Abstract
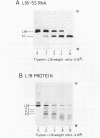
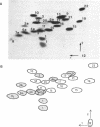
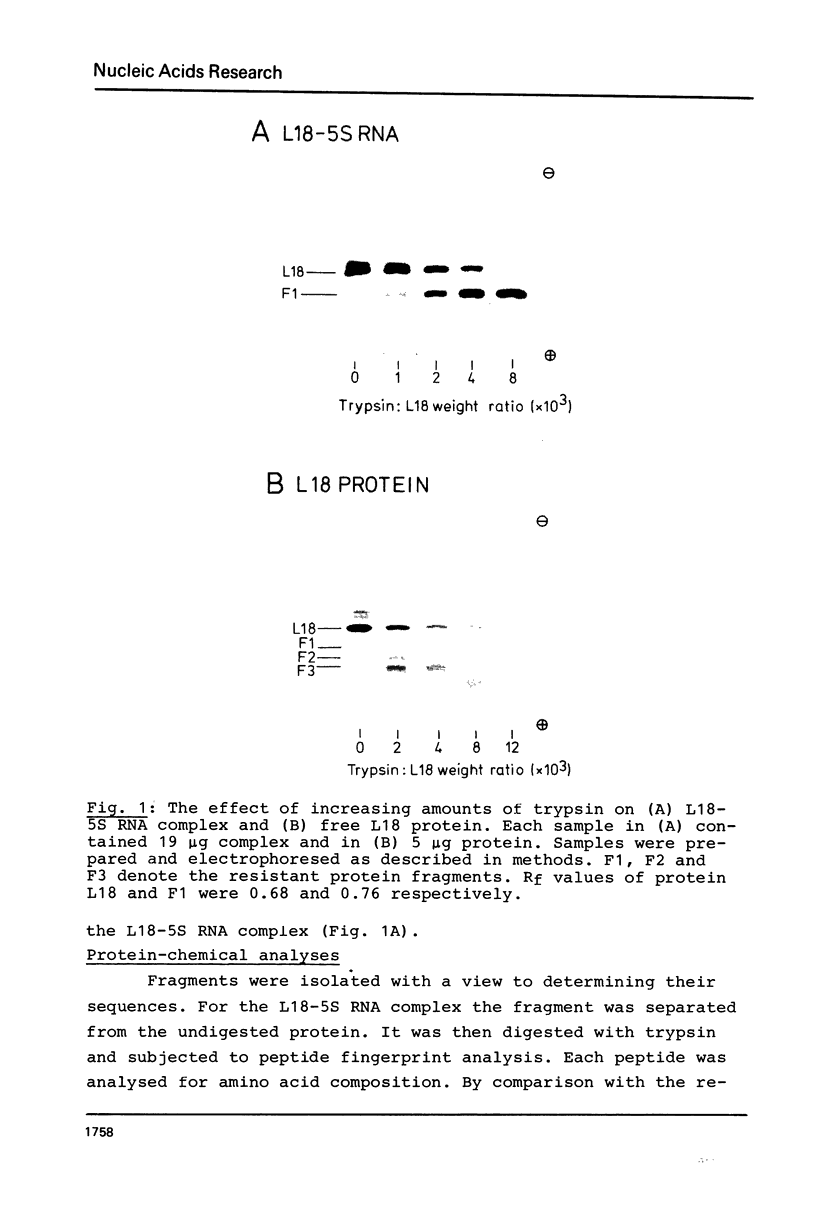
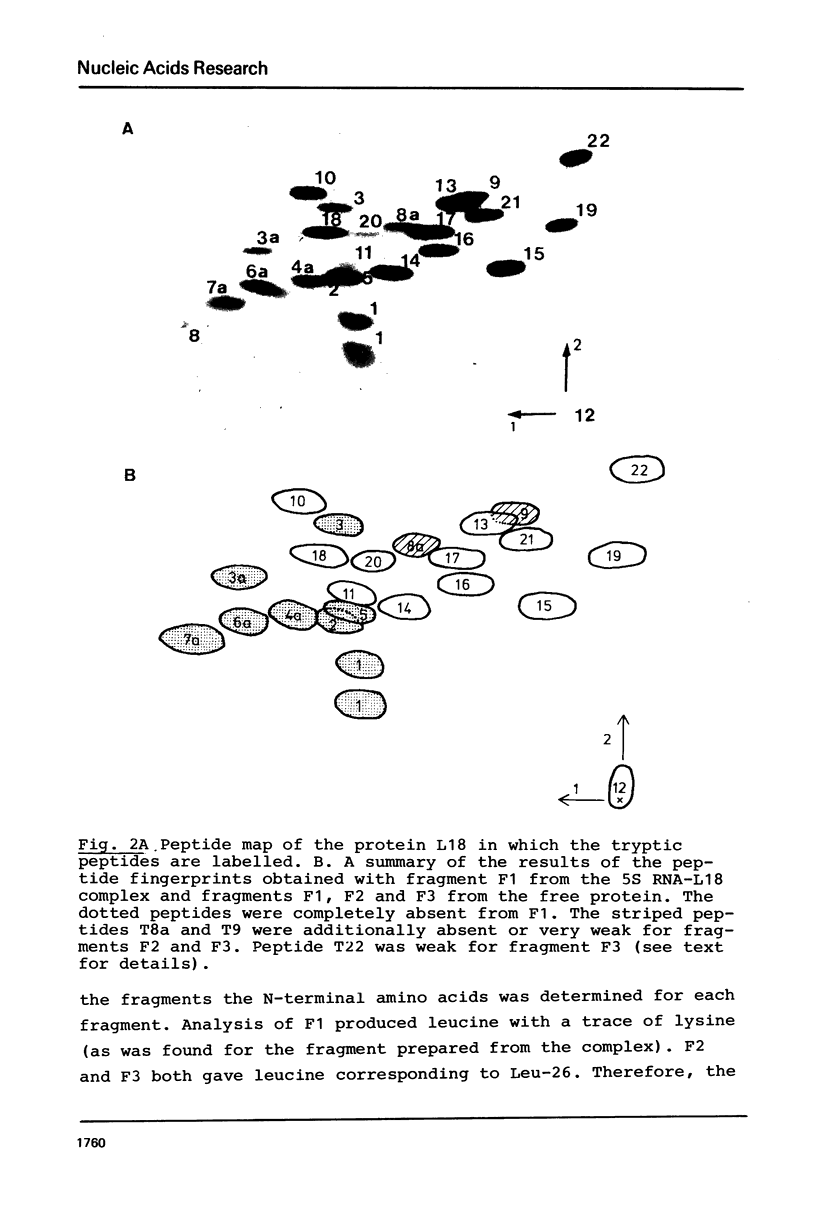
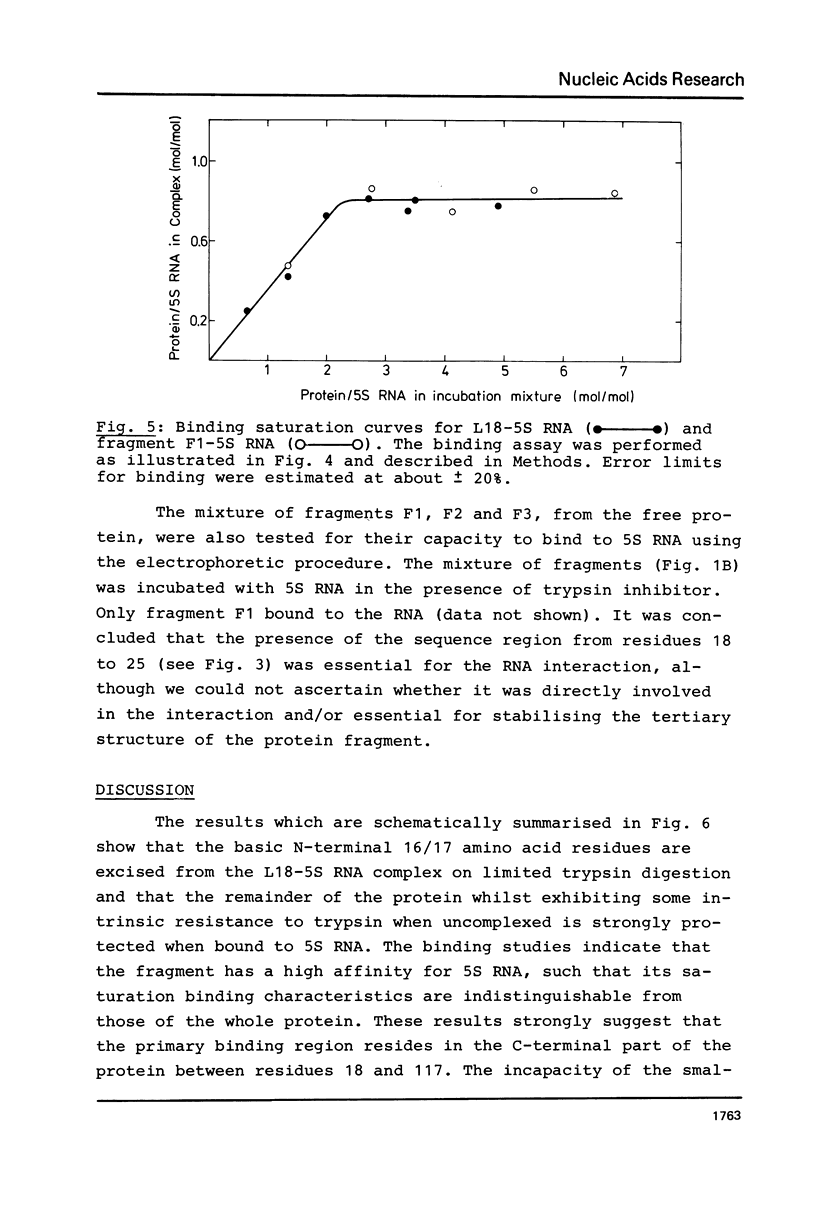
A fragment of ribosomal protein L18 was prepared by limited trypsin digestion of a specific complex of L18 and 5S RNA. It was characterised for sequence and the very basic N-terminal region of the protein was found to be absent. No smaller resistant fragments were produced. 5S RNA binding experiments indicated that the basic N-terminal region, from amino acid residues 1 to 17, was not important for the L18-5S RNA association. Under milder trypsin digestion conditions three resistant fragments were produced from the free protein. The largest corresponded to that isolated from the complex. The smaller ones were trimmed slightly further at both N- and C-terminal ends. These smaller fragments did not reassociate with 5S RNA. It was concluded on the basis of the trypsin protection observations and the 5S RNA binding results that the region extending from residues 18 to 117 approximates to the minimum amount of protein required for a specific and stable protein-RNA interaction. The accessibility of the very basic N-terminal region of L18, in the L18-5S RNA complex, suggests that it may be involved, in some way, in the interaction of 5S RNA with 23S RNA.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear D. G., Schleich T., Noller H. F., Garrett R. A. Alteration of 5S RNA conformation by ribosomal proteins L18 and L25. Nucleic Acids Res. 1977 Jul;4(7):2511–2526. doi: 10.1093/nar/4.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J. Primary structure of Escherichia coli ribosomal protein L31. Biochemistry. 1978 Feb 7;17(3):501–508. doi: 10.1021/bi00596a020. [DOI] [PubMed] [Google Scholar]
- Brosius J., Schiltz E., Chen R. The primary structure of the 5S RNA binding protein L18 from Escherichia coli ribosomes. FEBS Lett. 1975 Aug 15;56(2):359–361. doi: 10.1016/0014-5793(75)81127-6. [DOI] [PubMed] [Google Scholar]
- Changchien L. M., Craven G. R. The function of the N-terminal region of ribosomal protein S4. J Mol Biol. 1976 Dec;108(2):381–401. doi: 10.1016/s0022-2836(76)80126-x. [DOI] [PubMed] [Google Scholar]
- Chen-Schmeisser U., Garrett R. A. A new method for the isolation of a 5 S RNA complex with proteins L5, L18 and L25 from Escherichia coli ribosomes. FEBS Lett. 1977 Mar 1;74(2):287–291. doi: 10.1016/0014-5793(77)80866-1. [DOI] [PubMed] [Google Scholar]
- Fox J. W., Wong K. P. Changes in the conformation and stability of 5 S RNA upon the binding of ribosomal proteins. J Biol Chem. 1978 Jan 10;253(1):18–20. [PubMed] [Google Scholar]
- Fuenteun J., Monier R., Garrett R., Le Bret M., Le Pecq J. B. Effect of 50 S subunit proteins L5, L18 and L25 on the fluorescence of 5 S RNA-bound ethidium bromide. J Mol Biol. 1975 Apr 25;93(4):535–541. doi: 10.1016/0022-2836(75)90245-4. [DOI] [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- Garrett R. A., Rak K. H., Daya L., Stöffler G. Ribosomal proteins. XXIX. Specific protein binding sites on 16S rRNA of Escherichia coli. Mol Gen Genet. 1972;114(2):112–124. doi: 10.1007/BF00332782. [DOI] [PubMed] [Google Scholar]
- Garrett R. A., Ungewickell E., Newberry V., Hunter J., Wagner R. An RNA core in the 30S ribosomal subunit of Escherichia coli and its structural and functional significance. Cell Biol Int Rep. 1977 Nov;1(6):487–502. doi: 10.1016/0309-1651(77)90086-8. [DOI] [PubMed] [Google Scholar]
- Gray P. N., Bellemare G., Monier R., Garrett R. A., Stöffler G. Identification of the nucleotide sequences involved in the interaction between Escherichia coli 5 RNA and specific 50 S subunit proteins. J Mol Biol. 1973 Jun 15;77(1):133–152. doi: 10.1016/0022-2836(73)90367-7. [DOI] [PubMed] [Google Scholar]
- Gray P. N., Garrett R. A., Stoffler G., Monier R. An attempt at the identification of the proteins involved in the incorporation of 5-S RNA during 50-S ribosomal subunit assembly. Eur J Biochem. 1972 Jul 24;28(3):412–421. doi: 10.1111/j.1432-1033.1972.tb01927.x. [DOI] [PubMed] [Google Scholar]
- Gray P. N., Monier R. Partial localization of the 5S RNA binding site on 23S RNA. Biochimie. 1972;54(1):41–45. doi: 10.1016/s0300-9084(72)80036-1. [DOI] [PubMed] [Google Scholar]
- Hardy S. J. The stoichiometry of the ribosomal proteins of Escherichia coli. Mol Gen Genet. 1975 Oct 3;140(3):253–274. doi: 10.1007/BF00334270. [DOI] [PubMed] [Google Scholar]
- Herr W., Noller H. F. A fragment of 23S RNA containing a nucleotide sequence complementary to a region of 5S RNA. FEBS Lett. 1975 May 1;53(2):248–252. doi: 10.1016/0014-5793(75)80030-5. [DOI] [PubMed] [Google Scholar]
- Hindennach I., Kaltschmidt E., Wittmann H. G. Ribosomal proteins. Isolation of proteins from 50S ribosomal subunits of Escherichia coli. Eur J Biochem. 1971 Nov 11;23(1):12–16. doi: 10.1111/j.1432-1033.1971.tb01585.x. [DOI] [PubMed] [Google Scholar]
- Horne J. R., Erdmann V. A. Isolation and characterization of 5S RNA-protein complexes from Bacillus stearothermophilus and Escherichia coli ribosomes. Mol Gen Genet. 1972;119(4):337–344. doi: 10.1007/BF00272091. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morrison C. A., Bradbury E. M., Garrett R. A. A comparison of the structures of several acid-urea extracted ribosomal proteins from Escherichia coli using proton NMR. FEBS Lett. 1977 Sep 15;81(2):435–439. doi: 10.1016/0014-5793(77)80572-3. [DOI] [PubMed] [Google Scholar]
- Newberry V., Yaguchi M., Garrett R. A. A trypsin-resistant fragment from complexes of ribosomal protein S4 with 16-S RNA of Escherichia coli and from the uncomplexed protein. Eur J Biochem. 1977 Jun 1;76(1):51–61. doi: 10.1111/j.1432-1033.1977.tb11569.x. [DOI] [PubMed] [Google Scholar]
- Osterberg R., Garrett R. A. Small-angle X-ray titration study on the complex formation between 5-S RNA and the L18 protein of the Escherichia coli 50-S ribosome particle. Eur J Biochem. 1977 Sep 15;79(1):56–72. doi: 10.1111/j.1432-1033.1977.tb11784.x. [DOI] [PubMed] [Google Scholar]
- Osterberg R., Sjöberg B. Small-angle x-ray scattering study of the 5S RNA binding proteins L18 and L25 from Escherichia coli ribosomes. FEBS Lett. 1976 May 15;65(1):73–76. doi: 10.1016/0014-5793(76)80624-2. [DOI] [PubMed] [Google Scholar]
- Schulte C., Schiltz E., Garrent R. The characterisation of a fragment of ribosomal protein S4 that is protected against trypsin digestion by 16S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1975 Jun;2(6):931–941. doi: 10.1093/nar/2.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Weber H. J. Stoichiometric measurements of 30S and 50S ribosomal proteins from Escherichia coli. Mol Gen Genet. 1972;119(3):233–248. doi: 10.1007/BF00333861. [DOI] [PubMed] [Google Scholar]
- Yu R. S., Wittmann H. G. The sequence of steps in the attachment of 5-S RNA to cores of Escherichia coli ribosomes. Biochim Biophys Acta. 1973 Oct 26;324(3):375–385. doi: 10.1016/0005-2787(73)90282-7. [DOI] [PubMed] [Google Scholar]