Supercomputers can receive millions of jobs every minute that have to be processed following very specific rules. Similarly, cells are constantly flooded with external and internal cues that need to be sensed, integrated and decided upon.
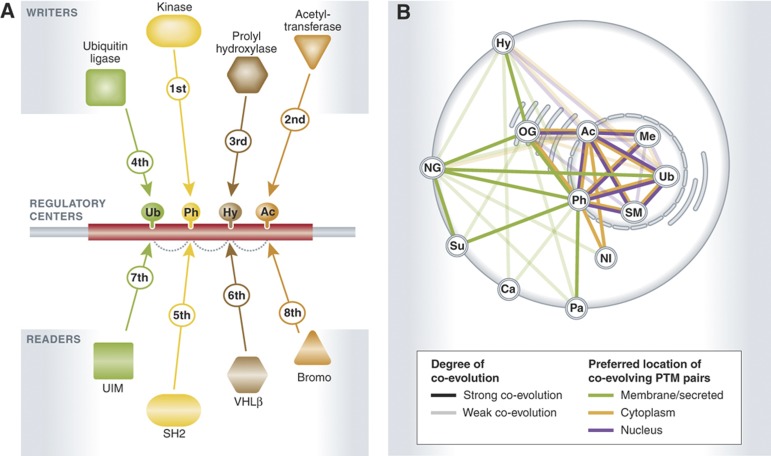
Analogous to computers' circuit boards, memory banks and bit-encoded electric pulses, eukaryotic cells rely on proteins composed of modular domains (globular structures larger than >30 residues) and linear motifs (<10 residue colinear sequences that often reside in disordered segments) harboring post-translational modifications (PTMs) to generate signaling fluxes with which they transmit and process information (Pawson and Nash, 2003; Janes et al, 2005). Cells use linear motifs, which typically evolve through convergent evolution and faster than modular domains, as storage devices, where the domains can ‘write', ‘read' and ‘erase' PTMs (Lim and Pawson, 2010 and Figure 1A) effectively generating logic gates (Lim, 2002) that modulate protein activity, directional and dynamic protein–protein interactions or allosteric effects (Hunter, 2007). Protein phosphorylation can, for example, modulate the binding of modular domains (e.g., SH2 domains) to a tyrosine- or serine-/threonine-containing linear motif, and thereby control the dynamics, timing and strength of a physical interaction. In this example, the kinase domain acts as the ‘writer' and the SH2 domain is the ‘reader', while a phosphatase domain would function as an ‘eraser' of the PTM. Similar functions as ‘writers', ‘readers' and ‘erasers' have been assigned to the components of many other PTMs (Figure 1A and Seet et al, 2006). The study of (co-)evolution of linear motifs, PTMs and their associated readers, writers and eraser domains are thus of great importance, as evidenced in the work from Bork and colleagues recently published at Molecular Systems Biology (Minguez et al, 2012).
Figure 1.
Post-translational regulatory centers and co-evolution of PTMs. (A) The interplay between PTMs (represented as dashed lines) and their location in post-transcriptional regulatory centers is a key feature that would facilitate an explosion of the degree of regulation and number of functional states that a protein can reach by combining multiple PTM-driven logic gates. The timing and order of events (represented by one number for each writer and reader domain) would be crucial to determine cellular outcome. (B) Co-evolution between different PTMs is represented as a network where line strength indicates degree of co-evolution and color denote location preference of the respective proteins.
Some of these different signaling and regulatory systems, such as ubiquitination and phosphorylation (Hunter, 2007), have previously been found to functionally interact—for example, by competition, co-regulation or other types of interplay. Nonetheless, the work by Minguez et al (2012) presents the first global survey of associations between 13 different PTM types spanning 8 eukaryotes. From this large-scale comparison, the authors report different degrees of sequence conservation for different PTMs. While this could lead the reader to consider that some PTMs and their functions are more conserved than others, it has been shown in other studies that sequence conservation is not required for functional conservation (Tan et al, 2009). In addition, by measuring co-evolution between PTMs, Minguez et al. also demonstrate that extensive interplay exists between different signaling and regulatory systems (Figure 1B). Finally, the team uncovers that, post-translationally modified residues tend to be closer to each other than expected by chance (clustering of PTMs), demonstrating the presence of ‘regulatory centers', i.e., protein segments that would accumulate several PTMs in reduced space (Figure 1A). This agrees with previous reports where it has been shown that PTMs tend to fall within disordered regions that can function as regulatory hot-spots (Neduva et al, 2005; Tan et al, 2009), while providing the first systematic study of this phenomenon.
The combination of these regulatory centers and the widespread interplay between PTMs advances our understanding of signaling systems in that, in analogy to shared memory computers, the different writers and readers of PTMs would access a shared protein segment, which enables combinatorial information encoding and leads thus to a considerable increase in computational power. More specifically, there are two areas where this could be beneficial. First, as briefly described earlier, these ‘centers of computation' lead to a factorial number of logic gates being possible (Lim, 2002). For example, the acetylation of a protein could depend on whether this protein has been phosphorylated, hydroxylated or ubiquitinated beforehand—some examples of this type of positive and negative cross-talk have been described in the literature for different pairs of PTMs (Hunter, 2007). This strategy is of great importance because the degree of regulation and number of functional states that a protein can reach will probably increase dramatically, thereby massively increasing the ‘control potential' of cells. Second, the presence of multiple PTMs and the new binding motifs that they form could result in lower specificity requirements for writer domains. For example, unspecific ubiquitin ligases could achieve specificity by containing a SH2 domain in its sequence that would bind with high specificity and in a conditional manner (only after tyrosine phosphorylation) to its substrate. If this is a general principle, it provides an important mechanism for decoupling catalytic activity from specificity in proteins: catalytic domains could focus solely on their catalytic function, while other domains would specifically bind to the substrate of the catalytic reaction. While several examples of ‘secondary PTM-binding domain driven specificity' exist (Hunter, 2007), different proteins will probably distribute their specificity and catalytic functions differently among their different domains and motifs. Finally, combinatorial logic gates and newly acquired specificities are not mutually exclusive features and can be often found combined like in the case of phosphodegrons (Hunter, 2007).
While biological entities are often conserved across millennia, cells do not live in evolutionary time; they live in the moment. Thus, operational freedom is needed in biological systems to establish responsive and emergent properties that enable cells to respond to changes in the environment, genomic lesions or other perturbations and cues. The study by Minguez et al (2012) demonstrates how, by systematically studying the evolutionary patterns that have accumulated in the past, one can provide insights into how cells compute their responses today.
One important missing piece in our understanding of cells' computational processes is how protein logic gates operate in time- (Figure 1A) and space-dependent networks. Perhaps the fact that cellular decisions seem to be concentrated around regulatory centers will facilitate their monitoring and lead to an easier understanding of how cells compute responses under normal circumstances as well as what leads to short-circuits in disease (Pawson and Linding, 2008).
Acknowledgments
RL is supported by a Sapere Aude Starting Grant from The Danish Council for Independent Research, a Lundbeck Foundation Group Leader Fellowship and a Career Development Award from Human Frontier Science Program. See http://www.networkbio.org and http://www.lindinglab.org for more information on cancer related network biology.
Footnotes
The authors declare that they have no conflict of interest.
References
- Hunter T (2007) The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol Cell 28: 730–738 [DOI] [PubMed] [Google Scholar]
- Janes KA, Albeck JG, Gaudet S, Sorger PK, Lauffenburger DA, Yaffe MB (2005) A systems model of signaling identifies a molecular basis set for cytokine-induced apoptosis. Science 310: 1646–1653 [DOI] [PubMed] [Google Scholar]
- Lim WA (2002) The modular logic of signaling proteins: building allosteric switches from simple binding domains. Curr Opin Struct Biol 12: 61–88 [DOI] [PubMed] [Google Scholar]
- Lim WA, Pawson T (2010) Phosphotyrosine signaling: evolving a new cellular communication system. Cell 142: 661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez P, Parca L, Diella F, Mende DR, Kumar R, Helmer-Citterich M, Gavin A-C, van Noort V, Bork P (2012) Deciphering a global network of functionally associated post-translational modifications. Mol Syst Biol 8: 59910.1038/msb.2012.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neduva V, Linding R, Su-Angrand I, Stark A, de Masi F, Gibson TJ, Serrano L, Russell RB (2005) Systematic discovery of new recognition peptides mediating protein interaction networks. PLoS Biol 3: e405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T, Linding R (2008) Network medicine. FEBS Lett 582: 1266–1270 [DOI] [PubMed] [Google Scholar]
- Pawson T, Nash P (2003) Assembly of cell regulatory systems through protein interaction domains. Science 300: 445–452 [DOI] [PubMed] [Google Scholar]
- Seet BT, Dikic I, Zhou M, Pawson T (2006) Reading protein modifications with interaction domains. Nat Rev Mol Cell Biol 7: 473–483 [DOI] [PubMed] [Google Scholar]
- Tan CS, Bodenmiller B, Pasculescu A, Jovanovic M, Hengartner MO, Jørgensen C, Bader GD, Aebersold R, Pawson T, Linding R (2009) Comparative analysis reveals conserved protein phosphorylation networks implicated in multiple diseases. Sci Signal 2: ra39. [DOI] [PubMed] [Google Scholar]