Abstract
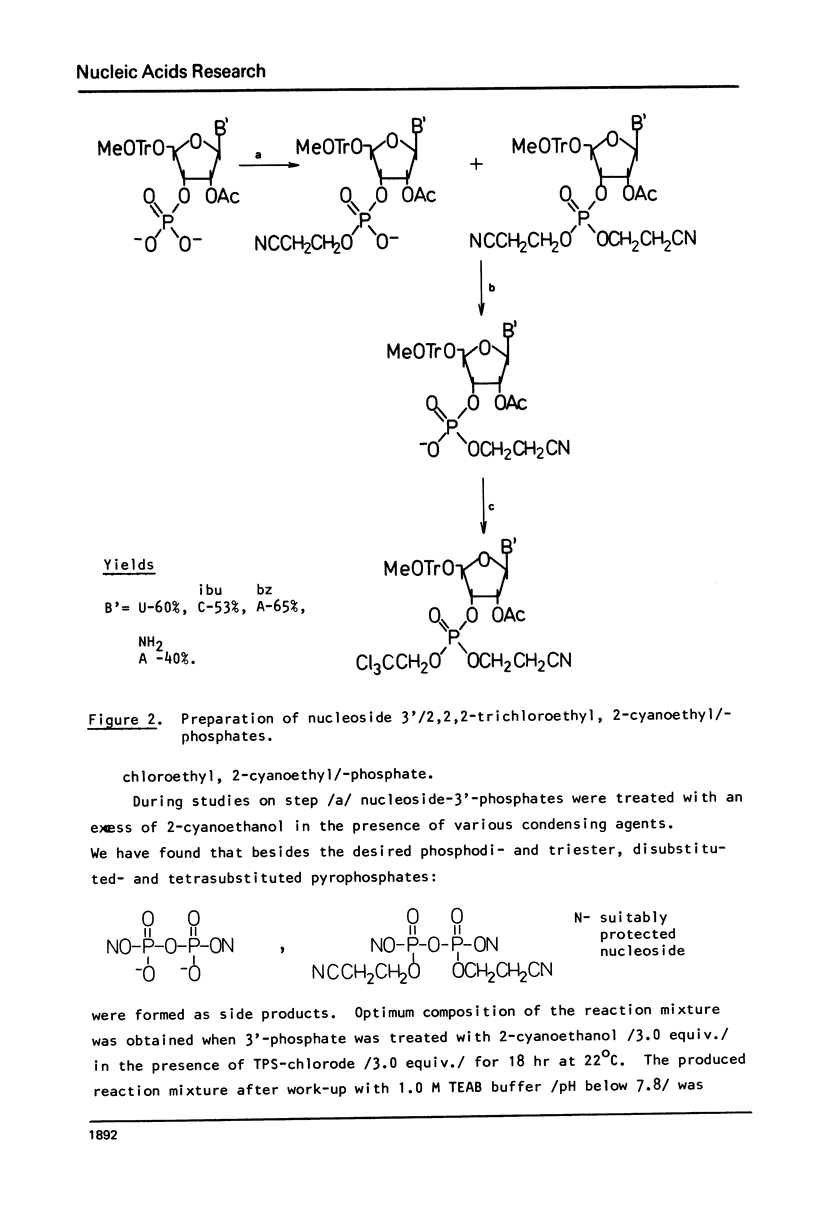
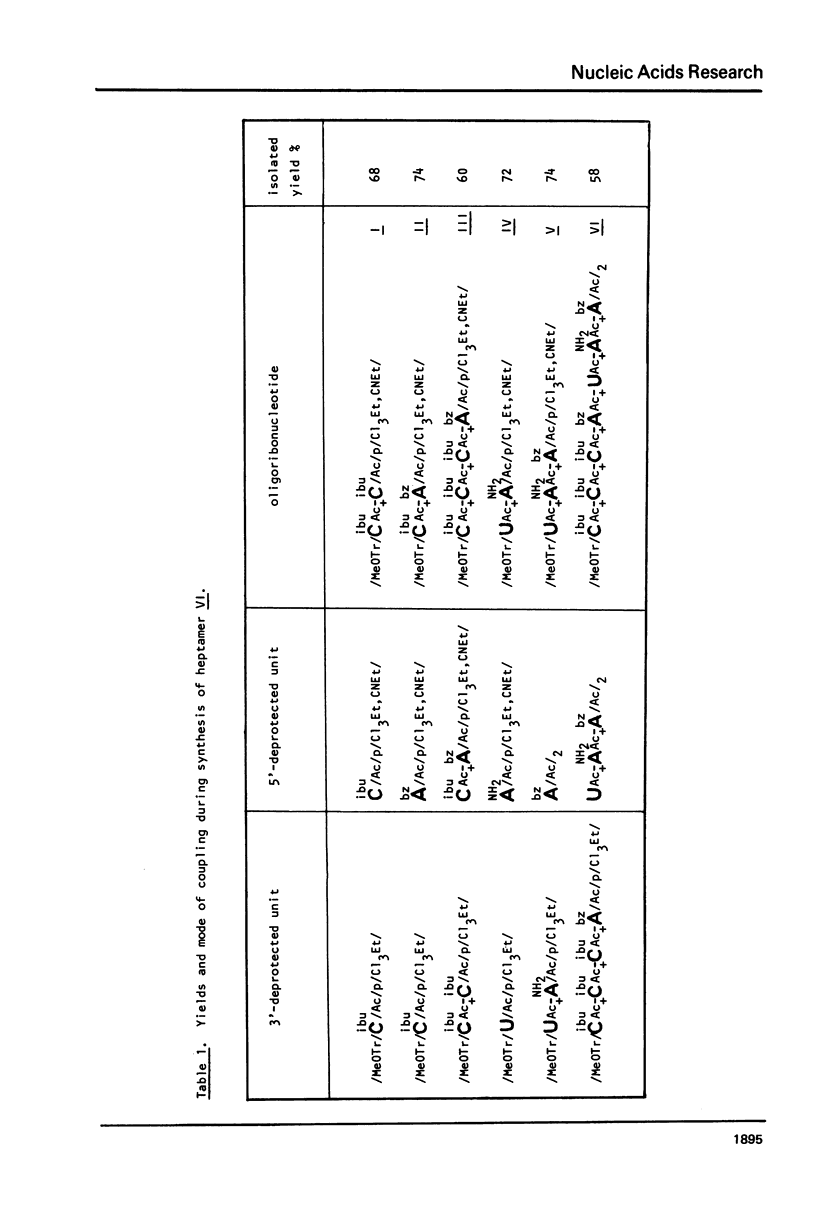
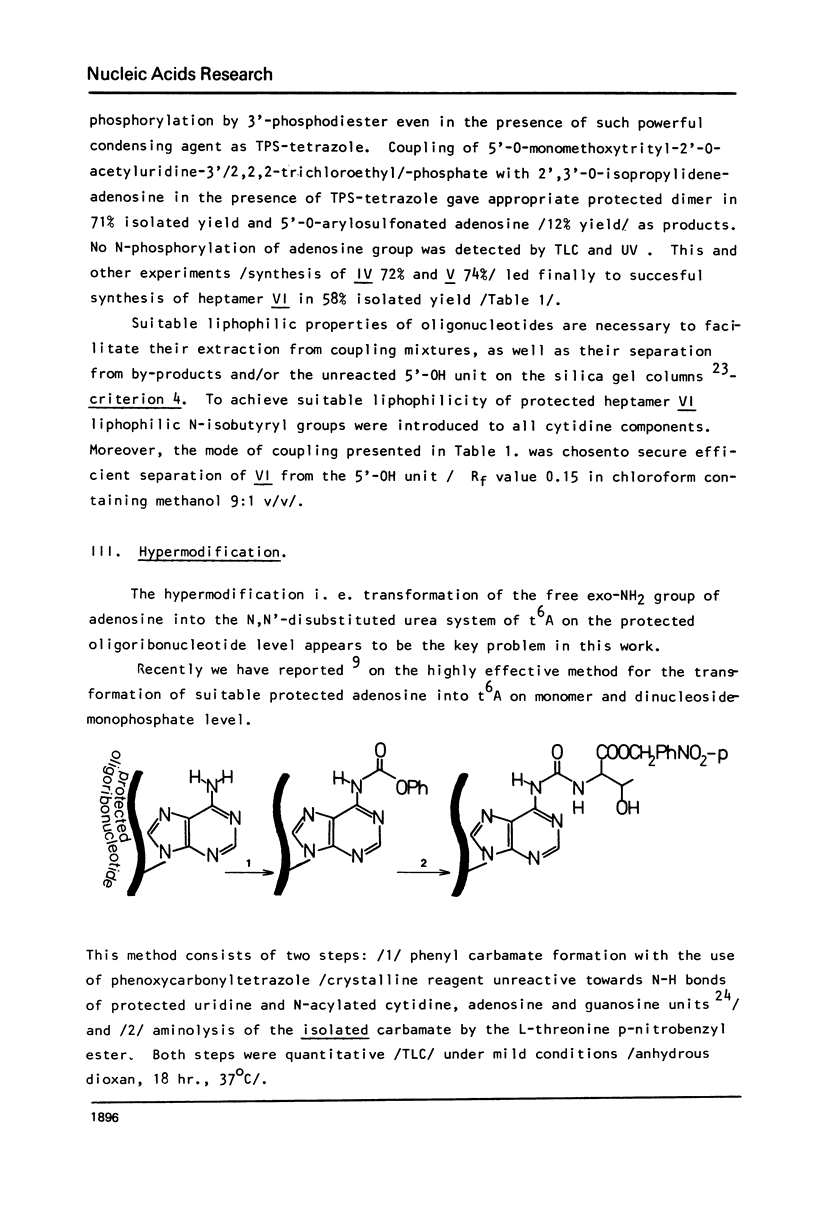
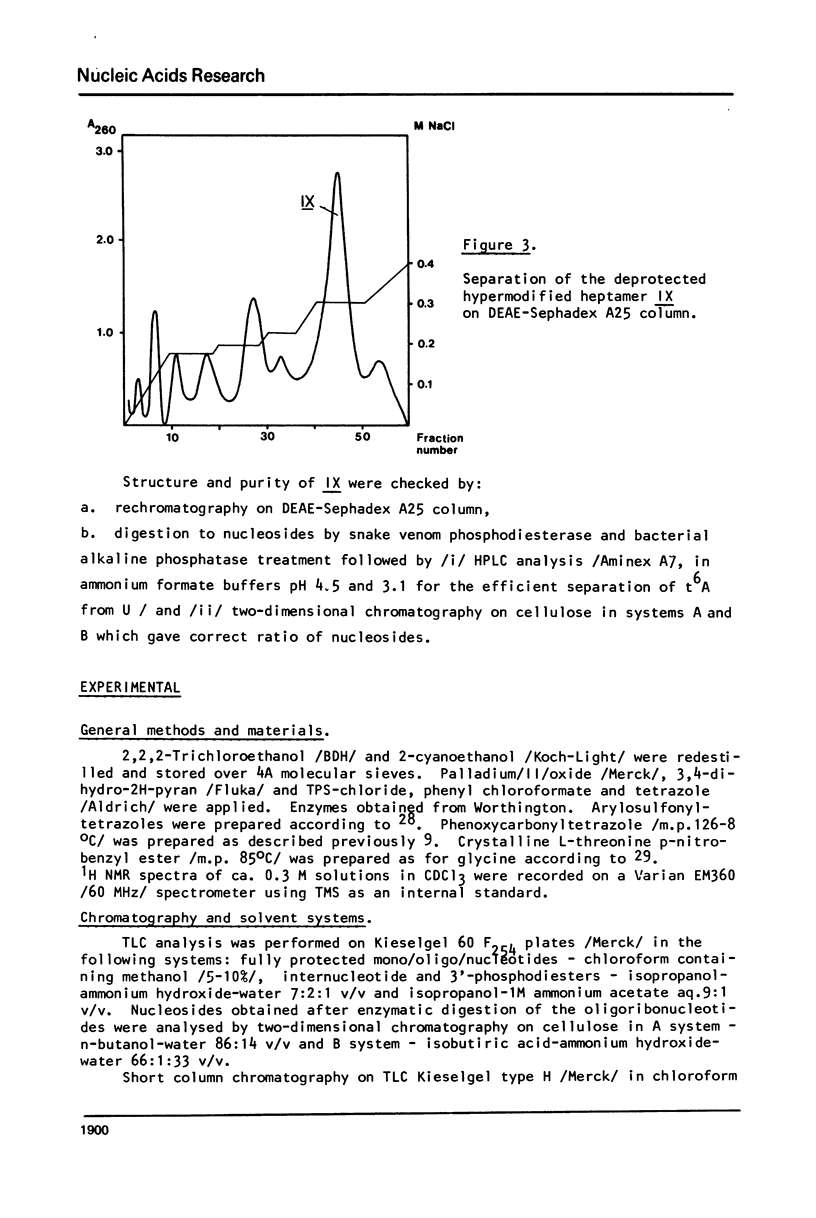
In this work, the first example of chemical synthesis of oligoribonucleotide containing the hypermodified nucleoside N6-/N-threonylcarbonyl/-adenosine /t6A/ is presented. Synthesis of the heptamer C-C-C-A-U-t6A-A IX, the sequence of which is related to the anticodon loop of the initiator tRNA from yellow lupine, was achieved by: /i/ phosphotriester block synthesis of suitably protected heptamer VI containing an adenosine unit with a free exo-NH2 group, /ii/ highly effective "one-flask" procedure for the transformation of the free exo-NH2 group of adenosine unit of heptamer VI into a N,N'-disubstituted urea system of t6A of heptamer VII /hypermodification/, and /iii/ final deprotection of VIII /32% total yield/ with the use of a new approach for simultaneous hydrogenolysis /PdO-hydrogen-pyridine/ of the p-nitrobenzyl group and 2,2,2-trichloroethyl groups from carboxyl function of t6A and internucleotide phosphates respectively.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamiak R. W., Barciszewska M. Z., Biala E., Grzéskowiak K., Kierzek R., Kraszewski A., Markiewicz W. T., Wiewiórowski M. Nucleoside-3'-phosphotriesters as key intermediates for the oligoribonucleotide synthesis. III. An improved preparation of nucleoside 3'-phosphotriesters, their 1H NMR characterization and new conditions for removal of 2-cyanoethyl group. Nucleic Acids Res. 1976 Dec;3(12):3397–3408. doi: 10.1093/nar/3.12.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamiak R. W., Biala E., Grześkowiak, Kierzek R., Kraszewski A., Markiewicz W. T., Stawiński J., Wiewiórowski Nucleoside 3'-phosphotriesters as key intermediates for the oligoribonucleotide synthesis. IV. New method for removal of 2,2,2-trichloroethyl group and 31P NMR as a new tool for analysis of deblocking of internucleotide phosphate protecting groups. Nucleic Acids Res. 1977 Jul;4(7):2321–2329. doi: 10.1093/nar/4.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin J. C., Cramer F. Deoxy oligonucleotide synthesis via the triester method. J Org Chem. 1973 Jan 26;38(2):245–250. doi: 10.1021/jo00942a011. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Jarman M., Reese C. B. The synthesis of oligoribonucleotides. IV. Preparation of dinucleoside phosphates from 2',5'-protected ribonucleoside derivatives. Tetrahedron. 1968 Jan;24(2):639–662. doi: 10.1016/0040-4020(68)88015-9. [DOI] [PubMed] [Google Scholar]
- Letsinger R. L., Lunsford W. B. Synthesis of thymidine oligonucleotides by phosphite triester intermediates. J Am Chem Soc. 1976 Jun 9;98(12):3655–3661. doi: 10.1021/ja00428a045. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Schweizer M. P. Determination of N-(9-( -D-ribofuranosyl)purin-6-ylcarbamoly)threonine at the picomole level in transfer-RNA. Anal Biochem. 1972 Dec;50(2):327–336. doi: 10.1016/0003-2697(72)90041-3. [DOI] [PubMed] [Google Scholar]
- Neilson T., Werstink E. S. Synthesis of the anticodon loop of Escherichia coli methionine transfer ribonucleic acid. J Am Chem Soc. 1974 Apr 3;96(7):2295–2297. doi: 10.1021/ja00814a074. [DOI] [PubMed] [Google Scholar]
- Ogilvie K. K., Theriault N., Sadana K. L. Synthesis of oligoribonucleotides. J Am Chem Soc. 1977 Nov 9;99(23):7741–7743. doi: 10.1021/ja00465a073. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Fujiyama K., Ohashi M., Ikehara M. Studies on transfer ribonucleic acids and related compounds. X. synthesis of the yeast tyrosine tRNA 5'-terminal oligonucleotides. Chem Pharm Bull (Tokyo) 1976 Apr;24(4):570–579. doi: 10.1248/cpb.24.570. [DOI] [PubMed] [Google Scholar]
- Otsuka E., Ubasawa M., Morioka S., Ikehara M. Studies on transfer ribonucleic acids and related compounds. VI. Synthesis of yeast alanine transfer ribonucleic acid 3'-terminal nonanucleotides and 5'-terminal hexanucleotides. J Am Chem Soc. 1973 Jul 11;95(14):4725–4733. doi: 10.1021/ja00795a042. [DOI] [PubMed] [Google Scholar]
- van Boom J. H., Burgers P. M., van der Marel G., Wille G. Synthesis of oligonucleotides with sequences identical with or analogous to the 3'-end of 16S ribosomal RNA of Escherichia coli: preparation of m-6-2-A-C-C-U-C-C and A-C-C-U-C-m-4-2C via phosphotriester intermediates. Nucleic Acids Res. 1977 Mar;4(3):747–759. doi: 10.1093/nar/4.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]



