Alpha1-Antitrypsin (AAT) is a major circulating inhibitor of neutrophil proteases, specifically elastase that has broad substrate specificity and can degrade many connective tissue matrix constituents. Severe inherited Z (Glu342Lys) deficiency of AAT (AATD) is characterized by a low serum AAT level (less than 20% of normal which is 11μM/L), the protease/antiprotease balance shift in favor of the elastase and an increased risk of early onset pulmonary emphysema. This lends support to the protease-antiprotease imbalance theory of the pathogenesis of emphysema and to AAT augmentation therapy.
Apart from the anti-protease activity, AAT is also a broad regulator of neutrophil functions, such as chemotaxis, adhesion, and release of free radicals and cytokines. In this way, AAT is an important controller of neutrophilic inflammation, particularly in the lungs 1. Recent discoveries revealed that stimulated neutrophils can produce Neutrophil Extracellular Traps (NETs) composed of chromatin with attached granular proteins (like neutrophil elastase). NETs can capture and kill gram-positive and gram-negative bacteria, and fungi 2. However, in a chronic setting NETs might also propagate unwanted inflammatory reactions 3.
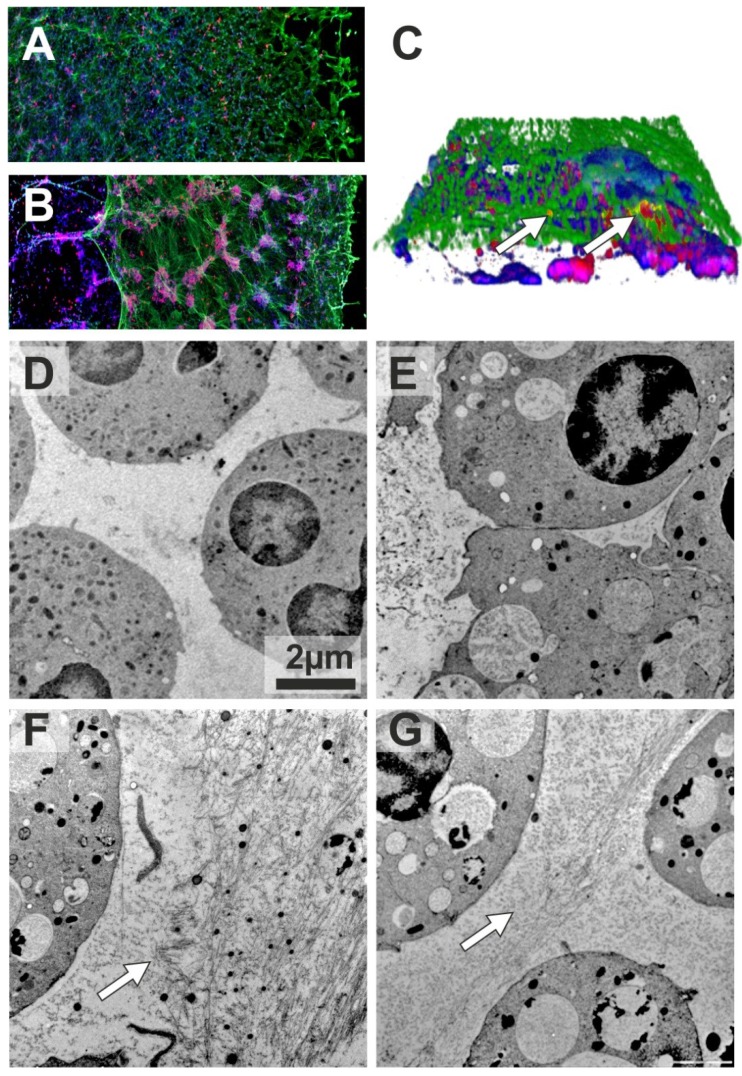
As mentioned above, in addition to anti-elastase activity, AAT can also suppress the generation of reactive oxygen species 4 required for NET formation. However, no studies have been carried out so far to assess the direct impact of AAT on NETs. To address this question we employed an ex-vivo model in which we used phorbol myristate acetate (PMA), a well characterized inducer of NETosis. We obtained blood neutrophils from ZZ AATD-related emphysema patient before and directly after augmentation therapy, and incubated with PMA for 4 hours in vitro. As judged by confocal microscopy, prior to augmentation therapy, PMA-induced NETs co-immunostained for AAT and elastase formed typical “smooth” stretches decorated with elastase (Figure 1A). Remarkably, after therapy, PMA-induced NETs contained large neutrophil aggregates, and were mostly detached from the coverslip (Figure 1B). Neutrophil aggregates were also present in the NETs formed 3 and 5 days after augmentation therapy, although they gradually became smaller. Closer investigation of the NETs revealed that elastase and AAT are only partially co-localized (Figure 1C).
Figure 1.
Confocal (A-C) and Transmission electron (D-G) microscopy of NETs. Blood neutrophils were isolated from 72-years-old woman with ZZ AATD-related COPD having 0.18-0.22 mg/ml serum levels of AAT. When augmentation therapy was initiated (60 mg/kg body weight once weekly), she was in a clinically stable condition, and 2 h after therapy her plasma levels of AAT were 1.56 mg/ml. Neutrophils (1x107/ml) were treated with PMA (20nM) or AAT (1mg/ml) separately or in a combination for 4 h at 37°C. After, specimens were fixed with 3 % formaldehyde and permeabilized with 0.5 % Triton X-100. AAT and NE were labelled using polyclonal rabbit anti-human AAT and monoclonal mouse anti-human elastase Clone NP57 (Dako, Denmark) antibodies. Applied secondary antibodies were conjugated with AlexaFluor 546 (Anti rabbit; MoBiTec GmbH, Göttingen, Germany) or AlexaFluor 488 (Anti mouse; Invitrogen Life Technologies, CA, USA). DNA was stained using 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) (Sigma-Aldrich, St. Louis, USA). Confocal images were obtained with an Olympus FluoView FV1000 laser scanning microscope using 20× objective lens. 3D stacks were acquired using 60× objective lens and reconstructed in Imaris software (Bitplane). NETs were formed by neutrophils isolated the same day before (A) and after (B) augmentation therapy. Immunolabeling for AAT (red), elastase (green) and DNA (blue). (C) 3D reconstruction of AAT/elastase co-localization in the NETs. White arrows indicate co-localized proteins (yellow). Neutrophils were isolated from a 41-years-old woman with ZZ AATD-related COPD (serum concentrations of AAT 0.47-0.67 mg/ml) under regular augmentation therapy since 6 month (D-E) and from healthy MM AAT donor (F-G). After treatment with the respective conditions cells were directly high-pressure-frozen using a Leica HPM100. Freeze substitution was carried out in a Leica AFS2 at -90°C in 0.1% tannic acid overnight and another 2 h in 2 % OsO4, each in dry acetone. Temperature was raised slowly to 4 °C, samples were washed and embedded in EPON. 50 nm ultrathin sections were post-stained with 4 % Uranyl acetate. Images were recorded in a Morgagni 268 TEM (FEI), operated at 80 kV, using a Veleta CCD 2K camera. Arrows indicate fibril-like structures. Scale bars are 2 μm.
In a second experiment we isolated blood neutrophils from healthy MM donor and augmented ZZ AATD patient, and investigated PMA-induced NET formation in solution by using transmission electron microscopy. Using this approach, we found that PMA-stimulated ZZ neutrophils from augmented patient form infrequent and short fibres-like stretches (Figure 1D and E). In a similar case the MM neutrophils from healthy control, formed large amount of long and densely packed fibres (Figure 1F). It is noteworthy, when in vitro MM neutrophils were treated with PMA and AAT simultaneously; the pattern of fibres became very similar to that in ZZ neutrophils from augmented patient (Figure 1G).
Taken together, our ex-vivo findings provide new evidence that AAT does not inhibit PMA-induced NETs formation, but induces remarkable NET shape changes. Although highly speculative, positive effects of AAT augmentation therapy on neutrophilic inflammation and frequencies of exacerbations 5 might be related to AAT effect on NETs formation, length, adhesion and subsequent clearance in vivo. This hypothesis encourages further studies.
References
- 1.Stockley RA. Neutrophils and the pathogenesis of COPD. Chest. 2002;121(5 Suppl):151S–155S. doi: 10.1378/chest.121.5_suppl.151s. [DOI] [PubMed] [Google Scholar]
- 2.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:883. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 3.Marcos V, Zhou Z, Yildirim AO. et al. CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat Med. 2010;16:1018–23. doi: 10.1038/nm.2209. [DOI] [PubMed] [Google Scholar]
- 4.Bucurenci N, Blake DR, Chidwick K. et al. Inhibition of neutrophil superoxide production by human plasma alpha 1-antitrypsin. FEBS Lett. 1992;300:21–4. doi: 10.1016/0014-5793(92)80156-b. [DOI] [PubMed] [Google Scholar]
- 5.Stockley RA, Parr DG, Piitulainen E. et al. Therapeutic efficacy of α-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res. 2010;5:136. doi: 10.1186/1465-9921-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]