Abstract
A fast and accurate assay procedure for DNA-RNA hybrids is described in which exhaustive digestion of unhybridized DNA with S1 nuclease is followed by binding of hybrids to filter discs of DEAE-cellulose. The digested DNA can be efficiently washed from the filters so that background levels of 0.1-0.2% of input tracer DNA can be achieved, in contrast to the much higher (approximately 1-5%) backgrounds obtained using TCA precipitation procedures. Short duplexes, as small as 36 nucleotides in length, which are inefficiently bound to hydroxyapatite, are quantitatively bound to the DEAE-cellulose filters.
Full text
PDF


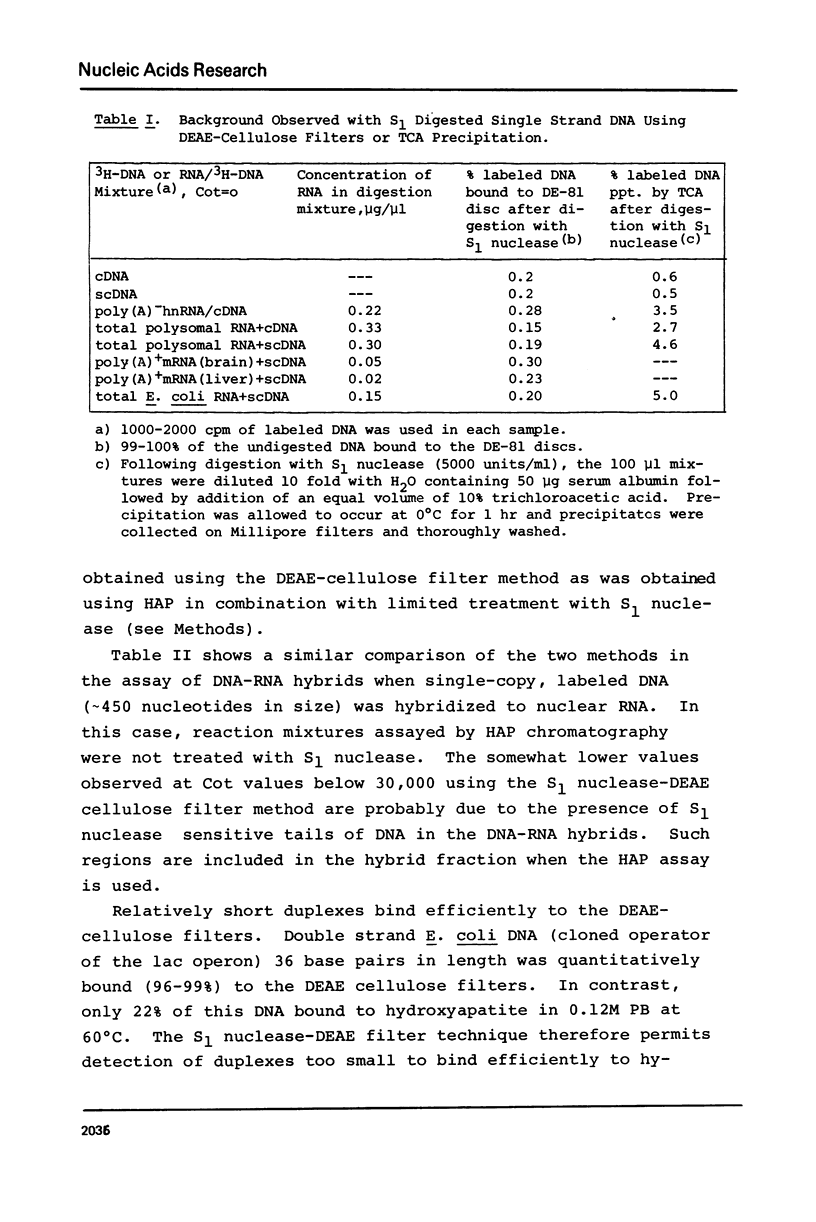
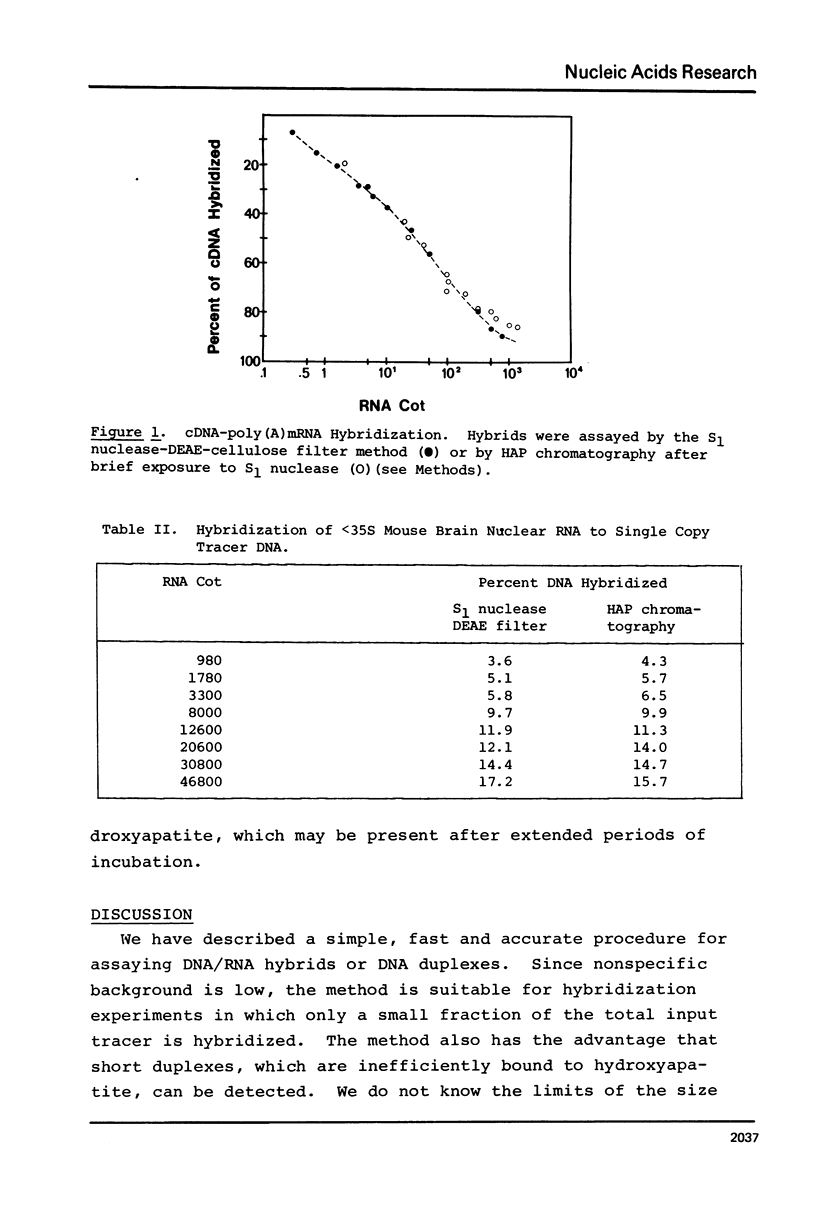

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affara N. A., Jacquet M., Jakob H., Jacob F., Gros F. Comparison of polysomal polyadenylated RNA from embryonal carcinoma and committed myogenic and erythropoietic cell lines. Cell. 1977 Oct;12(2):509–520. doi: 10.1016/0092-8674(77)90127-1. [DOI] [PubMed] [Google Scholar]
- Bantle J. A., Hahn W. E. Complexity and characterization of polyadenylated RNA in the mouse brain. Cell. 1976 May;8(1):139–150. doi: 10.1016/0092-8674(76)90195-1. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Britten R. J., Davidson E. H. A measurement of the sequence complexity of polysomal messenger RNA in sea urchin embryos. Cell. 1974 May;2(1):9–20. doi: 10.1016/0092-8674(74)90003-8. [DOI] [PubMed] [Google Scholar]
- Hahn W. E., Van Ness J. Elimination of double strand nuclease activity from S1 nuclease prepared from crude alpha amylase. Nucleic Acids Res. 1976 May;3(5):1419–1423. doi: 10.1093/nar/3.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson H. G. The nucleic acid-hydroxylapatite interaction. II. Phase transitions in the deoxyribonucleic acid-hydroxylapatite system. Biochemistry. 1973 Jan 2;12(1):145–150. doi: 10.1021/bi00725a024. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S., Kacian D. L. Synthesis of full-length DNA copies of avian myeloblastosis virus RNA in high yields. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2840–2843. doi: 10.1073/pnas.74.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Hoffman R., Penman S. The extensive homology between mRNA sequences of normal and SV40-transformed human fibroblasts. Cell. 1977 Aug;11(4):901–907. doi: 10.1016/0092-8674(77)90301-4. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Hydroxyapatite chromatography of short double-helical DNA. Biochim Biophys Acta. 1973 Dec 21;331(3):333–340. doi: 10.1016/0005-2787(73)90019-1. [DOI] [PubMed] [Google Scholar]



