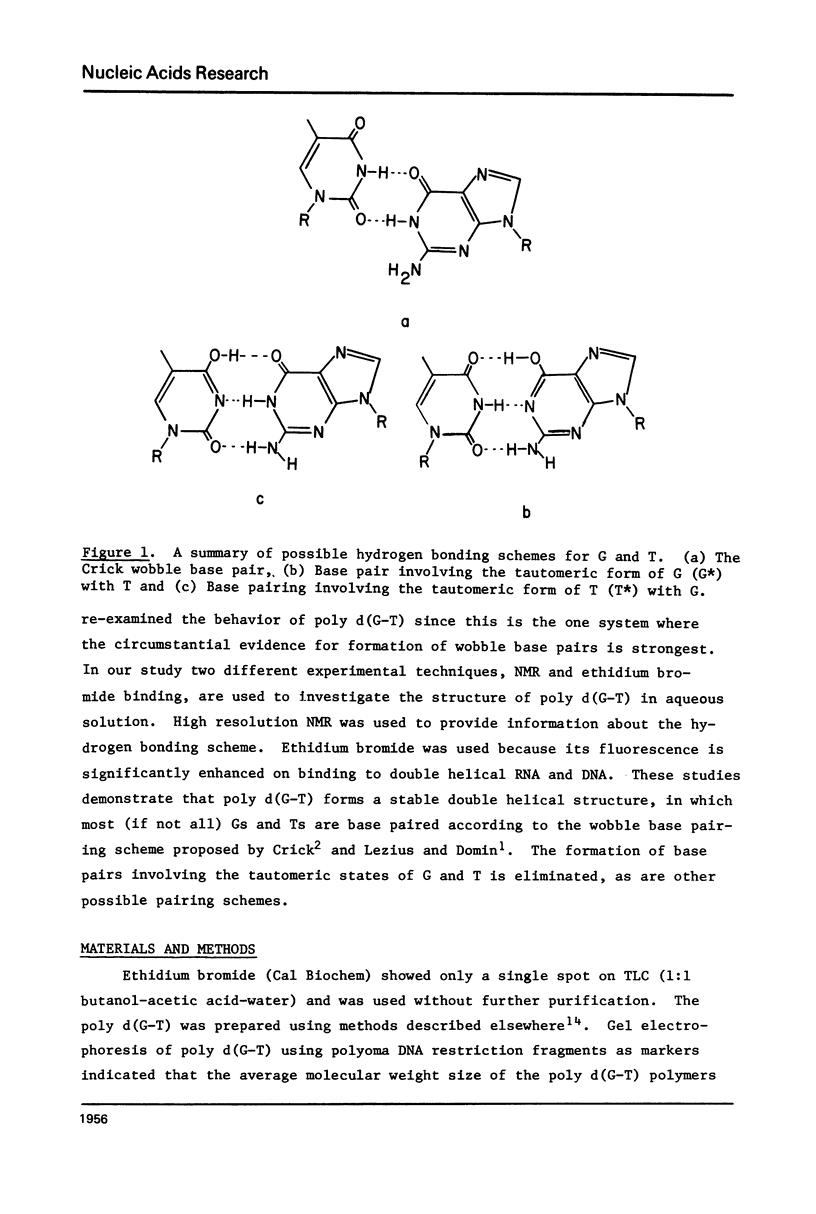
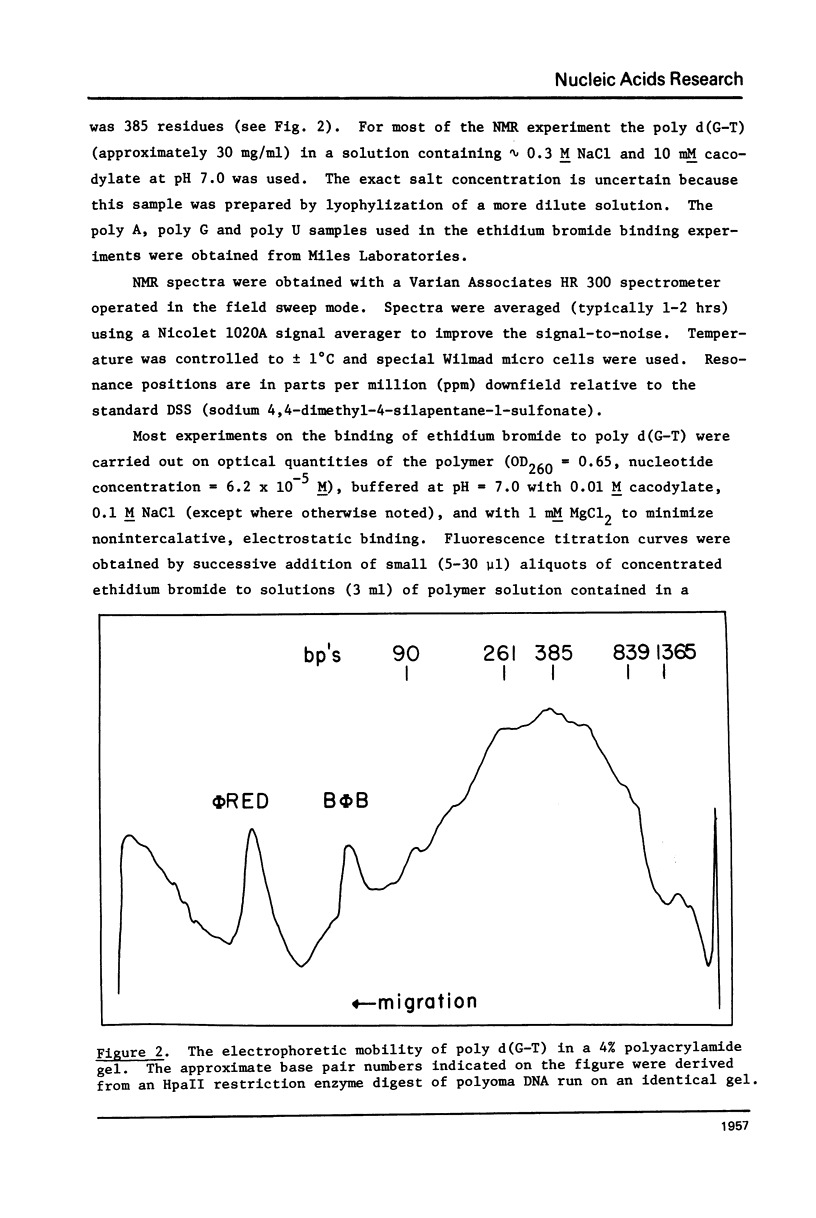
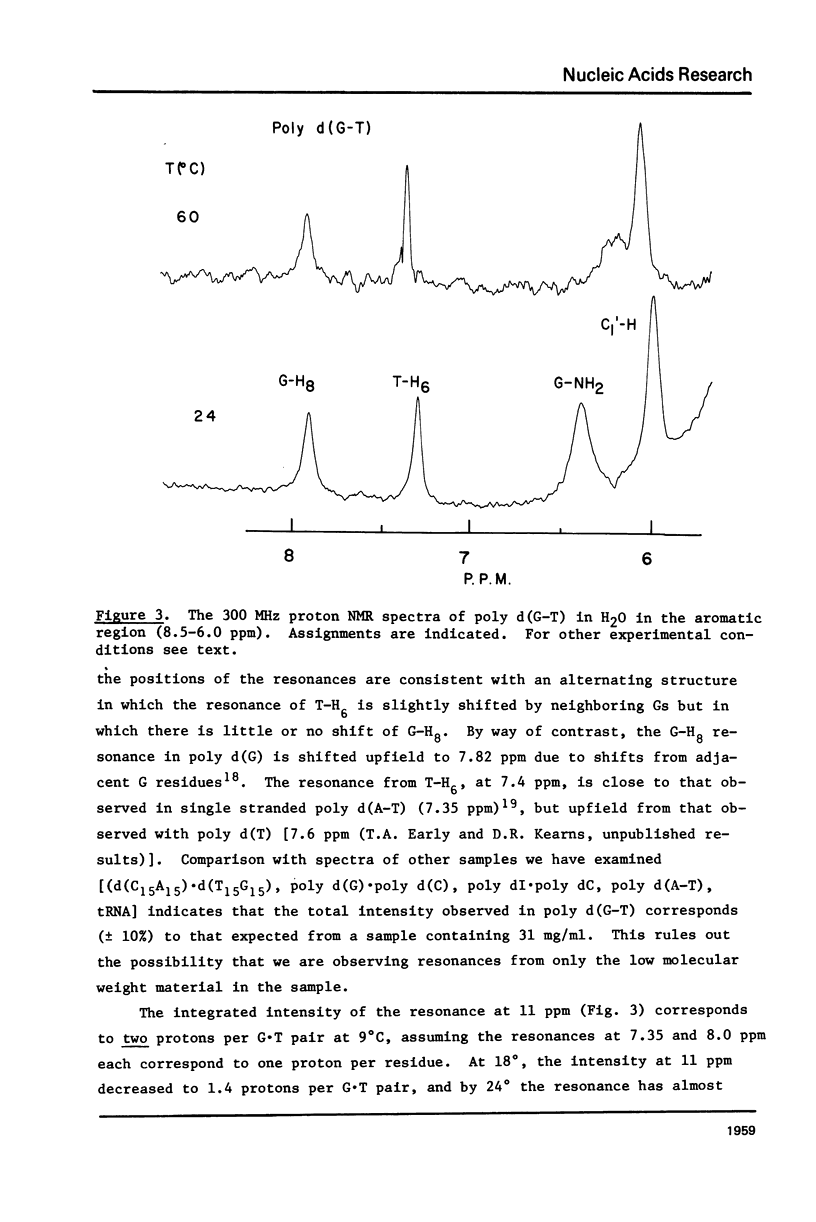
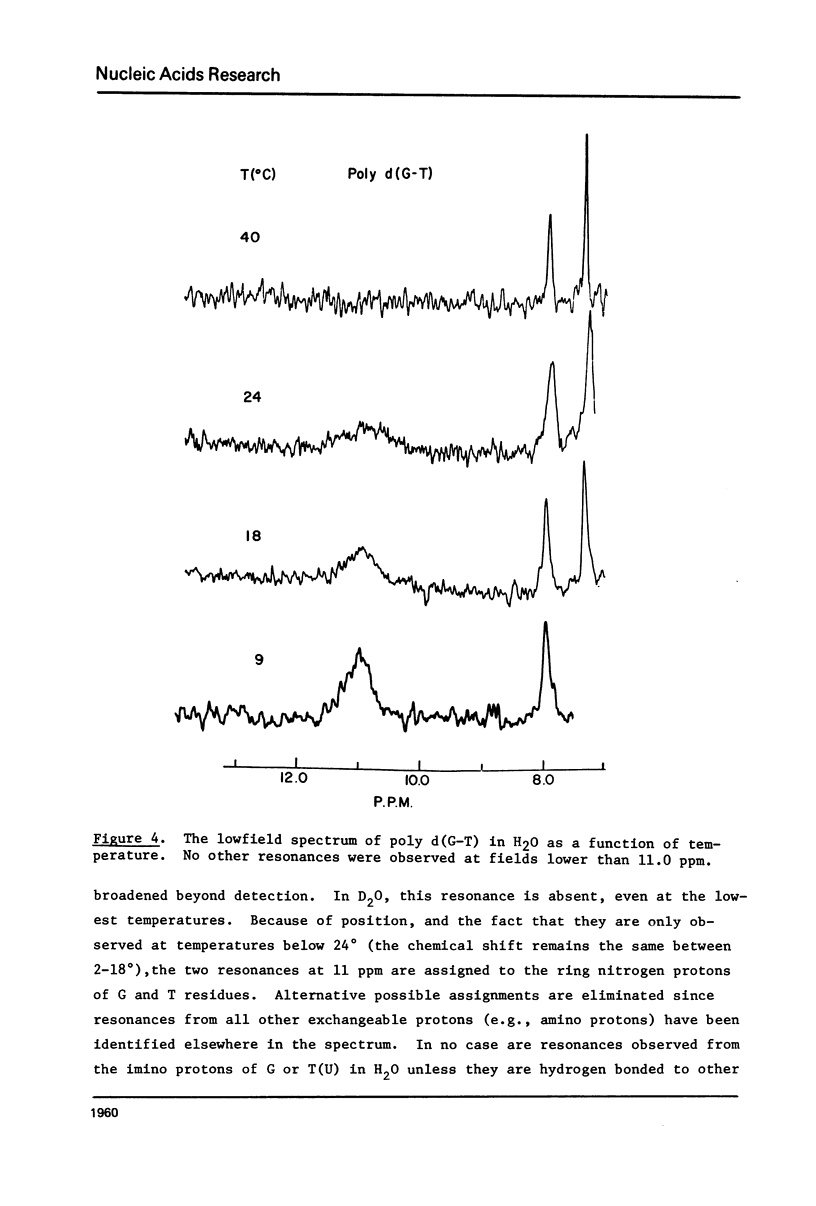
Abstract
High resolution nuclear magnetic resonance (NMR) and ethidium bromide binding studies are used to demonstrate that poly d(G-T) forms an ordered double helical structure at low temperatures (below 24 degrees C in 0.3 M NaCl) in which G and T are hydrogen bonded together in a wobble base pair hydrogen bonding scheme as proposed earlier by Lezius and Domin. Alternative hydrogen bonding schemes involving the tautomeric form of either T or G, such as have been proposed to account for mutation rates in DNA synthesis, are eliminated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktipis S., Kindelis A. Optical properties of the deoxyribonucleic acid-ethidium bromide complex. Effect of salt. Biochemistry. 1973 Mar 13;12(6):1213–1221. doi: 10.1021/bi00730a031. [DOI] [PubMed] [Google Scholar]
- Bittman R. Studies of the binding of ethidium bromide to transfer ribonucleic acid: absorption, fluorescence, ultracentrifugation and kinetic investigations. J Mol Biol. 1969 Dec 14;46(2):251–268. doi: 10.1016/0022-2836(69)90420-3. [DOI] [PubMed] [Google Scholar]
- Chan S. I., Lee G. C., Schmidt C. F., Kreishman G. P. Guanine-uracil base-pairing. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1536–1543. doi: 10.1016/0006-291x(72)90782-6. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Hogness D. S. Separation of the strands of poly d-TG: AC in alkaline CsCl. J Mol Biol. 1965 Nov;14(1):237–240. doi: 10.1016/s0022-2836(65)80243-1. [DOI] [PubMed] [Google Scholar]
- Early T. A., Kearns D. R., Burd J. F., Larson J. E., Wells R. D. High resolution proton nuclear magnetic resonance investigation of the structural and dynamic properties of d(C15A15)-d(T15G15). Biochemistry. 1977 Feb 8;16(3):541–551. doi: 10.1021/bi00622a031. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Ratliff R. L. Circular dichroism evidence for G-U and G-T base pairing in poly[r(G-U)] and poly[d(G-T)]. Biopolymers. 1977 Jun;16(6):1331–1342. doi: 10.1002/bip.1977.360160613. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Tinoco I., Jr, Chamberlin M. J. The circular dichroism of synthetic ribonucleic acids and the influence of uracil on conformation. Biopolymers. 1972;11(6):1235–1258. doi: 10.1002/bip.1972.360110609. [DOI] [PubMed] [Google Scholar]
- Kearns D. R. High-resolution nuclear magnetic resonance investigations of the structure of tRNA in solution. Prog Nucleic Acid Res Mol Biol. 1976;18:91–149. doi: 10.1016/s0079-6603(08)60587-5. [DOI] [PubMed] [Google Scholar]
- Krugh T. R., Young M. A. Nuclear magnetic resonance studies of hydrogen bonded complexes of oligonucleotides in aqueous solution. I. pdG-dC and pdG-dT. Biochem Biophys Res Commun. 1975 Feb 17;62(4):1025–1031. doi: 10.1016/0006-291x(75)90425-8. [DOI] [PubMed] [Google Scholar]
- Ladner J. E., Jack A., Robertus J. D., Brown R. S., Rhodes D., Clark B. F., Klug A. Atomic co-ordinates for yeast phenylalanine tRNA. Nucleic Acids Res. 1975 Sep;2(9):1629–1637. doi: 10.1093/nar/2.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Weber G. Quenching of fluorescence by oxygen. A probe for structural fluctuations in macromolecules. Biochemistry. 1973 Oct 9;12(21):4161–4170. doi: 10.1021/bi00745a020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pecq J. B., Paoletti C. Interaction du bromhydrate d'éthidium (BET) avec les polyribonucléotides. Applications à l'étude des réactions d'hybridation. C R Acad Sci Hebd Seances Acad Sci D. 1965 Jun 28;260(26):7033–7036. [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Lezius A. G., Domin E. A wobbly double helix. Nat New Biol. 1973 Aug 8;244(136):169–170. doi: 10.1038/newbio244169a0. [DOI] [PubMed] [Google Scholar]
- Lomant A. J., Fresco J. R. Structural and energetic consequences of noncomplementary base oppositions in nucleic acid helices. Prog Nucleic Acid Res Mol Biol. 1975;15(0):185–218. doi: 10.1016/s0079-6603(08)60120-8. [DOI] [PubMed] [Google Scholar]
- Olmsted J., 3rd, Kearns D. R. Mechanism of ethidium bromide fluorescence enhancement on binding to nucleic acids. Biochemistry. 1977 Aug 9;16(16):3647–3654. doi: 10.1021/bi00635a022. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. Nuclear magnetic resonance studies of the helix-coil transition of poly (dA-dT) in aqueous solution. Proc Natl Acad Sci U S A. 1976 Mar;73(3):674–678. doi: 10.1073/pnas.73.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J. Proton and phosphorus NMR studies of d-CpG(pCpG)n duplexes in solution. Helix-coil transition and complex formation with actinomycin-D. Biopolymers. 1976 Mar;15(3):533–558. doi: 10.1002/bip.1976.360150310. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley G. J., Seeman N. C., Wang A. H., Suddath F. L., Rich A. Yeast phenylalanine transfer RNA: atomic coordinates and torsion angles. Nucleic Acids Res. 1975 Dec;2(12):2329–2341. doi: 10.1093/nar/2.12.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard G. T., Hilbers C. W., Reid B. R., Gangloff J., Dirheimer G., Shulman R. G. A study of secondary and tertiary solution structure of yeast tRNA(Asp) by nuclear magnetic resonance. Assignment of G.U ring NH and hydrogen-bonded base pair proton resonances. Biochemistry. 1976 May 4;15(9):1883–1888. doi: 10.1021/bi00654a014. [DOI] [PubMed] [Google Scholar]
- Stannard B. S., Felsenfeld G. The conformation of polyriboadenylic acid at low temperature and neutral pH. A single-stranded rodlike structure. Biopolymers. 1975 Feb;14(2):299–307. doi: 10.1002/bip.1975.360140205. [DOI] [PubMed] [Google Scholar]
- Sussman J. L., Kim S. H. Idealized atomic coordinates of yeast phenylalanine transfer RNA. Biochem Biophys Res Commun. 1976 Jan 12;68(1):89–96. doi: 10.1016/0006-291x(76)90014-0. [DOI] [PubMed] [Google Scholar]
- Thrierr J. C., Deubel V., Leng M. Interaction between polyribouridylic acid and spermine. Biochimie. 1972;54(9):1115–1119. doi: 10.1016/s0300-9084(72)80015-4. [DOI] [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Base pairing and fidelity in codon-anticodon interaction. Nature. 1976 Sep 23;263(5575):289–293. doi: 10.1038/263289a0. [DOI] [PubMed] [Google Scholar]
- Yguerabide J. Nanosecond fluorescence spectroscopy of macromolecules. Methods Enzymol. 1972;26:498–578. doi: 10.1016/s0076-6879(72)26026-8. [DOI] [PubMed] [Google Scholar]



