Abstract
Up to 50% of bipolar disorder (BD) patients present a lifetime diagnosis of alcohol use disorders (AUD). BD patients with comorbid AUD, even when in remission from the AUD, have a poorer outcome and functional impairment than patients with BD alone. The neurobiological abnormalities that potentially characterize this severe subgroup of BD patients are unknown. Our goal was to investigate gray matter (GM) volume abnormalities in BD I patients with comorbid AUD. Twenty-one BD-AUD patients, 21 BD-nonAUD BD patients, and 25 healthy controls (HC), matched by age, gender, and handedness were studied. The BD-AUD patients were in remission from AUD on average for 6.8 years. 3D SPGR MRIs (TR=25 ms, TE=5 ms, slice thickness=1.5 mm) were acquired from all subjects using a 1.5 T GE Signa Imaging System. We used an optimized voxel-based morphometry protocol to compare GM volumes among the groups. BD-AUD patients presented smaller GM volumes in the left medial frontal and the right anterior cingulate gyri compared to BD-nonAUD patients. BDnon-AUD patients did not present GM volume differences compared to HC. These findings provide evidence for an effect of comorbid AUD on regional brain structure of BD I patients and warrant further research on neurobiological aspects of this prevalent and severe comorbidity.
Keywords: Bipolar disorder, alcoholism, comorbidity, physiopathology, cerebral cortex, magnetic resonance imaging
Bipolar disorder (BD) is a severe and debilitating psychiatric disorder, with unpredictable course and high economical burden to society [8]. A substantial portion of patients with BD (between 33 and 46%) also suffers from alcohol use disorders (AUD) [17,23]. The presence of AUD is associated with a significant negative impact on the course of BD. BD patients with comorbid AUD typically have a poorer treatment response, a greater number of episodes and hospitalizations, a higher tendency to chronicity and disability, greater impulsivity, and are more likely to attempt suicide in comparison to BD patients who have never had AUD [5,11,28].
Surprisingly, in spite of the high prevalence and clinical significance of the comorbidity between BD and AUD, the neurobiological abnormalities that may underlie the co-existence of these two conditions have been poorly investigated. Regarding BD, extensive research suggests that structural abnormalities in brain areas pertaining to a fronto-limbic circuit underlie the mood and cognitive disturbance seen in that condition [25]. On the other hand, AUD per se is also associated with structural brain changes, such as total brain shrinkage and reduction in gray and white matter volumes, particularly in frontal lobes, which can be partially reverted with abstinence [16,24]. However, even patients with long-term remitted AUD present reductions in gray matter (GM) volumes of regional prefrontal areas that regulate positive and negative reinforcement and decision-making [16,29]. These findings suggest that structural brain changes associated with AUD may be persistent and may potentially underlie the behaviors that characterize the compulsive drug seeking.
To the best of our knowledge, no study has investigated any structural brain abnormalities that may exist in BD patients with comorbid AUD. Such a study would be particularly important as it could help to disentangle in which brain regions there is any GM abnormality that might be specific to BD with comorbid AUD and possibly underlie the worse clinical features and outcome of this comorbidity. Thus, our objective in this study was to evaluate whether BD patients with comorbid AUD present specific GM abnormalities in comparison to BD patients who never developed an AUD and to healthy controls (HC). Based on previous report of decreased GM volumes of prefrontal areas in alcoholic patients [16,29], we hypothesized that BD patients with comorbid AUD would present smaller GM volumes in brain areas involved in mood regulation and cognitive control when compared to BD patients without comorbid AUD and to HC.
The sample comprised 21 BD patients with comorbid AUD (BD-AUD group) (mean age±S.D.=40.1±11.6 years; males: 33.3%), 21 BD patients without comorbid AUD (BD-nonAUD group) (mean age±S.D.=40.5±11.9 years; males: 23.8%) and 25 HC (mean age±S.D.=40.9±10.6 years; males: 32%). The groups were matched by age, gender, ethnicity and handedness. All the patients were on medication. Inclusion criteria for patients were a DSM-IV diagnosis of BD type I, in any mood state and age of 18 to 99 years. BD-AUD patients also met DSM-IV criteria for abuse or dependence of alcohol, and had to be in remission from AUD for at least 6 months. Exclusion criteria for all BD subjects were the presence of current drug/alcohol abuse or dependence or any other Axis I diagnosis, except a lifetime diagnosis of any anxiety disorders. The exclusion criteria for HC were the presence of any past or current Axis I psychiatric disorder, presence of significant neurological or medical problems and presence of any Axis I diagnosis in first-degree relatives. Additional exclusion criteria for all BD subjects and HC were the presence of significant medical problems (such as hypertension, diabetes mellitus, renal or active liver disease) or neurological disorders (such as epilepsy, stroke, dyslexia or head trauma with loss of consciousness for more than 1 hour). All of the patients and HC were residents of the San Antonio metropolitan area or surrounding cities. The subjects were recruited through local media advertisements and flyers posted in the medical center. We conducted all investigation in accordance with the Declaration of Helsinki, and the local institutional review board approved the study. After complete description of the study to the subjects, written informed consent was obtained.
The diagnostic assessments were conducted using the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID), versions for patients and non-patients [7]. The 21-item Hamilton Depression Rating Scale (HDRS) and the Young Mania Rating Scale (YMRS) were administered to all BD patients and HC to assess severity of mood symptoms [10,32].
Brain scans were performed on a Philips 1.5 T MR system (Philips Medical System, Andover, MA). Images were collected by means of an axial 3-dimensional T1 weighted field fast echo sequence (field of view: 256 mm; view matrix: 256×256; repetition time, 24 msec: echo time: 5 msec; flip angle: 40 degree, slice thickness: 1 mm).
Image preprocessing was performed using SPM2 software (Wellcome Department of Imaging Neuroscience, London, United Kingdom) running under Matlab 7.1.0 (MathWorks, Natick, MA) and following the optimized voxel-based morphometry (VBM) protocol [9]. A local T1 template was created by averaging all the images of the 67 participants using an optimized VBM script (http://dbm.neuro.uni-jena.de/vbm.html). The original images were analyzed using this local template as follows: the extracted segmented images were normalized with the own gray matter (GM) template, and deformation parameters were applied to the original images, followed by a second segmentation step in stereotactic space. This procedure automatically removed non-brain tissues including scalp tissue, skull and dural venous sinus. Finally, the segmented images were modulated by Jacobian determinants derived from the spatial normalization. Images were obtained in 1×1×1 mm resolution by the optimized VBM script. These images were smoothed with a 12 mm Gaussian filter.
For image analysis, we used SPM2 software to implement a General Linear Model. Age and sex were treated as covariates because both of them may affect brain structure [9,26]. Total GM volumes were calculated by the optimized VBM script and treated as a nuisance variable in SPM2. We performed two different 2-group comparisons: BD-AUD patients versus BD-nonAUD patients; BD-nonAUD patients versus HC. The results set voxel values for each contrast constituted an SPM t statistic (SPM {t}). The suprathreshold was set at Puncorrected < 0.001 with an extent threshold k value > 50 voxels in the whole brain analysis. Based on previous literature, we selected three a priori regions of interest (ROIs): ventromedial prefrontal cortex, dorsolateral prefrontal cortex, and anterior cingulate cortex. Then, we corrected the volumes of ROIs using the small volume correction with family wise error (FWE) in SPM to confirm our hypothesis. We identified these regions using automated anatomical labeling [31] via WFU PickAtlas version 2 [15]. All results were reported as the MNI coordinates. Statistical comparisons of the demographic and clinical characteristics of the groups were performed using the SPSS for Windows statistical software, version 14.0 (SPSS, Inc., Chicago, IL).
The 3 groups were matched by age (ANOVA, F(2,72)=0.03, p=0.97), gender (Chi square test, p=0.76), ethnicity (Chi square, p=0.91) and handedness (Chi square, p=0.42). Details of demographic and clinical characteristics of the sample are displayed in Table 1.
Table 1.
Demographic and clinical characteristics of the sample
| Characteristics | BD-AUD patients (n=21) | BD-nonAUD patients (n=21) | HC (n=25) | P value |
|---|---|---|---|---|
| Age, y (mean±S.D.) | 40.1±11.6 | 40.5±11.9 | 40.9±10.6 | 0.97 |
| Gender: males (%) | 7 (33.3) | 5 (23.8) | 8 (32) | 0.76 |
| Ethnicity: Caucasian (%) | 13 (61.9) | 15 (71.4) | 15 (60) | 0.91 |
| Handedness: right-handed (%) | 20 (95.2) | 20 (95.2) | 23 (92) | 0.42 |
| Mood state, (%) | 0.94 | |||
| Euthymic | 8 (38.1) | 8 (38.1) | ||
| Depressed | 10 (47.6) | 9 (42.9) | ||
| Manic/hypomanic/mixed | 3 (14.3) | 4 (19) | ||
| HDRS score (mean±S.D.) | 14.8±10.2 | 14.7±9.5 | - | 0.95 |
| YMRS score (mean±S.D.) | 5.1±5.7 | 9.8±9.4 | - | 0.13 |
| Age at onset of BD, y (mean±S.D.) | 18.2±7.2 | 20.0±10.1 | - | 0.9 |
| Length of BD illness, y (mean±S.D.) | 21.6±13.2 | 20.6±13.9 | - | 0.65 |
| Number of hospitalizations, (mean±S.D.) | 2.3±2.2 | 2.3±3.7 | - | 0.41 |
| History of psychotic symptoms, (%) | 9 (42.9) | 3 (14.3) | - | 0.04 |
| Comorbid anxiety disorders, (%) | 13 (61.9) | 15 (71.4) | - | 0.74 |
| Panic disorder, (%) | 8 (38.1) | 8 (38.1) | - | |
| Agoraphobia without panic disorder, (%) | 6 (24) | 7 (28) | - | |
| Specific phobia, (%) | 4 (19) | 5 (23.8) | - | |
| Social phobia, (%) | 4 (19) | 3 (14.3) | - | |
| Obsessive-compulsive disorder, (%) | 3 (14.3) | 3 (14.3) | - | |
| Generalized anxiety disorder, (%) | 3 (14.3) | 7 (33.3) | - | |
| Posttraumatic stress disorder, (%) | 3 (14.3) | 6 (28.6) | - | |
| Comorbid substance use disorder (%) | 10 (47.6) | 2 (9.5) | - | 0.02 |
| Marijuana, (%) | 7 (33.3) | 0 | - | |
| Cocaine, (%) | 4 (19) | 1 (4.8) | - | |
| Stimulants, (%) | 3 (14.3) | 1 (4.8) | - | |
| Opioids, (%) | 1 (4.8) | 0 | - | |
| Medication in use, (%) | ||||
| Lithium | 2 (9.5) | 0 | - | 0.49 |
| Anticonvulsants | 9 (42.9) | 10 (47.6) | - | 1.0 |
| Atypical antipsychotics | 9 (42.9) | 6 (28.6) | - | 0.52 |
| Antidepressants | 8 (38.1) | 8 (38.1) | - | 1.0 |
| Benzodiazepines | 4 (19) | 2 (9.5) | - | 0.66 |
Abbreviations: BD-AUD: bipolar disorder with comorbid alcohol use disorders; BD-nonAUD: bipolar disorder without comorbid alcohol use disorders; HC: healthy controls; S.D.: standard deviation; HDRS: Hamilton Depression Rating Scale; YMRS: Young Mania Rating Scale
Of the BD-AUD patients, 11 (52.4%) presented an alcohol abuse diagnosis and 10 (47.6%) presented an alcohol dependence diagnosis. In the BD-AUD group, the mean age of onset of AUD was 23.6±9.4 years (range: 13 to 48 y), the mean length of alcohol use was 8.1±10.6 years (range: 1 to 44 y) and the mean time in remission from AUD was 6.8±7.7 years (range: 0.5 to 25 y). Of the BD-nonAUD patients, two (9.5%) presented a diagnosis of past cocaine abuse and past stimulant abuse. Because their drug use disorder was of mild severity, they were abstinent for more than 6 months, and we were mainly interested in comorbid AUD effects, we decided to keep them in the sample.
The BD-AUD patients presented smaller GM volumes of the left medial frontal gyrus (MNI coordinates of the voxel of maximum statistical significance: x=12, y=12, z=48, t=3.48, k=167, PFWEcorrected=0.01) (Figure 1) and right anterior cingulate gyrus (x=−8, y=19, z=24, t=3.48, k=171, PFWEcorrected=0.01) (Figure 2) compared to the BD-nonAUD patients. There were no ROIs with suprathreshold clusters between the BD-nonAUD patients and HC in the whole brain analysis.
Figure 1.
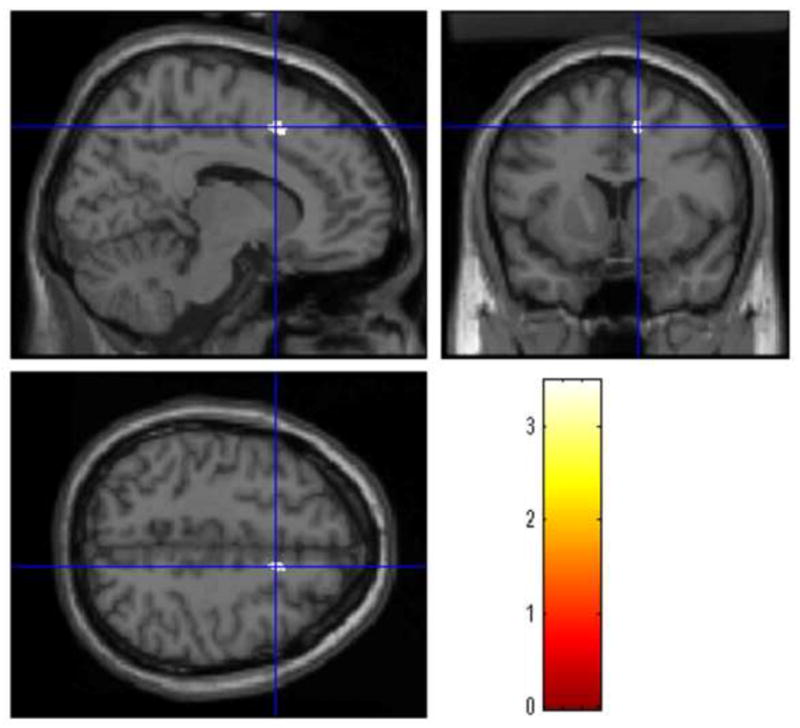
The BD-AUD patients presented small GM volumes of the left medial frontal gyrus compared to the BD-nonAUD patients. The cross-hair represents the coordinate with maximum threshold.
Figure 2.
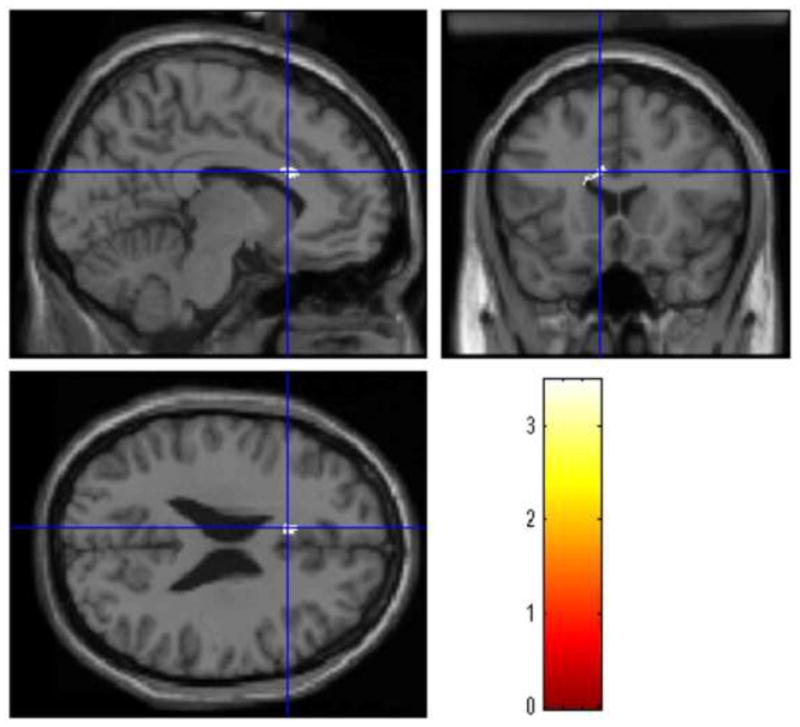
The BD-AUD patients presented small GM volumes of the right anterior cingulate gyrus compared to the BD-nonAUD patients. The cross-hair represents the coordinate with maximum threshold.
In the present study, BD-AUD patients presented smaller regional GM volumes of the left medial frontal and right anterior cingulate gyri compared to BD-nonAUD patients. These brain regions are frontal lobe subareas that have extensive intrinsic and extrinsic connections with other prefrontal areas and high-order association regions, such as the orbitofrontal cortex, temporal and parietal lobe, and subcortical structures [20]. The prefrontal cortex is functionally responsible for the temporal organization of behavior, and plays an important role in the inhibitory control of inappropriate behaviors, including internal and external sensory-driven compulsive behaviors, including addiction [1]. It has been proposed that addictive behaviors are characterized by decreased “top-down” inhibitory control in prefrontal brain areas [13]. We speculate that GM decrements in prefrontal areas, as seen in this study, may represent a structural correlate of decreased inhibitory control that distinguishes BD-AUD patients from BD patients who never develop AUD.
Our finding of smaller GM volumes in the left medial frontal gyrus is consistent with our previous report of neurochemical abnormalities in the left dorsolateral prefrontal cortex of BD-AUD patients detected by magnetic resonance spectroscopy [18]. In that study, using a sample that partially overlaps the present sample, we found significantly lower Glu+Gln and glutamate levels in BD-AUD patients compared to BD-nonAUD patients[18]. Both findings add to the evidence that brain abnormalities exist in the prefrontal cortex and may characterize BD patients who develop comorbid AUD.
It is noteworthy that our BD-AUD patients were in remission from their AUD on average for 6.8 years, which excludes a current effect of alcohol on GM volumes. Our findings may represent a long-term effect of heavy alcohol drinking. For instance, patients with primary AUD who have been in long-term remission from alcohol misuse also present decreased GM volumes in brain areas functionally responsible for drug seeking behaviors [14,29]. Alternatively, it is possible that reduced frontal GM volumes may represent a vulnerability factor for AUD, as reduced GM volumes of the superior frontal gyri have been found in alcohol-naïve individuals at high risk for developing AUD [2]. We should note that these are speculative explanations for our findings, as a prospective study would be needed to clarify the temporal relationships between heavy alcohol drinking and brain abnormalities in BD.
It is also noteworthy that 48% of the BD-AUD patients also presented an additional past drug use disorder comorbidity, which raises issues about how specific our findings are regarding effects of alcohol rather than substances in general. Studies in primary cannabis use disorder patients have not consistently found GM changes associated with cannabis misuse [3,22]. On the other hand, studies in primary cocaine use disorder patients describe decreased GM volumes in total prefrontal cortex [6], and in dorsolateral prefrontal cortex [19]. It should be noted that the presence of cocaine use disorders in our sample was very small and of mild severity, decreasing the possibility that our findings are confounded by their presence. However, we cannot completely exclude the possibility that the additional drug use disorders contributed, at least to some extent, to the GM decrements seen in our BD-AUD patients.
BD-nonAUD patients presented no differences in regional GM reductions compared to HC, which is different than reported by previous studies. Regional GM reductions in BD are still a matter of controversy. While a region-of-interest based meta-analysis found no evidence for robust gray matter abnormalities [12], a recent voxelwise meta-analysis of VBM studies in BD patients found that only two regions are consistently reduced across studies, i.e., the left anterior cingulate and the right anterior insula [4]. Differences in sample characteristics (i.e., lithium effects on GM volumes, number of episodes, length of illness), or in VBM techniques (i.e., optimized versus traditional protocol, smoothing or statistical threshold methods) have all been proposed as explanations for the variation in findings across studies [4] and may also potentially explain the differences between our and previous findings.
Our findings also have to be considered at the light of the heterogeneity of our sample. For example, 9 of the BD-AUD patients and 3 of the BD-nonAUD patients presented a lifetime history of psychotic symptoms. Few neuroimaging studies suggest that, in BD patients, lifetime history of hallucinations and delusions is associated with reduced GM density in the left middle temporal lobe [27] and the left dorsolateral prefrontal cortex [30]. While the brain areas identified in our study do not closely correspond to the areas associated with psychosis, we cannot exclude the possibility that history of psychosis may affect these regions.
Some limitations of this study should be considered. The cross-sectional design cannot exclude the possibility that the differences in GM volumes between BD-AUD and BD-nonAUD patients antecede the onset of alcohol problems and potentially predispose BD patients to develop AUD. Because this was an exploratory study with relatively small sample size, we could not compare subgroups of BD-AUD patients with adequate protection against type I errors. Lastly, exposure to psychiatric medication represents a confounding factor. Current use of lithium and valproate have been associated with increased GM volumes in key brain regions involved in BD pathophysiology, including the anterior cingulate gyrus, amygdala and hippocampus [20]. With these limitations in mind, our results should be considered preliminary until replicated by further studies.
This study also has considerable strengths. This is the first study to report effects of comorbid AUD on GM volumes of BD patients. Moreover, our sample was well characterized clinically and reasonably well matched for demographic and clinical characteristics of BD, also minimizing the confounding effects of these variables. Our results suggest that effects of AUD may be detected in BD patients even after long-term remission from the alcohol misuse. These results may have general implications for structural neuroimaging studies in BD. Our findings suggest that potential confounding effects of comorbid AUD should be considered when planning or analyzing neuroimaging studies in BD.
In conclusion, we found that a prior AUD diagnosis may be associated with smaller GM volumes in the left medial frontal and right anterior cingulate gyri of BD I patients. These findings are preliminary, but they warrant further investigation on neurobiological aspects of BD comorbid with AUD, a prevalent, severe and difficult-to-treat condition.
Highlights.
Bipolar disorder often co-occurs with alcohol use disorders.
We investigated the brain structure of alcoholic and non-alcoholic bipolar patients.
Alcoholic bipolar patients have smaller gray matter volumes in frontal lobe areas.
The affected areas are the left medial frontal gyrus and the right anterior cingulate gyrus.
These areas may be key brain regions in the neurobiology of this comorbidity.
Acknowledgments
This research was partly supported by grants MH 01736, MH 068662, RR 020571, UTHSCSA GCRC (M01-RR-01346), the Krus Endowed Chair in Psychiatry (UTHSCSA), the Veterans Administration (VA Merit Review), KAKENHI-C 21591519 (to K. Matsuo) and a private donation from Thompson Motta’s family (to PROMAN). E. Serap Monkul is an employee of Eli Lilly Brazil.
Footnotes
The other authors do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abernathy K, Chandler LJ, Woodward JJ. Alcohol and the prefrontal cortex. Int Rev Neurobiol. 2010;91:289–320. doi: 10.1016/S0074-7742(10)91009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- 3.Block RI, O’Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11:491–496. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- 4.Bora E, Fornito A, Yucel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry. 2010;67:1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso BM, Sant’Anna M, Dias VV, Andreazza AC, Cereser KM, Kapczinski F. The impact of co-morbid alcohol use disorders in bipolar patients. Alcohol. 2008;42:451–457. doi: 10.1016/j.alcohol.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. New York, NY: State Psychiatric Institute, Biometrics Research Department; 2002. [Google Scholar]
- 8.Gershon S, Soares JC. Commentary - Current therapeutic profile of lithium. Arch Gen Psychiatry. 1997;54:16–20. doi: 10.1001/archpsyc.1997.01830130020004. [DOI] [PubMed] [Google Scholar]
- 9.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton M. Hamilton Psychiatric Rating Scale for Depression. In: Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Washington DC: U.S. Department of Health, Education and Welfare; 1976. pp. 179–192. [Google Scholar]
- 11.Holmes KH, Bearden CE, Barguil M, Fonseca M, Serap Monkul E, Nery FG, Soares JC, Mintz J, Glahn DC. Conceptualizing impulsivity and risk taking in bipolar disorder: importance of history of alcohol abuse. Bipolar Disord. 2009;11:30–40. doi: 10.1111/j.1399-5618.2008.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 13.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makris M, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An authomated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI datasets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 16.Mann K, Agartz I, Harper C, Shoaf S, Rawlings RR, Momenan R, Hommer DW, Pfefferbaum A, Sullivan EV, Anton RF, Drobes DJ, George MS, Bares R, Machulla HJ, Mundle G, Reimold M, Heinz A. Neuroimaging in alcoholism: ethanol and brain damage. Alcohol Clin Exp Res. 2001;25:104S–109S. doi: 10.1097/00000374-200105051-00019. [DOI] [PubMed] [Google Scholar]
- 17.McElroy SL, Altshuler LL, Suppes T, Keck PE, Jr, Frye MA, Denicoff KD, Nolen WA, Kupka RW, Leverich GS, Rochussen JR, Rush AJ, Post RM. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry. 2001;158:420–426. doi: 10.1176/appi.ajp.158.3.420. [DOI] [PubMed] [Google Scholar]
- 18.Nery FG, Stanley JA, Chen HH, Hatch JP, Nicoletti MA, Monkul ES, Lafer B, Soares JC. Bipolar disorder comorbid with alcoholism: a 1H magnetic resonance spectroscopy study. J Psychiatr Res. 2010;44:278–285. doi: 10.1016/j.jpsychires.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- 20.Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Jones EG, Peters A, editors. Cerebral cortex. Vol. 2. Plenum Press; New York: 1985. pp. 3–55. [Google Scholar]
- 21.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quickfall J, Crockford D. Brain neuroimaging in cannabis use: a review. J Neuropsychiatry Clin Neurosci. 2006;18:318–332. doi: 10.1176/jnp.2006.18.3.318. [DOI] [PubMed] [Google Scholar]
- 23.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 24.Rosenbloom M, Sullivan EV, Pfefferbaum A. Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alc Res Health. 2003;27:146–152. [PMC free article] [PubMed] [Google Scholar]
- 25.Soares JC. Contributions from brain imaging to the elucidation of pathophysiology of bipolar disorder. Int J Neuropsychopharmacol. 2003;6:171–180. doi: 10.1017/S1461145703003390. [DOI] [PubMed] [Google Scholar]
- 26.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AT, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 27.Stanfield AC, Moorhead TW, Job DE, McKirdy J, Sussmann JE, Hall J, Giles S, Johnstone EC, Lawrie SM, McIntosh AM. Structural abnormalities of ventrolateral and orbitofrontal cortex in patients with familial bipolar disorder. Bipolar Disord. 2009;11:135–144. doi: 10.1111/j.1399-5618.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 28.Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. Am J Psychiatry. 2005;162:1680–1687. doi: 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- 29.Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tost H, Ruf M, Schmal C, Schulze TG, Knorr C, Vollmert C, Bosshenz K, Ende G, Meyer-Lindenberg A, Henn FA, Rietschel M. Prefrontal-temporal gray matter deficits in bipolar disorder patients with persecutory delusions. J Affect Disord. 2010;120:54–61. doi: 10.1016/j.jad.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 32.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensibility. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]


