Abstract
One of our highest evolved functions as human beings is our capacity to switch between multiple tasks effectively. A body of research has identified a distributed frontoparietal network of brain regions which contribute to task switching. However, relatively less is known about whether some brain regions may contribute to switching in a domain‐general manner while others may be more preferential for different kinds of switching. To explore this issue, we conducted three meta‐analyses focusing on different types of task switching frequently used in the literature (perceptual, response, and context switching), and created a conjunction map of these distinct switch types. A total of 36 switching studies with 562 activation coordinates were analyzed using the activation likelihood estimation method. Common areas associated with switching across switch type included the inferior frontal junction and posterior parietal cortex. In contrast, domain‐preferential activation was observed for perceptual switching in the dorsal portion of the premotor cortex and for context switching in frontopolar cortex. Our results suggest that some regions within the frontoparietal network contribute to domain‐general switching processes while others contribute to more domain‐preferential processes, according to the type of task switch performed. Hum Brain Mapp, 2011. © 2011 Wiley Periodicals, Inc.
Keywords: cognitive control, brain imaging, task switching, meta‐analysis, switch type, perceptual switching, response switching, context switching
INTRODUCTION
Cognitive control refers to a set of processes that enable humans to flexibly coordinate thoughts and behaviors in order to accomplish internal goals [Miller and Cohen,2001]. An important component of cognitive control involves the ability to reconfigure task sets in a flexible manner in order to meet shifting demands [Monsell,2003]. One of the most common ways to explore this cognitive ability is through the use of the task switching paradigm, in which a participant's reaction time (RT) is compared when they switch between performing two simple tasks versus performing either task alone [Jersild,1927]. The requirement to switch between performing two tasks results in a RT increase compared with performing either task in isolation, termed a switch cost [Kramer et al.,1999; Rogers and Monsell,1995].
Functional neuroimaging studies have identified a distributed frontoparietal network of brain regions which contribute to task switching. Within the frontoparietal network, there appear to be several key cortical hubs that are active across a variety of stimuli and paradigms including dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), frontopolar cortex (FPC), and posterior parietal cortex (PPC) [e.g., Badre and Wagner,2006; Braver et al.,2003; Crone et al.,2006; Dove et al.,2000; Gold et al.,2010; Liston et al.,2006; Sohn et al.,2000]. However, less is known about whether some of these regions may contribute to switching in a domain‐general manner whereas others may be more preferential for different kinds of switching. One reason for this knowledge gap is that neuroimaging studies have tended to describe switching as a unitary process, with limited consideration of the potential role of switch type on brain activation patterns.
Nevertheless, a few neuroimaging studies that have directly compared different switch types have tended to report distinct neural correlates associated with different switch types [Nagahama et al.,2001; Ravizza and Carter,2008; Rushworth et al.,2002]. For example, Ravizza and Carter [2008] tested whether neural networks are dissociable when switching between perceptual features or between response rules. These authors found that DLPFC (BA 9/46) showed greater activity for response switching than for perceptual switching while the dorsal premotor cortex (BA 6) showed greater activity for perceptual than response switching.
Such findings raise the question of whether some regions within the frontoparietal task switching network may be relatively specialized for particular kinds of task switches. The present meta‐analysis was conducted to explore this issue. Specifically, we aimed to test whether some frontoparietal brain regions contribute to task switching irrespective of the kind of switch being performed, whereas others may be preferential for the type of switch being performed. A meta‐analysis is appropriate to address this question because it incorporates results obtained from different scanners, participants, and statistical analysis approaches and is thus insensitive to idiosyncracies associated with any one study. In surveying the literature, we identified three distinct switch types that have been employed frequently in studies of task switching: perceptual, response, and context.
Perceptual switching refers to switching attention between perceptual features of a stimulus or between stimulus selection rules in order to make a task‐appropriate decision about the properties of a stimulus. For example, subjects are instructed to respond based on shape (e.g., a circle indicates a left button press, and a square indicates a right button press), or to respond based on the direction of an arrow (e.g., a left pointing arrow indicates a left button press and a right pointing arrow indicates a right button press). The defining feature of perceptual switching is that attention must be actively switched between perceptual features (i.e., shape and direction) in order to select the appropriate, task‐relevant response.
Response switching refers to switching between two or more arbitrary or opposing S‐R mappings (sometimes called S‐R reversal paradigms). In a typical response switching task, participants may be asked to select the higher of two numbers, with the S‐R mappings depending upon a cue. For example, a red color cue might indicate that the left button is associated with a lower digit whereas the right button is associated with a higher digit. Conversely, a green color cue might indicate that the left button is associated with a higher digit and the right button is associated with a lower digit. To perform accurately participants must maintain two arbitrarily assigned S‐R contingencies in mind and actively switch between them based on a cue.
Context switching (sometimes called set shifting) refers to shifting between task rules or cognitive sets. A prototypical context switching task is the Wisconsin Card Sorting Task (WCST), which has been used in the study of patients with PFC lesions [e.g., Nelson,1976; Stuss et al.,2000] and in many functional neuroimaging studies [e.g., Monchi et al.,2001; Nagahama et al.,2001; Rogers et al.,2000]. On the WCST, a participant is instructed to match a target card to one of four simultaneously presented cards, according to one out of three possible matching rules (i.e., color, shape, or number of stimuli). The participant is not told how to match the cards but is informed whether a particular match is correct or incorrect following each trial. Thus, context switching emphasizes endogenous control processes associated with the maintenance and switching between multiple cognitive sets.
In the present study, we first conducted a meta‐analysis of switching studies collapsed across each of these three switch types to identify the broad network of brain regions that contribute to task switching. We then performed three separate meta‐analyses to identify the broad network of regions that contribute to each switch type. Next, a conjunction map was created from these individual meta‐analyses to identify domain‐general switch regions. Finally, we carried out three additional meta‐analyses focusing on direct comparisons between switch types to identify regions which contribute to specific switch types in a domain‐preferential manner.
METHODS
Selection of Studies and Contrasts of Interest
In our meta‐analyses, we included functional neuroimaging switching studies (fMRI or PET) published in English between the years of 1995 and 2009. These studies were identified via searches on Medline and PsycInfo. Studies were included if they reported whole‐brain comparisons of switching tasks in which the control task required processing stimuli of similar complexity and similar motor output as the switch task (i.e., switch vs. nonswitch trials but not switch vs. passive fixation or rest baselines). We considered studies involving healthy, young adults. For the studies in which young adults were compared to older adults or young adults were compared with patients, we included only the results from the healthy, young adults. For fMRI studies, both block and event‐related designs were included.
We included only studies that reported peaks of activation in standardized coordinate space (Talairach or MNI) based on group analyses. We excluded studies that used emotional stimuli in the tasks. All studies meeting these inclusion criteria were reviewed independently by each of the authors to determine switch type designation (using criteria described in the Introduction section for each of the switch types). Only studies in which the authors unanimously agreed upon the switch type designation were included in the reported analyses. Using these criteria, we were able to identify 48 studies. Among these studies, 12 studies were excluded since these studies involved multidimensional kinds of switching (i.e., both perceptual and response switching). As a result, 36 studies were included in this study (see Table I for details).
Table I.
The list of switching studies included in the ALE meta‐analyses
| Author (year) | Method | N | Contrast | Task | Foci |
|---|---|---|---|---|---|
| Context switching | |||||
| Berman et al. [1995] | PET | 40 | Shifting > control | WCST | 35 |
| Braver et al. [2003] | fMRI | 13 | Mixed > single state | Semantic classification | 3 |
| Goldberg et al. [1998] | PET | 12 | Shifting > control | WCST | 17 |
| Graham et al. [2009] | fMRI | 18 | 2+NF > 2 + PF | Modified WCST | 14 |
| Koechlin et al. [1999] | fMRI | 6 | Branching > control | Letter matching | 2 |
| Koechlin et al. [2000] | fMRI | 6 | Predictive and random > control | Letter matching | 12 |
| Konishi et al. [2002] | fMRI | 16 | Dimensional change > no‐change | Modified WCST | 9 |
| Monchi et al. [2001] | fMRI | 11 | Shifting > control | WCST | 30 |
| Monchi et al. [2001] | fMRI | 11 | Shifting > no shifting | WCST | 9 |
| Monchi et al. [2004] | fMRI | 9 | Shifting > control | WCST | 17 |
| Monchi et al. [2004] | fMRI | 9 | Shifting > no shifting | WCST | 6 |
| Nagahama et al. [1997] | PET | 6 | MCST > number‐matching task | MCST | 26 |
| Nagahama et al. [2001] | fMRI | 6 | Set shift > no‐shift | Modified WCST | 14 |
| Nakahara et al. [2002] | fMRI | 10 | Shifting > maintenance | Modified WCST | 13 |
| Pollmann et al. [2000b] | fMRI | 10 | Dimension change > no change | Visual detection (odd one out) | 16 |
| Pollmann et al. [2000b] | fMRI | 10 | Cross‐dimensional change > control | Visual detection (odd one out) | 24 |
| Rogers et al. [2000] | PET | 12 | Extra‐ > interdimensional shift | ID/ED task | 4 |
| Rogers et al. [2000] | PET | 12 | Extradimensional shift > reversal | ID/ED task | 3 |
| Weidner et al. [2002] | fMRI | 10 | Cross‐dimension change > no‐changes | Visual detection (odd one out) | 16 |
| Zanolie et al. [2008a] | fMRI | 18 | Extradimensional shift > no‐shift | Modified ID/ED | 17 |
| Response switching | |||||
| Barber and Carter [2005] | fMRI | 13 | Switch > repeat (preparation phase) | S‐R incompatibility task | 1 |
| Barber and Carter [2005] | fMRI | 13 | Switch > repeat (target phase) | S‐R incompatibility task | 4 |
| Brass and von Cramon [2004] | fMRI | 14 | Meaning switch > cue switch | Digit discrimination | 3 |
| Crone et al. [2006] | fMRI | 20 | Bivalent switches > repetitions | Visual discrimination | 23 |
| Dove et al. [2000] | fMRI | 16 | Switch> repetition | Visual detection | 13 |
| Dreher et al. [2002] | fMRI | 8 | Switching > baseline (nonswitch) | Letter discrimination | 9 |
| Jancke et al. [2000] | fMRI | 6 | Switch > fixed task (repetitive) | Finger tapping | 3 |
| Jancke et al. [2000] | fMRI | 6 | Switch > fixed task (sequential) | Finger tapping | 6 |
| Luks et al. [2002] | fMRI | 11 | Informative cues > neutral cues | Digit discrimination | 4 |
| Luks et al. [2002] | fMRI | 11 | Neutrally cued switch > repeat targets | Digit discrimination | 2 |
| Luks et al. [2002] | fMRI | 11 | Switch > repeat targets | Digit discrimination | 2 |
| Nagahama et al. [2001] | fMRI | 6 | Response shift > no‐shift | Modified WCST | 13 |
| Parris et al. [2007] | fMRI | 22 | Flip > hold | Switch response rules | 20 |
| Pollmann et al. [2000a] | fMRI | 11 | Switch > basic task | Visual detection | 6 |
| Pollmann et al. [2006] | fMRI | 20 | Change > stay (response) | Visual detection (odd one out) | 15 |
| Ravizza and Carter [2008] | fMRI | 14 | Shift > repetition (response rule) | Visual detection (odd one out) | 4 |
| Rogers et al. [2000] | PET | 12 | Reversal > interdimensional shift | ID/ED task | 5 |
| Rogers et al. [2000] | PET | 12 | Reversal > extradimensional shift | ID/ED task | 3 |
| Rushworth et al. [2002] | fMRI | 10 | Switch > stay | Visual detection | 4 |
| Sohn et al. [2000] | fMRI | 12 | Repetition and switch x scan | Letter‐digit task | 4 |
| Zanolie et al. [2008b] | fMRI | 20 | Shift > no‐shift | Location rule switch | 12 |
| Perceptual switching | |||||
| Brass and von Cramon [2004] | fMRI | 14 | Cue switch > cue repetition | Digit discrimination | 4 |
| Crone et al. [2006] | fMRI | 20 | Univalent switches > repetitions | Visual discrimination | 2 |
| Gurd et al. [2002] | fMRI | 11 | Switching > no switching | Verbal fluency | 2 |
| Liston et al. [2006] | fMRI | 19 | Shift > repeat (color and motion) | Visual detection | 7 |
| Liston et al. [2006] | fMRI | 19 | Shift > repeat (color) | Visual detection | 2 |
| Liston et al. [2006] | fMRI | 19 | Shift > repeat (motion) | Visual detection | 2 |
| Pessoa et al. [2009] | fMRI | 20 | Switch > nonswitch | Visual detection | 8 |
| Pessoa et al. [2009] | fMRI | 20 | Switch > nonswitch (color cue) | Visual detection | 10 |
| Pessoa et al. [2009] | fMRI | 20 | Switch > nonswitch (pop‐out) | Visual detection | 2 |
| Pollmann et al. [2006] | fMRI | 20 | Dimension change > dimension stay | Visual detection (odd one out) | 5 |
| Ravizza and Carter [2008] | fMRI | 14 | Shift > repetition (perceptual) | Visual detection (odd one out) | 5 |
| Rogers et al. [2000] | PET | 12 | Interdimensional shift > reversal | ID/ED task | 3 |
| Rogers et al. [2000] | PET | 12 | Inter‐ > extra‐dimensional shift | ID/ED task | 6 |
| Rushworth et al. [2002] | fMRI | 8 | Switch > stay | Visual detection | |
| Serences et al. [2004] | fMRI | 15 | Switching > holding attention | Attention shift | 5 |
| Serences et al. [2004] | fMRI | 8 | Switching > holding attention | Attention shift | 2 |
| Vandenberghe et al. [2001] | fMRI | 11 | Shifting > maintaining | Spatial shift of attention | 4 |
| Weidner et al. [2002] | fMRI | 10 | Within‐dimension change > no‐changes | Visual detection (odd one out) | 1 |
| Wilkinson et al. [2001] | fMRI | 12 | Internal and external switch > no‐switch | Visual discrimination | 7 |
| Wylie et al. [2006] | fMRI | 13 | Color switch > color repeat (cues) | Visual detection | 11 |
| Wylie et al. [2006] | fMRI | 13 | Speed switch > speed repeat (cues) | Visual detection | 2 |
| Wylie et al. [2006] | fMRI | 13 | Color switch > color repeat (targets) | Visual detection | 19 |
| Wylie et al. [2006] | fMRI | 13 | Speed switch > speed repeat (targets) | Visual detection | 4 |
| Zanolie et al. [2008a] | fMRI | 18 | Intradimensional shift > no‐shift | Modified ID/ED | 4 |
Activation Likelihood Estimation Meta‐Analyses
For the meta‐analyses, we used GingerALE 1.2 in conducting activation likelihood estimation (ALE) analyses for the contrasts of interest [Laird et al.,2005; Turkeltaub et al.,2002]. The ALE method is a voxel‐based meta‐analysis technique used for a quantitative evaluation of the reliability of spatial patterns of activation foci across independently conducted neuroimaging studies. The method yields a statistical map that indicates the set of brain voxels that are more active than would be expected by chance alone, using a permutation‐testing framework to assess the extent to which the spatial location of activation foci are correlated across independently conducted studies. Each reported locus of maximal activation from each contrast was modeled as a center of a three‐dimensional Gaussian probability distribution with a full‐width half‐maximum (FWHM) of 10 mm. For studies reporting MNI coordinates, a conversion tool [Lancaster et al.,2007] was used to convert the coordinates into Talairach space. Every three‐dimensional Gaussian distribution from the set of studies contributed to create a statistical map to estimate the likelihood of activation in each voxel (2 mm3 in Talairach space). ALE maps were then thresholded at P < 0.05 using the false discovery rate (FDR) method to correct multiple comparisons. Clusters exceeding 120 mm3 (15 continuous voxels) were applied for all analyses. Thresholded ALE maps were overlaid onto the “colinbrain” Talairach template [Kochunov et al.,2002] using the MRIcron software (http://www.cabiatl.com/mricro/).
Four individual ALE analyses were performed: (1) all switch types (65 contrasts with 562 foci); (2) perceptual switching (24 contrasts with 119 foci); (3) response switching (21 contrasts with 156 foci); and (4) context switching (20 contrasts with 287 foci). The ALE analysis for all switch types included all of the above perceptual, response, and context switching foci. Details of these studies are shown in Table I. Additionally, three direct comparisons were performed. For the three direct comparisons, 118 foci were randomly selected per switch type in order to equate power. The direct comparisons were: (1) perceptual switching (118 foci) versus response (59 foci) and context switching (59 foci); (2) response switching (118 foci) versus perceptual (59 foci) and context switching (59 foci); and (3) context switching (118 foci) versus perceptual (59 foci) and response switching (59 foci). The 59 foci used in the direct comparisons were randomly selected from the total of 118 foci per switch type.
RESULTS
The results of the ALE analysis for all switching studies are shown in Figure 1. The results showed that switching was associated with a distributed network of brain regions including medial PFC (BAs 6 and 32), lateral PFC (BAs 6, 9, 46, and 10), and parietal (BAs 7 and 40), temporal (BA 37), and occipital cortices (BAs 18 and 19), as well as subcortical structures (caudate nucleus and thalamus).
Figure 1.
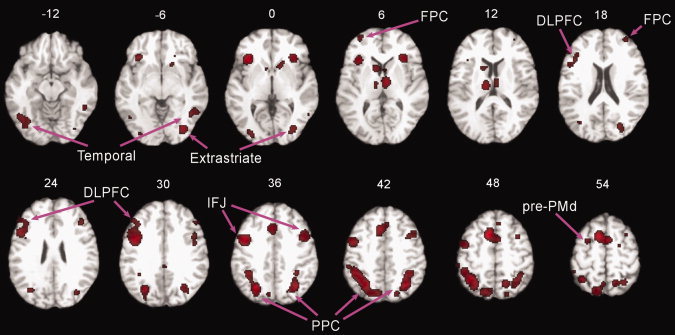
Activations associated with task switching contrasts collapsed across switch type. The ALE activation map thresholded at P < 0.05 (corrected) is overlaid onto the “colinbrain” template. Numbers indicate z‐coordinates of axial planes.
The results of the individual ALE analyses for perceptual, response, and context switching contrasts are presented in Figure 2A. The ALE analysis of perceptual switching revealed activation of a distributed set of brain regions. Within frontal cortex, perceptual switching activation was observed in a rostral portion of the dorsal premotor cortex (pre‐PMd; BA 6), anterior cingulate cortex (ACC)/pre‐supplementary motor area (pre‐SMA) (BA 32/6), inferior frontal junction (IFJ; BA 44/6/9). In addition, perceptual switching activations were observed in several posterior regions: PPC (BAs 7 and 40), and temporal and visual areas (BAs 37, 19, 18, and 17). The ALE analysis of response switching revealed a frontoparietal activation pattern. Specifically, we found activations in pre‐SMA/ACC (BAs 6 and 32), inferior and middle frontal gyri (BAs 44, 45, 46, 9, 10, and 47), IFJ (BA 44/6), PPC (BAs 7 and 40), and precuneus. The ALE analysis of context switching also revealed activation of a frontoparietal network. Activation was observed in diverse PFC regions, such as lateral and medial FPC (BA 10), IFJ (BAs 6/44), inferior and middle frontal gyri (BAs 8, 9, 46, and 47), pre‐SMA and ACC (BAs 6, 32, and 8). Additional activations were found in PPC, precuneus and cuneus (BAs 40, 7, and 19), and inferior temporal (BAs 19 and 37) and visual areas (BAs 18 and 19).
Figure 2.
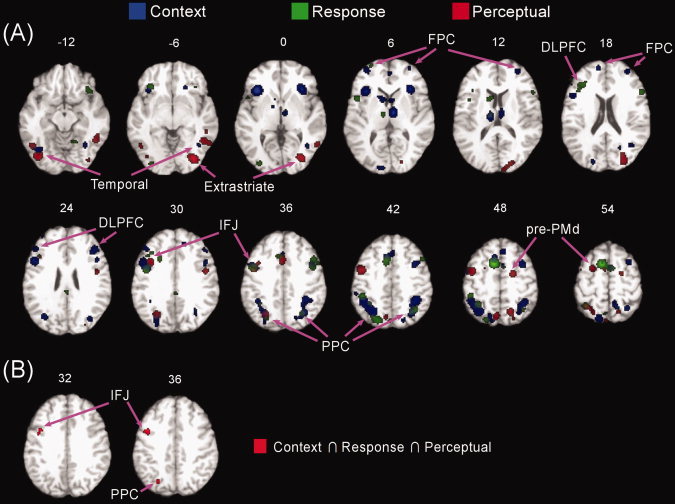
Activations associated with each switch type and their conjunction. The ALE activation maps of separate context, response, and perceptual switching contrasts compared with nonswitching overlaid on a common “colinbrain” template (A). The conjunction of context, response, and perceptual switching contrasts (B). Numbers indicate z‐coordinates of axial planes.
Using the above individual analyses, the conjunction map was calculated by taking the voxel‐wise intersection of three individual ALE maps in order to identify common areas activated by perceptual, response, and context switching contrasts. As shown in Figure 2B, the left IFJ (BA 6/44; centered at −40, 3, 33) and PPC (BA 7; centered at −25, −68, 36) were the only regions that showed overlap of significant voxels across perceptual, response, and context switching.
The next analyses focused on direct comparisons between three switching contrasts in order to identify regions preferentially activated by each switch type. The results of the direct comparisons between switch types are presented in Figure 3 and cluster details are listed in Tables II, III to IV. Preferential activation associated with perceptual switching was observed in caudal PFC regions, such as bilateral pre‐PMd (BA 6), left middle frontal gyrus (BA 6), precentral gyrus (BAs 6 and 9), medial frontal gyrus (BA 6) and cingulate gyrus (BA 32). Additionally, PPC (BA 7) and inferior temporal areas (BAs 19 and 37) were also preferentially activated by perceptual switching contrasts.
Figure 3.
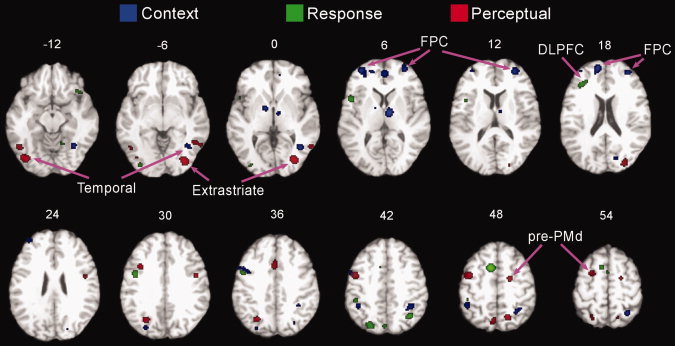
Domain‐preferential activations according to switch type. The ALE activation maps of three direct comparisons: (1) context switching versus response and perceptual switching; (2) response switching versus context and perceptual switching; (3) perceptual switching versus context and response switching. The activation maps thresholded at P < 0.05 (corrected) are overlaid onto the “colinbrain” template. Numbers indicate z‐coordinates of axial planes.
Table II.
Brain regions preferentially activated by perceptual switching compared to context and response switching
| Region | Hem | Talairach | BA | ALE | Volume (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Middle frontal gyrus | L | −40 | −2 | 48 | 6 | 0.0119 | 1,256 |
| Pre‐PMd | L | −22 | 2 | 54 | 6 | 0.0102 | 400 |
| Pre‐PMd | R | 20 | −6 | 50 | 6 | 0.0116 | 392 |
| Precentral gyrus | R | 46 | −2 | 28 | 6 | 0.0111 | 384 |
| Precentral gyrus | L | −36 | 10 | 30 | 9 | 0.0115 | 360 |
| Medial frontal gyrus | L | −8 | 0 | 60 | 6 | 0.0096 | 312 |
| Cingulate gyrus | L | 0 | 14 | 34 | 32 | 0.0106 | 512 |
| Precuneus | L | −26 | −66 | 32 | 7 | 0.0153 | 632 |
| Superior parietal lobule | L | −20 | −66 | 58 | 7 | 0.0090 | 496 |
| Precuneus | R | 16 | −62 | 50 | 7 | 0.0119 | 456 |
| Precuneus | L | −4 | −68 | 48 | 7 | 0.0097 | 384 |
| Fusiform gyrus | L | −38 | −78 | −12 | 19 | 0.0138 | 912 |
| Fusiform gyrus | R | 48 | −54 | −8 | 37 | 0.0104 | 640 |
| Inferior temporal gyrus | R | 56 | −58 | −2 | 37 | 0.0096 | SC |
| Fusiform gyrus | L | −46 | −62 | −10 | 37 | 0.0092 | 624 |
| Inferior occipital gyrus | R | 32 | −80 | −2 | 18 | 0.0165 | 1,320 |
| Middle occipital gyrus | R | 30 | −84 | 20 | 19 | 0.0103 | 496 |
Hem, hemisphere; BA, Brodmann area; ALE, activation likelihood estimation; pre‐PMd, the rostral portion of the dorsal premotor cortex; SC, same cluster.
Table III.
Brain regions preferentially activated by response switching compared to context and perceptual switching
| Region | Hem | Talairach | BA | ALE | Volume (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Superior frontal gyrus | L | −8 | 10 | 48 | 6 | 0.0192 | 1,064 |
| Precentral gyrus | L | −42 | 0 | 30 | 6 | 0.0101 | 640 |
| Precentral gyrus | L | −48 | 10 | 4 | 44 | 0.0111 | 568 |
| Middle frontal gyrus (DLPFC) | L | −34 | 30 | 18 | 46 | 0.0097 | 456 |
| Medial frontal gyrus | R | 2 | 2 | 58 | 6 | 0.0087 | 216 |
| Inferior frontal gyrus | R | 38 | 20 | −12 | 47 | 0.0077 | 144 |
| Inferior frontal gyrus | R | 42 | 18 | −10 | 47 | 0.0077 | SC |
| Precuneus | L | −22 | −74 | 44 | 7 | 0.0122 | 624 |
| Inferior parietal lobule | R | 36 | −60 | 44 | 7 | 0.0113 | 368 |
| Inferior parietal lobule | L | −44 | −38 | 42 | 40 | 0.0090 | 232 |
| Precuneus | L | −2 | −76 | 40 | 7 | 0.0086 | 184 |
| Inferior occipital gyrus | L | −32 | −86 | −4 | 18 | 0.0096 | 248 |
| Cerebellum (declive) | R | 16 | −56 | −14 | 0.0079 | 152 | |
Hem, hemisphere; BA, Brodmann area; ALE, activation likelihood estimation; DLPFC, dorsolateral prefrontal cortex; SC, same cluster.
Table IV.
Brain regions preferentially activated by context switching compared to perceptual and response switching
| Region | Hem | Talairach | BA | ALE | Volume (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Middle frontal gyrus (lateral FPC) | R | 32 | 48 | 16 | 10 | 0.0125 | 1,280 |
| Middle frontal gyrus (lateral FPC) | L | −32 | 50 | 8 | 10 | 0.0124 | 680 |
| Medial frontal gyrus (medial FPC) | L | −10 | 54 | 18 | 10 | 0.0113 | 600 |
| Anterior cingulate | R | 2 | 46 | 6 | 32 | 0.0107 | 584 |
| Superior frontal gyrus (lateral FPC) | L | −34 | 50 | 22 | 10 | 0.0096 | 368 |
| Precentral gyrus | L | −40 | 4 | 36 | 9 | 0.0079 | 320 |
| Middle frontal gyrus | L | −54 | 10 | 36 | 9 | 0.0072 | SC |
| Medial frontal gyrus (medial FPC) | L | −12 | 48 | 4 | 10 | 0.0077 | 176 |
| Middle frontal gyrus | L | −50 | 2 | 42 | 6 | 0.0081 | 152 |
| Superior parietal lobule | R | 30 | −56 | 52 | 7 | 0.0113 | 1,176 |
| Inferior parietal lobule | R | 36 | −46 | 40 | 40 | 0.0095 | SC |
| Inferior parietal lobule | L | −42 | −46 | 46 | 40 | 0.0105 | 624 |
| Precuneus | R | 28 | −70 | 38 | 19 | 0.0079 | 120 |
| Cuneus | L | −28 | −78 | 32 | 19 | 0.0104 | 280 |
| Cuneus | R | 18 | −78 | 20 | 18 | 0.0077 | 152 |
| Inferior temporal gyrus | R | 40 | −62 | −2 | 37 | 0.0116 | 1,024 |
| Fusiform gyrus | R | 32 | −58 | −12 | 37 | 0.0105 | SC |
| Thalamus | R | 8 | −10 | 6 | 0.0156 | 1,016 | |
| Lentiform nucleus | L | −12 | −4 | 2 | 0.0110 | 320 | |
Hem, hemisphere; BA, Brodmann area; ALE, activation likelihood estimation; FPC, frontopolar cortex; SC, same cluster.
Preferential activation associated with response switching was observed in a left mid‐PFC region centered on DLPFC (BA 46). Other PFC regions, such as left superior frontal gyrus (BA 6), left precentral gyrus (BAs 6 and 44), right inferior frontal gyrus (BA 47), and medial frontal gyrus (BA 6), and bilateral PPC (BAs 7 and 40) regions were also activated by response switching.
Finally, preferential activation associated with context switching was observed in rostral PFC regions, such as lateral and medial FPC (BA 10) and ACC (BA 32). Context switching was also associated with activations in middle frontal gyrus (BAs 6 and 9), PPC (BA 7 and 40), cuneus (BAs 18 and 19), inferior temporal areas (BA 37), visual areas (BAs 18 and 19), and subcortical structures (thalamus and lentiform nucleus).
DISCUSSION
The current meta‐analysis sought to distinguish brain regions within the distributed frontoparietal task switching network that contribute to switching in a domain‐general manner from those that may be more specialized for different kinds of switching. Three switch types were explored which have been frequently used in the task switching literature (perceptual, response, and context). The results of the ALE meta‐analysis that collapsed across all (perceptual, response, and contextual) switch types revealed a distributed frontoparietal network associated with switching. These results are consistent with those from previous meta‐analysis studies that have collapsed across distinct switch types [Buchsbaum et al.,2005; Derrfuss et al.,2005; Wager et al.,2004].
However, results from our meta‐analyses also provide new evidence related to the on‐going debate about the degrees of domain‐specificity of brain regions within the distributed frontoparietal task switching network. We observed three main patterns in our meta‐analyses: (1) regions exhibiting domain general patterns across all switching tasks (i.e., putative domain‐general switch regions); (2) regions showing a pattern of clear domain preferentiality/specificity according to switch type (i.e., putative domain‐preferential switch regions); and (3) regions showing an activation pattern that was preferential, but not selective, for one switch type. Below we discuss each of these three main patterns and their contribution to understanding the neural bases of cognitive control processes involved in task switching.
Domain‐General Switching Mechanisms Within the Frontoparietal Network
Within the distributed network of frontoparietal brain regions associated with task switching, there were two regions, the inferior frontal junction (IFJ) and posterior parietal cortex (PPC), which were found to contribute to each of the three (perceptual, response, and context) forms of switching explored in our meta‐analyses. The IFJ is a posterior lateral region of frontal cortex near the junction of the inferior frontal sulcus and the inferior precentral sulcus (∼BA 44/6/9) [Derrfuss et al.,2004]. The PPC comprises a wide expanse of parietal cortex (∼BA 40/7) including much of the inferior and superior parietal lobules. The observation that a circumscribed set of regions (IFJ and PPC) from within the widespread frontoparietal switching network were involved in all three forms of switching suggests that these regions contribute to cognitive processes common to attention shifting in a domain‐general manner. Two cognitive processes which are thought to contribute to all forms of switching are representing and updating task sets [Miyake et al.,2000], making IFJ and PPC potential contributors to these domain‐general switch processes.
If IFJ and PPC contribute to representing and updating task sets during task switching, then two expectations should follow. The first expectation is that there should be existing evidence supporting a role for IFJ and PPC in representing and updating task sets from previous studies. There is support for this expectation. Specifically, previous functional neuroimaging studies have suggested that IFJ appears to be involved in updating representations of task rules or sets across a range of cognitive control tasks [Brass and von Cramon,2004; Derrfuss et al.,2004,2005; Roth and Courtney,2007; Roth et al.,2006,2009]. Analogously, a body of evidence has demonstrated a role for PPC in representing task sets [Bunge et al.,2002,2003; Cavina‐Pratesi et al.,2006]. For example, PPC has shown sustained neural activity during a delay period before the stimulus presentation [Cavina‐Pratesi et al.,2006] and has shown increased brain activity when comparing two stimulus‐response alternatives with a single stimulus‐response mapping [Bunge et al.,2003].
The intimate relationship between the cognitive processes of representing and updating task sets suggests a coordinated role of regions supporting these processes (i.e., IFJ and PPC). Thus, if IFJ and PPC contribute to the highly related cognitive processes of representing and updating task sets relevant to task switching, we would expect that task switching performance would be influenced by the strength of anatomical connectivity between these regions. There is support for this expectation. First, results from diffusion tensor imaging (DTI) tractography studies have demonstrated that portions of the IFJ (∼BA 44/6) and PPC (∼BA 40/7) are anatomically connected via the superior longitudinal fasciculus (SLF) [Catani et al.,2005; Makris et al.,2005]. Secondly, we recently found that the strength of anatomical connectivity (assessed via the DTI metric of fractional anisotropy) along the SLF tract is negatively correlated with switch cost RT in both young and older groups [Gold et al.,2010]. This finding suggests that faster task switching is associated with ‘more direct’ information flow between IFJ and PPC, in keeping with a view that these regions play a coordinated role during task switching.
Dorsal Premotor Cortex Shows Domain‐Preferentiality for Perceptual Switching
Our meta‐analyses found that pre‐PMd was preferentially recruited for perceptual switching. The pre‐PMd region comprises a rostral portion of premotor cortex (BA 6), which is situated anterior to motor cortex (M1; BA 4). However, pre‐PMd is more heavily interconnected with PFC than M1 [Barbas and Pandya,1987]. In accordance with this anatomical feature, recent evidence suggests that pre‐PMd is involved in nonmotoric cognitive processes in addition to motor planning while caudal premotor cortex (i.e., premotor proper) is engaged in actual motor execution [Abe and Hanakawa,2009]. Data from a body of lesion studies in the monkey and humans suggest that pre‐PMd's role in stimulus‐motor relationships relates to learning perceptually‐driven, arbitrary stimulus associations as opposed to motor output [Abe et al.,2007; Amiez et al.,2006; Hanakawa et al.,2002; Hopfinger et al.,2000; Petrides,2005]. Based on such evidence, an emerging theory is that pre‐PMd is involved in learning and applying rule‐based associations between perceptual features of stimuli and responses [Badre and D'Esposito,2009].
The pre‐PMd activation associated with perceptual switching was close (particularly in the left hemisphere) to the frontal eye fields (FEF; BA 8). The FEF have a well‐established role in saccadic eye movements [Keating,1991]. However, there is accumulating evidence that FEF are involved in attention and working memory processes beyond saccadic eye movements [Awh et al.,2006]. Of particular relevance, a recent study demonstrated that FEF activity persists when maintaining auditory‐cued space, even for locations behind the head to which it is impossible to make saccades [Tark and Curtis,2009]. These data suggest that FEF contributes to high‐level working memory processes which broadly support spatial attention shifts, even in the absence of saccades.
However, it has been unclear from individual functional neuroimaging studies of task switching whether pre‐PMd (or FEF) is involved in perceptual switching per se. There are likely several reasons for this knowledge gap. First, some functional neuroimaging studies used passive control conditions (i.e., fixation or rest) or control conditions which had perceptual and motor demands that were much simpler than those of the switching condition. Second, some studies reporting pre‐PMd activation have utilized multidimensional perceptual‐and‐response switching tasks (such as the color/shape task), making it difficult to tease apart pre‐PMd's contribution. Finally, pre‐PMd activation in any one particular imaging study could be idiosyncratic to one group of subjects or switch task.
In the present meta‐analyses, all contrasts compared conditions which required similar perceptual and motor requirements (i.e., switch vs. nonswitch trials but not switch vs. passive fixation or rest baselines). In addition, care was taken in the present meta‐analysis to insure that perceptual switching studies did not include response switching. Finally, because our meta‐analyses included multiple samples of participants, and multiple kinds of perceptual switching tasks, it is likely to be less affected by statistical idosyncrasies than individual studies. Thus, results from the present meta‐analysis suggest that pre‐PMd (and possibly FEF) is actively involved in the switching processes in a domain‐preferential manner: it appears to contribute to switching attention between perceptual features of stimuli (e.g., shape and direction) as opposed to switching between response mappings or contextual rules.
Frontopolar Cortex Shows Domain‐Preferentiality for Context Switching
The present meta‐analysis revealed that context switching preferentially activated lateral and medial regions of FPC (BA 10). The context switching studies classified here emphasized shifting between cognitive sets or rules. Unlike perceptual or response switches, context switches are not triggered by a direct cue‐task association. Context switches thus emphasize endogenous control processes associated with the internal generation and maintenance of task sets [Dreher et al.,2002]. The present finding of preferential FPC activation during context switching is consistent with a body of work linking FPC with the internal generation of high‐level cognitive representations. For example, FPC has been associated with planning [Koechlin et al.,1999,2000; van den Heuvel et al.,2003] envisioning/predicting future events [Okuda et al.,2003; Partiot et al.,1995], reasoning [Christoff et al.,2001; Green et al.,2006; Kroger et al.,2002], maintaining rules guiding subsequent cognitive activity [Sakai and Passingham,2006], and endogenous set switching [Rogers et al.,2000; Weidner et al.,2002].
In the present meta‐analysis, FPC activation was specific to context switching. This region was not activated during perceptual switching or response switching (the latter is discussed below). The preferential response of FPC to context switching suggests that it, like pre‐PMd, constitutes a region of the brain that is sensitive to the type of switch performed as opposed to IFJ and PPC, which appear to contribute to switching in a more domain‐general manner. The dissociation between FPC and pre‐PMd according to switch type demonstrates that switching is not controlled by a single set of brain regions. Instead, our results demonstrate that multiple brain regions are capable of guiding switches, with regional specialization depending upon the kind of switching being performed.
Dorsolateral Prefrontal Cortex Contributes to Both Response and Context Switching
Results from the direct comparison showed that DLPFC was activated most strongly by response switching. Also, results from individual ALE analyses found DLPFC activation during response switching but not perceptual switching. This finding is consistent with several recent studies which have directly contrasted perceptual‐based and response‐based cognitive control processes. For example, Ravizza and Carter [2008] found greater DLPFC activity for response switching than for perceptual switching and greater pre‐PMd activity for perceptual than response switching. Similarly, in our recent work using a modified version of the Stroop task, DLPFC was activated during response conflict but not perceptual conflict [Kim et al.,2010a; Kim et al., 2010b].
However, in the present meta‐analyses, DLPFC recruitment was not specific to response switching. Instead, DLPFC was also activated during context switching. One possible reason for the activation DLPFC during both response and context switching, but not perceptual switching, is that the former two switch types likely place greater emphasis on the maintenance and manipulation of information within working memory, processes which have been linked with the DLPFC [e.g., D'Esposito et al.,1998,1999]. A different possibility is that only specific portions of the DLPFC are preferential for response switching. For example, the peak DLPFC activation we observed for context switching was dorsal and anterior to that observed for response switching (see Figs. 2 and 3). These different possibilities should be contrasted in future studies which include both response and context switching tasks and explicitly manipulate levels of working memory within these tasks.
An Anterior‐Posterior PFC Gradient Associated With Endogenous Switching Processes
Overall, the present results suggest an anterior‐to‐posterior gradient within PFC for task switching, according to the degree of endogenous control processes. The most anterior activations were observed for context switches, which are not triggered by a direct cue‐task association. Context switches thus emphasize endogenous control processes associated with the internal generation and maintenance of task sets [Dreher et al.,2002]. In contrast, the most posterior activations were observed for perceptual switching. Perceptual switches can be viewed as relatively low in the need for endogenous control processes because they are driven by external cues that directly indicate the upcoming task [Logan and Bundesen,2003]. Perceptual switches thus minimize the need for endogenous control processes because of the facilitative contribution of the cue to the retrieval and maintenance of task set [Koch,2003]. The anterior‐to‐posterior task switching gradient we observed according to the level of endogenous control is consistent with evidence for a similar gradient across several other cognitive domains [Badre and D'Esposito,2007].
The present meta‐analysis had several caveats that highlight open questions and may help guide future research. First, as with all meta‐analyses, results will need to be tested in functional neuroimaging studies that compare activation patterns of each switch type in the same group of participants. Second, our analyses were restricted to only three kinds of task switching. The switch types were chosen because they have been used frequently in the task switching literature. However, there are other forms of task switching that were not explored in the present meta‐analysis, some of which may be associated with different neural correlates than those reported here. For example, Wager et al. [2004] reported that the spatial distribution of peak activation clusters within the parietal lobe differs between location switching and object attribute switching. The fact that our meta‐analyses did not explore attention shifts associated with spatial location may be one reason why no differences between switch types were observed in the parietal lobes.
CONCLUSION
The current meta‐analysis provides evidence concerning the degrees of domain‐preferentiality of frontoparietal brain regions during task switching. We found that IFJ and PPC were commonly activated by perceptual, response, and context switching tasks, suggesting that these regions contribute to domain‐general task switching processes (such as task representation and updating). Results also indicated more domain‐preferential roles for two PFC regions: pre‐PMd was preferentially associated with perceptual switching, and FPC was preferentially associated with context switching. These findings suggest that switching is not controlled by a single set of brain regions. Instead, multiple brain regions appear to be capable of guiding task switches, depending upon the type of switch being performed. Consequently, our findings suggest that switching is not a unitary process and that future cognitive control studies employing task switching paradigms should consider the type of switch being performed. In addition, our results provide regions of interest that can be used in future functional neuroimaging studies to explore specific hypotheses about the functional neuroanatomy of task switching.
Acknowledgements
The authors thank three anonymous reviewers for helpful comments on an earlier version of the manuscript.
REFERENCES
- Abe M, Hanakawa T ( 2009): Functional coupling underlying motor and cognitive functions of the dorsal premotor cortex. Behav Brain Res 198: 13–23. [DOI] [PubMed] [Google Scholar]
- Abe M, Hanakawa T, Takayama Y, Kuroki C, Ogawa S, Fukuyama H ( 2007): Functional coupling of human prefrontal and premotor areas during cognitive manipulation. J Neurosci 27: 3429–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Kostopoulos P, Champod AS, Petrides M ( 2006): Local morphology predicts functional organization of the dorsal premotor region in the human brain. J Neurosci 26: 2724–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Armstrong KM, Moore T ( 2006): Visual and oculomotor selection: Links, causes and implications for spatial attention. Trends Cogn Sci 10: 124–130. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M ( 2009): Is the rostro‐caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci 10: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD ( 2006): Computational and neurobiological mechanisms underlying cognitive flexibility. Proc Natl Acad Sci USA 103: 7186–7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Pandya DN ( 1987): Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol 256: 211–228. [DOI] [PubMed] [Google Scholar]
- Barber AD, Carter CS ( 2005): Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cereb Cortex 15: 899–912. [DOI] [PubMed] [Google Scholar]
- Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, Carson RE, Herscovitch P, Weinberger DR ( 1995): Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: A positron emission tomography study. Neuropsychologia 33: 1027–1046. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY ( 2004): Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci 16: 609–620. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI ( 2003): Neural mechanisms of transient and sustained cognitive control during task switching. Neuron 39: 713–726. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF ( 2005): Meta‐analysis of neuroimaging studies of the Wisconsin card‐sorting task and component processes. Hum Brain Mapp 25: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD ( 2002): Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage 17: 1562–1571. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD ( 2003): Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol 90: 3419–3428. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH ( 2005): Perisylvian language networks of the human brain. Ann Neurol 57: 8–16. [DOI] [PubMed] [Google Scholar]
- Cavina‐Pratesi C, Valyear KF, Culham JC, Kohler S, Obhi SS, Marzi CA, Goodale MA ( 2006): Dissociating arbitrary stimulus‐response mapping from movement planning during preparatory period: Evidence from event‐related functional magnetic resonance imaging. J Neurosci 26: 2704–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD ( 2001): Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage 14: 1136–1149. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA ( 2006): Neural evidence for dissociable components of task‐switching. Cereb Cortex 16: 475–86. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J ( 1998): Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res 7: 1–13. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J ( 1999): Maintenance versus manipulation of information held in working memory: An event‐related fMRI study. Brain Cogn 41: 66–86. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY ( 2005): Involvement of the inferior frontal junction in cognitive control: Meta‐analyses of switching and Stroop studies. Hum Brain Mapp 25: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY ( 2004): Cognitive control in the posterior frontolateral cortex: Evidence from common activations in task coordination, interference control, and working memory. Neuroimage 23: 604–612. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY ( 2000): Prefrontal cortex activation in task switching: An event‐related fMRI study. Brain Res Cogn Brain Res 9: 103–109. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Koechlin E, Ali SO, Grafman J ( 2002): The roles of timing and task order during task switching. Neuroimage 17: 95–109. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD ( 2010): Age‐related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging 31: 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Berman KF, Fleming K, Ostrem J, Van Horn JD, Esposito G, Mattay VS, Gold JM, Weinberger DR ( 1998): Uncoupling cognitive workload and prefrontal cortical physiology: A PET rCBF study. Neuroimage 7: 296–303. [DOI] [PubMed] [Google Scholar]
- Graham S, Phua E, Soon CS, Oh T, Au C, Shuter B, Wang SC, Yeh IB ( 2009): Role of medial cortical, hippocampal and striatal interactions during cognitive set‐shifting. Neuroimage 45: 1359–1367. [DOI] [PubMed] [Google Scholar]
- Green AE, Fugelsang JA, Kraemer DJ, Shamosh NA, Dunbar KN ( 2006): Frontopolar cortex mediates abstract integration in analogy. Brain Res 1096: 125–137. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Amunts K, Weiss PH, Zafiris O, Zilles K, Marshall JC, Fink GR ( 2002): Posterior parietal cortex is implicated in continuous switching between verbal fluency tasks: An fMRI study with clinical implications. Brain 125: 1024–1038. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Sawamoto N, Okada T, Yonekura Y, Fukuyama H, Shibasaki H ( 2002): The role of Rostral Brodmann area 6 in mental‐operation tasks: An integrative neuroimaging approach. Cereb Cortex 12: 1157–1170. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR ( 2000): The neural mechanisms of top‐down attentional control. Nat Neurosci 3: 284–291. [DOI] [PubMed] [Google Scholar]
- Jancke L, Himmelbach M, Shah NJ, Zilles K ( 2000): The effect of switching between sequential and repetitive movements on cortical activation. Neuroimage 12: 528–537. [DOI] [PubMed] [Google Scholar]
- Jersild AT ( 1927): Mental set and shift. Arch Psychol 89: 5–82. [Google Scholar]
- Keating EG ( 1991): Frontal eye field lesions impair predictive and visually‐guided pursuit eye movements. Exp Brain Res 86: 311–323. [DOI] [PubMed] [Google Scholar]
- Kim C, Chung C, Kim J ( 2010a): Multiple cognitive control mechanisms associated with the nature of conflict. Neurosci Lett 496: 156–160. [DOI] [PubMed] [Google Scholar]
- Kim C, Kroger JK, Kim J ( 2010b): A functional dissociation of conflict processing within anterior cingulate cortex. Hum Brain Mapp:n/a‐n/a. doi: 10.1002/hbm.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch I ( 2003): The role of external cues for endogenous advance reconfiguration in task switching. Psychon Bull Rev 10: 488–492. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Lancaster J, Thompson P, Toga AW, Brewer P, Hardies J, Fox P ( 2002): An optimized individual target brain in the Talairach coordinate system. Neuroimage 17: 922–927. [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J ( 1999): The role of the anterior prefrontal cortex in human cognition. Nature 399: 148–151. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Corrado G, Pietrini P, Grafman J ( 2000): Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proc Natl Acad Sci USA 97: 7651–7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Hayashi T, Uchida I, Kikyo H, Takahashi E, Miyashita Y ( 2002): Hemispheric asymmetry in human lateral prefrontal cortex during cognitive set shifting. Proc Natl Acad Sci USA 99: 7803–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Gopher D ( 1999): Task coordination and aging: Explorations of executive control processes in the task switching paradigm. Acta Psychol (Amst) 101: 339–378. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ ( 2002): Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: A parametric study of relational complexity. Cereb Cortex 12: 477–485. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT ( 2005): ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT ( 2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28: 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ ( 2006): Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task‐switching paradigm. Neuron 50: 643–653. [DOI] [PubMed] [Google Scholar]
- Logan GD, Bundesen C ( 2003): Clever homunculus: is there an endogenous act of control in the explicit task‐cuing procedure? J Exp Psychol Hum Percept Perform 29: 575–599. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Feiwell RJ, Miller WL ( 2002): Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. Neuroimage 17: 792–802. [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, Pandya DN ( 2005): Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 15: 854–869. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD ( 2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD ( 2000): The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cogn Psychol 41: 49–100. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A ( 2004): Neural bases of set‐shifting deficits in Parkinson's disease. J Neurosci 24: 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A ( 2001): Wisconsin Card Sorting revisited: Distinct neural circuits participating in different stages of the task identified by event‐related functional magnetic resonance imaging. J Neurosci 21: 7733–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S ( 2003): Task switching. Trends Cogn Sci 7: 134–140. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Fukuyama H, Yamauchi H, Katsumi Y, Magata Y, Shibasaki H, Kimura J ( 1997): Age‐related changes in cerebral blood flow activation during a Card Sorting Test. Exp Brain Res 114: 571–577. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Konishi J, Fukuyama H, Shibasaki H ( 2001): Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb Cortex 11: 85–92. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Hayashi T, Konishi S, Miyashita Y ( 2002): Functional MRI of macaque monkeys performing a cognitive set‐shifting task. Science 295: 1532–1536. [DOI] [PubMed] [Google Scholar]
- Nelson HE ( 1976): A modified card sorting test sensitive to frontal lobe defects. Cortex 12: 313–324. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A ( 2003): Thinking of the future and past: The roles of the frontal pole and the medial temporal lobes. Neuroimage 19: 1369–1380. [DOI] [PubMed] [Google Scholar]
- Parris BA, Thai NJ, Benattayallah A, Summers IR, Hodgson TL ( 2007): The role of the lateral prefrontal cortex and anterior cingulate in stimulus‐response association reversals. J Cogn Neurosci 19: 13–24. [DOI] [PubMed] [Google Scholar]
- Partiot A, Grafman J, Sadato N, Wachs J, Hallett M ( 1995): Brain activation during the generation of non‐emotional and emotional plans. Neuroreport 6: 1397–1400. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Rossi A, Japee S, Desimone R, Ungerleider LG ( 2009): Attentional control during the transient updating of cue information. Brain Res 1247: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M ( 2005): Lateral prefrontal cortex: Architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci 360: 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann S, Dove A, Yves von Cramon D, Wiggins CJ ( 2000a) Event‐related fMRI: Comparison of conditions with varying BOLD overlap. Hum Brain Mapp 9: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann S, Weidner R, Muller HJ, Maertens M, von Cramon DY ( 2006): Selective and interactive neural correlates of visual dimension changes and response changes. Neuroimage 30: 254–265. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Weidner R, Muller HJ, von Cramon DY ( 2000b) A fronto‐posterior network involved in visual dimension changes. J Cogn Neurosci 12: 480–494. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Carter CS ( 2008): Shifting set about task switching: Behavioral and neural evidence for distinct forms of cognitive flexibility. Neuropsychologia 46: 2924–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW ( 2000): Contrasting cortical and subcortical activations produced by attentional‐set shifting and reversal learning in humans. J Cogn Neurosci 12: 142–162. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S ( 1995): Costs of a predictible switch between simple cognitive tasks. J Exp Psychol Gen 124: 207–231. [Google Scholar]
- Roth JK, Courtney SM ( 2007): Neural system for updating object working memory from different sources: Sensory stimuli or long‐term memory. Neuroimage 38: 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JK, Johnson MK, Raye CL, Constable RT ( 2009): Similar and dissociable mechanisms for attention to internal versus external information. Neuroimage 48: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JK, Serences JT, Courtney SM ( 2006): Neural system for controlling the contents of object working memory in humans. Cereb Cortex 16: 1595–1603. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK ( 2002): Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. J Neurophysiol 87: 2577–2592. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE ( 2006): Prefrontal set activity predicts rule‐specific neural processing during subsequent cognitive performance. J Neurosci 26: 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Schwarzbach J, Courtney SM, Golay X, Yantis S ( 2004): Control of object‐based attention in human cortex. Cereb Cortex 14: 1346–1357. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS ( 2000): Inaugural article: The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 97: 13448–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D ( 2000): Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: Effects of lesion location and test structure on separable cognitive processes. Neuropsychologia 38: 388–402. [DOI] [PubMed] [Google Scholar]
- Tark KJ, Curtis CE ( 2009): Persistent neural activity in the human frontal cortex when maintaining space that is off the map. Nat Neurosci 12: 1463–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA ( 2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. Neuroimage 16: 765–780. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Groenewegen HJ, Barkhof F, Lazeron RH, van Dyck R, Veltman DJ ( 2003): Frontostriatal system in planning complexity: A parametric functional magnetic resonance version of Tower of London task. Neuroimage 18: 367–374. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Gitelman DR, Parrish TB, Mesulam MM ( 2001): Functional specificity of superior parietal mediation of spatial shifting. Neuroimage 14: 661–673. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S ( 2004): Neuroimaging studies of shifting attention: A meta‐analysis. Neuroimage 22: 1679–1693. [DOI] [PubMed] [Google Scholar]
- Weidner R, Pollmann S, Muller HJ, von Cramon DY ( 2002): Top‐down controlled visual dimension weighting: An event‐related fMRI study. Cereb Cortex 12: 318–328. [DOI] [PubMed] [Google Scholar]
- Wilkinson DT, Halligan PW, Marshall JC, Buchel C, Dolan RJ ( 2001): Switching between the forest and the trees: Brain systems involved in local/global changed‐level judgments. Neuroimage 13: 56–67. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Javitt DC, Foxe JJ ( 2006): Jumping the gun: Is effective preparation contingent upon anticipatory activation in task‐relevant neural circuitry? Cereb Cortex 16: 394–404. [DOI] [PubMed] [Google Scholar]
- Zanolie K, Teng S, Donohue SE, van Duijvenvoorde AC, Band GP, Rombouts SA, Crone EA ( 2008a) Switching between colors and shapes on the basis of positive and negative feedback: An fMRI and EEG study on feedback‐based learning. Cortex 44: 537–547. [DOI] [PubMed] [Google Scholar]
- Zanolie K, Van Leijenhorst L, Rombouts SA, Crone EA ( 2008b) Separable neural mechanisms contribute to feedback processing in a rule‐learning task. Neuropsychologia 46: 117–126. [DOI] [PubMed] [Google Scholar]


