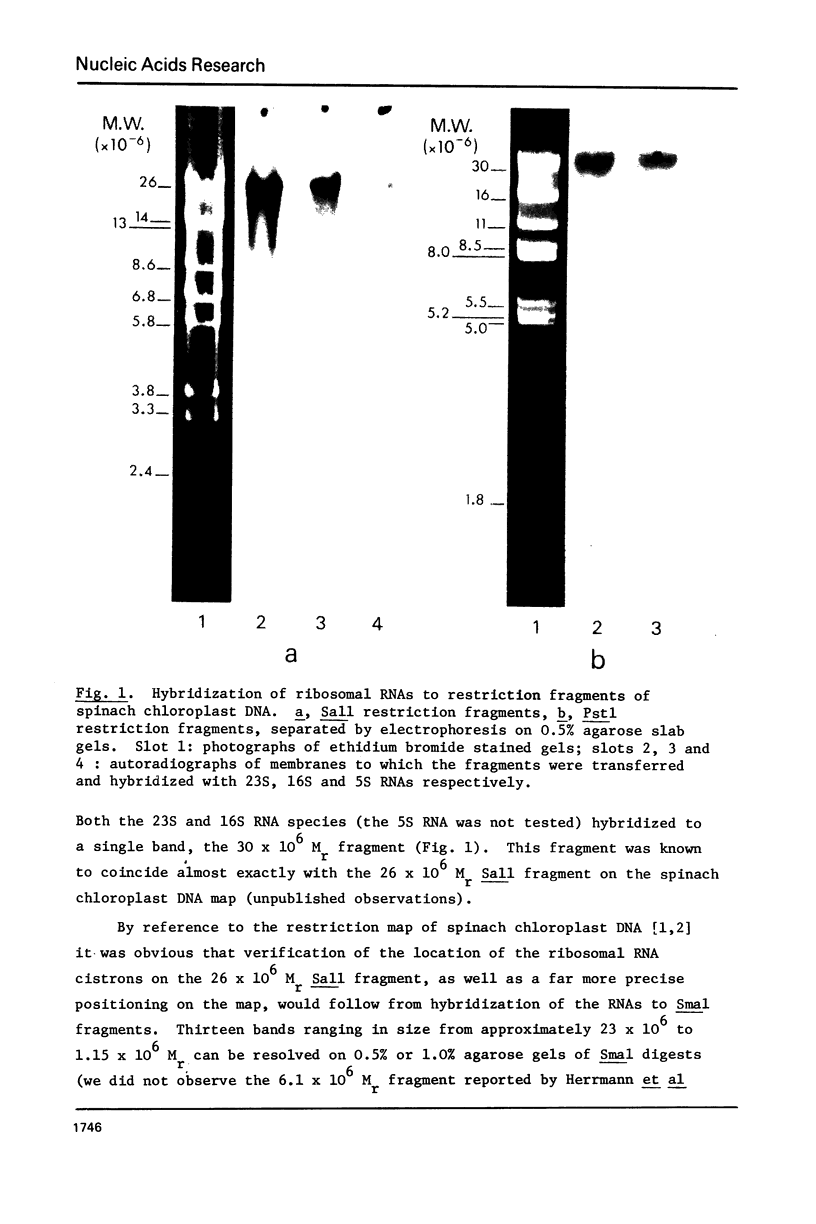
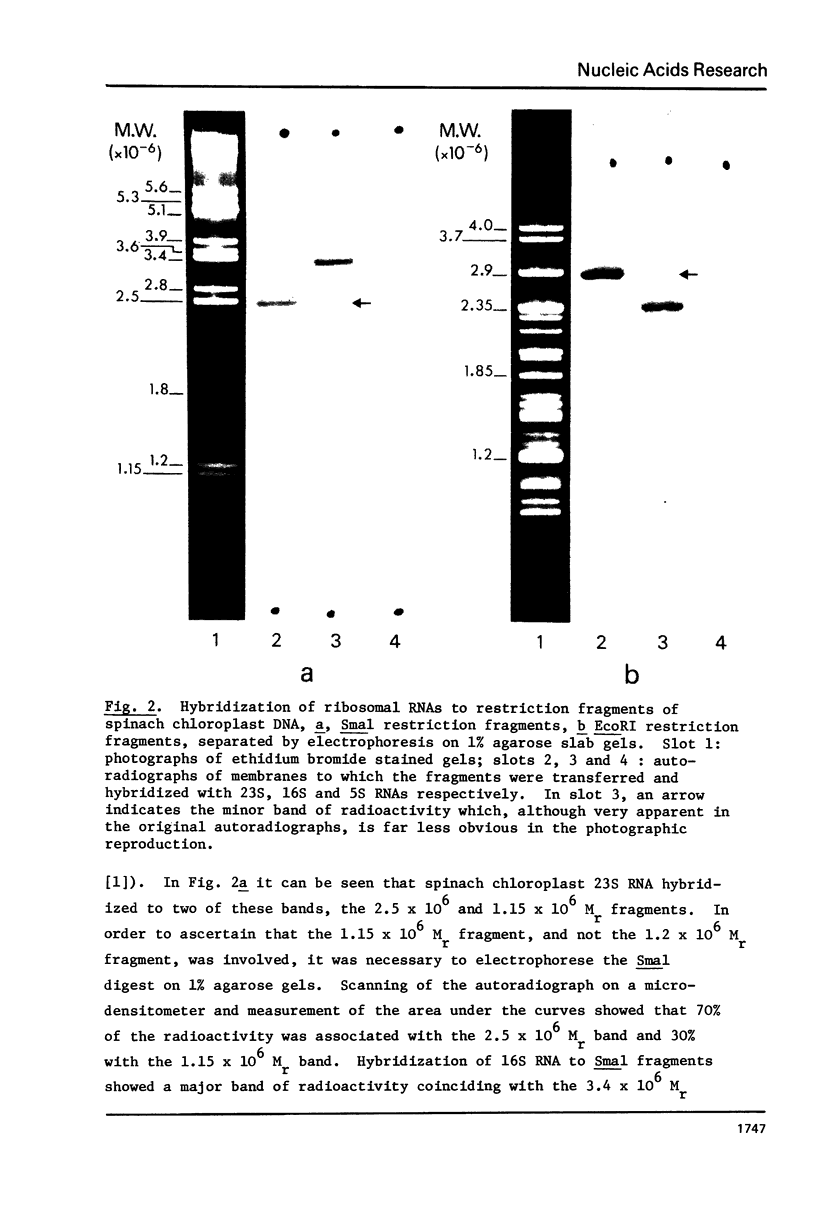
Spinach chloroplast ribosomal RNAs have been hybridized to restriction endonuclease fragments of spinach chloroplast DNA. All three RNA species (23S, 16S and 5S) hybridized to a single large fragment when the DNA was digested with either Sall or Pstl. Hybridization of 23S RNA to fragments produced by Smal yielded two radioactive bands which corresponded to the bi-molar 2.5 X 10(6) and 1.15 X 10(6) Mr fragments. 16S RNA also hybridized to two, bi-molar Smal fragments (3.4 X 10(6) and 2.5 X 10(6) Mr) and 5S RNA hybridized to the 1.15 X 10(6) Mr bi-molar Smal fragment. The 23S RNA and 16S RNA cistrons were each also shown to contain a single EcoRI site. From the data it was possible to conclude that the ribosomal RNA genes are located on the inverted repeat region of the spinach chloroplast DNA restriction map [1,2], that the sequence of the cistrons is 16S - 23S - 5S and that the size of the spacer between the 16S and 23S RNA cistrons is approximately 0.90 X 10(6) Mr.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedbrook J. R., Bogorad L. Endonuclease recognition sites mapped on Zea mays chloroplast DNA. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4309–4313. doi: 10.1073/pnas.73.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedbrook J. R., Kolodner R., Bogorad L. Zea mays chloroplast ribosomal RNA genes are part of a 22,000 base pair inverted repeat. Cell. 1977 Aug;11(4):739–749. doi: 10.1016/0092-8674(77)90288-4. [DOI] [PubMed] [Google Scholar]
- Expert-Bezançon A., Guérin M. F., Hayes D. H., Legault L., Thibault J. Preparation of E. coli ribosomal subunits without loss of biological activity. Biochimie. 1974;56(1):77–89. doi: 10.1016/s0300-9084(74)80357-3. [DOI] [PubMed] [Google Scholar]
- Getz M. J., Altenburg L. C., Saunders G. F. The use of RNA labeled in vitro with iodine-125 in molecular hybridization experiments. Biochim Biophys Acta. 1972 Dec 22;287(3):485–494. doi: 10.1016/0005-2787(72)90293-6. [DOI] [PubMed] [Google Scholar]
- Herrmann R. G., Bohnert H. J., Kowallik K. V., Schmitt J. M. Size, conformation and purity of chloroplast DNA of some higher plants. Biochim Biophys Acta. 1975 Jan 20;378(2):305–317. doi: 10.1016/0005-2787(75)90118-5. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Model P., Prensky W. Application of fingerprinting techniques to iodinated nucleic acids. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3260–3264. doi: 10.1073/pnas.70.11.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L., Gualerzi C. Non-random differences in the base composition of chloroplas and cytoplasmic ribosomal RNA from some higher plants. Life Sci II. 1970 Dec 22;9(24):1401–1407. doi: 10.1016/0024-3205(70)90100-1. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Gross K., Telford J., Birnstiel M. Molecular analysis of the histone gene cluster of psammechinus miliaris: II. The arrangement of the five histone-coding and spacer sequences. Cell. 1976 Aug;8(4):471–478. doi: 10.1016/0092-8674(76)90214-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spencer D., Whitfeld P. R. Ribonucleic acid synthesizing activity of spinach chloroplasts and nuclei. Arch Biochem Biophys. 1967 Aug;121(2):336–345. doi: 10.1016/0003-9861(67)90085-9. [DOI] [PubMed] [Google Scholar]
- Thomas J. R., Tewari K. K. Conservation of 70S ribosomal RNA genes in the chloroplast DNAs of higher plants. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3147–3151. doi: 10.1073/pnas.71.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]