Abstract
Background
The vasoactive peptide bradykinin (BK) acts as a potent growth factor for normal kidney cells, but there have been few studies on the role of BK in renal cell carcinomas.
Purpose
In this study, we tested the hypothesis that BK also acts as a mitogen in kidney carcinomas, and explored the effects of BK in human renal carcinoma A498 cells.
Methods
The presence of mRNAs for BK B1 and BK B2 receptors in A498 cells was demonstrated by reverse transcription–polymerase chain reaction. To study BK signaling pathways, we employed fluorescent measurements of intracellular Ca2+, measured changes in extracellular pH as a reflection of Na+/H+ exchange (NHE) with a Cytosensor microphysiometer, and assessed extracellular signal-regulated kinase (ERK) activation by Western blotting.
Results
Exposure to 100 nM of BK resulted in the rapid elevation of intracellular Ca2+, caused a ≥30% increase in NHE activity, and a ≥300% increase in ERK phosphorylation. All BK signals were blocked by HOE140, a BK B2 receptor antagonist, but not by a B1 receptor antagonist. Inhibitor studies suggest that BK-induced ERK activation requires phospholipase C and protein kinase C activities, and is Ca2+/calmodulin-dependent. The amiloride analog 5-(N-methyl-N-isobutyl)-amiloride (MIA) blocked short-term NHE activation and inhibited ERK phosphorylation, suggesting that NHE is critical for ERK activation by BK. BK induced an approximately 40% increase in the proliferation of A498 cells as assessed by bromodeoxyuridine uptake. This effect was blocked by the ERK inhibitor PD98059, and was dependent on NHE activity.
Conclusion
We conclude that BK exerts mitogenic effects in A498 cells via the BK B2 receptor activation of growth-associated NHE and ERK.
Keywords: A498 cells, G protein-coupled receptors, signal transduction, Na+/H+ exchange, extracellular signal-regulated protein kinase
Introduction
Renal cell carcinoma is the most common cancer of the kidney and is one of the most resistant to systemic therapy. Therefore, until recently, surgery was the only medical treatment for patients with limited metastatic disease.1 During the past 3 years, a better understanding of the molecular biology underlying metastatic renal cell carcinoma has led to the development of new therapeutic techniques. Several new drugs that target the vascular endothelial growth factor, its receptor, and the mammalian target of rapamycin have been shown to provide a clinical benefit in the treatment of advanced or metastatic renal cell carcinoma, but a significant improvement in survival has not yet been convincingly demonstrated across all clinical trials.2 Further studies of the specific signaling pathways that lead to the proliferation of renal carcinoma cells will increase the understanding of the biology of this tumor, and will help to develop new ways for the targeted inhibition of malignant cell proliferation, ultimately leading to more effective therapies.
The endogenous intrarenal kinin hormone bradykinin (BK) plays a significant role as a modulator of renal functions such as electrolyte and water excretion,3 as well as acting as a vasodilator.4 In addition to its vasoactive properties, BK plays a role in renal cell growth and proliferation.5,6 BK exerts its multiple pathophysiological functions via two known receptors, the bradykinin B1 (BK B1) and bradykinin B2 (BK B2) receptors, which belong to the superfamily of G protein-coupled receptors (GPCR).7,8 We have reported previously that BK is a potent mitogenic factor for a kidney cell line derived from the inner medullary collecting duct of mice (mIMCD-3 cells), and demonstrated that BK-induced cell proliferation was dependent on the activation of the epidermal growth factor receptor (EGFR) tyrosine kinase and the subsequent activation of mitogen- and extracellular signal-regulated kinase (MEK), and on extracellular signal-regulated protein kinase (ERK).9–11 There is evidence linking the mitogenic kinin peptides to the carcinogenic process.12 The histological presence of BK receptors has been demonstrated in clinical specimens of adenocarcinoma, squamous carcinoma, lymphoma, hepatoma, and carcinoid, and in experimental mouse sarcoma 180 and colon adenocarcinoma 38.13 In esophageal carcinomas, the expression of BK B1 and BK B2 receptors was upregulated in activated mast cells and giant tumor cells.14 The BK receptors were also highly expressed in small cell and non-small cell carcinomas of the lung.15 The mitogenic effects of BK have also been reported in primary cultured epithelial breast cancer cells, where BK-stimulated cell proliferation through ERK activation takes place.16 BK also exerted mitogenic effects in the MCF-7 breast cancer cell line, suggesting that BK has an important role in cancer endorsement and progression.17 In addition, the BK antagonist CU201 has been reported to inhibit the growth of human lung cancer cell lines.18 However, only limited studies on the role of BK in renal cell carcinomas have been done. There is one report suggesting that BK, among the other GPCR agonists, causes tyrosine phosphorylation of endogenous EGFR in several kidney cancer cell lines.19 Another study demonstrated an increased expression of the BK B1 and BK B2 receptors in the renal parenchymal tissue adjacent to the clear cell carcinoma.20 In the present study, we used A498 cells, a transformed cell line established from a clear cell renal cell carcinoma of a 52-year-old patient.21 A498 human renal cancer cells represent a model used widely for the in vitro study of renal carcinomas.22,23 Here we used these cells to explore the possible mitogenic effects and signaling pathways of BK.
Materials and methods
Reagents and cell culture
Bradykinin, des-Arg9 BK, HOE-140, des-Arg10-HOE-140, probenecid, ethidium bromide, and various salts were purchased from Sigma (St Louis, MO). 5-(N-methyl-N-isobutyl)-amiloride (MIA) was purchased from RBI (Natick, MA). U-73122 (1-[6-[((17β)-3-Methoxyestra-1,3,5[10]-trien-17-yl) amino] hexyl]-1H-pyrrole-2, 5-dione), U-73343 (1-[6-[((17β)-3-Methoxyestra-1,3,5[10]-trien-17-yl) amino] hexyl]-2,5-pyrrolidinedione), GF109203 (2-[1-(3-Dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)-maleimide), PD98059 (2′-Amino-3′-methoxyflavone), W-7 (N-(6-Aminohexyl)-5-chloro-1-naphthalenesulfonamide), AG1478 (4-(3-Chloroaniline)-6,7-dimethoxyquinazoline), and BAPTA-AM (1,2-bis (o-Aminophenoxy) ethane N, N,N′,N′-tetraacetic acid tetra (acetoxymethyl) ester) were purchased from Calbiochem (Gibbstown, NJ). The BK B1 antibody was purchased from Abcam Inc (Cambridge, MA), and the BK B2 antibody was purchased from BD Transduction Laboratories™ (Franklin Lakes, NJ). All cell culture media and supplements were purchased from Life Technologies (Carlsbad, CA). Polycarbonate cell culture inserts for microphysiometry were purchased from Corning Costar (Cambridge, MA). Fluo-4 AM was purchased from Molecular Probes (Eugene, OR).
The A498 cells were obtained from American Type Culture Collection (Rockville, MD). Cells were grown in Eagle’s minimum essential medium supplemented with 10% fetal bovine serum, 100 U/mL of penicillin, and 100 μg/mL of streptomycin (Life Technologies) in a cell culture incubator at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using the SAT-60 reagent (Tel-Test Inc, Friendswood, TX), and RT-PCR was performed using a one-step RT-PCR kit (Qiagen, Valencia, CA). Both methods were performed following the manufacturers’ instructions. Specific sequences for sense (S) and antisense (AS) primers for BK B1 and BK B2 receptors that were published previously24 were synthesized (IDT Technologies Inc, Coralville, IA). The primers for the BK B1 receptor were 5′-TTC TTA TTC CAG GTG CAA GCA G-3′ (S) and 5′-CTT TCC TAT GGG ATG AAG ATA T-3′ (AS), yielding a 213-bp fragment. The primers for the BK B2 receptor were 5′-TGC TGC TGC TAT TCA TCA TC-3′ (S) and 5′-CCA GTC CTG CAG TTT GTG AA-3′ (AS), yielding a 335-bp product. The GAPDH primer sets 5′-ACC ACA GTC CAT GCC ATC AC-3′ (S) and 5′-TCC ACC ACC CTG TTG CTG TA-3′ (AS), yielding a 452-bp product (IDT Technologies Inc), were used as an internal control. The PCR conditions for both the BK B1 and BK B2 primer sets were cDNA synthesis at 50°C for 30 minutes and 95°C for 15 minutes (one cycle), followed by 94°C for 30 seconds, 52.4°C for 30 seconds, and 72°C for 1 minute during 35 cycles, and 72°C for 10 minutes (one cycle). Amplified products were separated by electrophoresis on 4%–12% polyacrylamide gel (Life Technologies) and visualized after staining with ethidium bromide (1 μg/mL) under UV light using Fluorochem Image (Alpha Innotech Corp, San Leandro, CA).
Measurement of intracellular Ca2+
A498 cells were plated onto collagen-coated 25 mm cover slips placed into a single well of a six-well plate. The next day, the medium was changed to 0.1% fetal bovine serum. Cells were incubated 24 hours later with the calcium-sensitive fluorescent probe Fluo-4 AM (2 μM; Molecular Probes) in a Hank’s balanced salt solution (HBSS)—with a pH of 7.4 and containing 2.5 mM Probenecid and 0.1% bovine serum albumin—for 60 minutes at 37°C. At the end of the incubation, the cells were washed three times with HBSS and the cover slip was mounted into a thermo-regulated heating block at 37°C (Olympus America, Melville, NY), and 1 mL HBSS was added to the cells. Microscopic images were acquired using an Olympus Ultra View LCI High Resolution Workstation (PerkinElmer Life Sciences, Boston, MA) equipped with a laser-scanning confocal unit (Omnichrome, Chino, CA), 15-mW krypton three-line laser head, and a UAPO 340 40× 1.35 or PlanAPO 60× 1.4 NA (Olympus America) oil immersion objective. Fluorescence was excited by using a 488 nm argon laser emission line and collected using a standard fluorescein isothiocyanate filter set (530 ± 30 nm). An increase in fluorescence (gray-scale intensity) was considered an indicator of increased intracellular free calcium and was recorded and compared between samples.
Microphysiometry
Extracellular acidification rates (ECARs) were measured in real-time in intact cells placed in an eight-chamber Cytosensor™ microphysiometer (Molecular Devices, Sunnyvale, CA), as described previously.25,26 A microphysiometer uses a light-addressable silicon sensor to detect extracellular protons, which can be derived primarily from Na+/H+ exchange and glycolysis, and from other metabolic pathways. Rate data transformed by a personal computer running Cytosoft™ (version 2.0; Molecular Devices) were presented as ECARs in μVolts/s, which roughly correspond to milli-pH units/min. In order to facilitate the comparison of data between two channels, values were expressed as percentage increases of the baseline as determined by computerized analysis of the three data points after the exposure of the cell monolayers to test substances.
The day prior to experimentation, A498 cells were plated onto polycarbonate membranes (3 μ pore size, 12 mm size) at a density of 300,000 cells per insert. The day of the study, cells were washed with serum-free, bicarbonate-free Ham’s F-12 medium, placed into the microphysiometer chambers, and perfused at 37°C with the same medium. The pump cycle was set to perfuse cells for 60 seconds, followed by a 30-second “pump-off” phase, during which proton efflux was measured from the 6 seconds through to 28 seconds. Cells were exposed to the test agents for four cycles (360 seconds). Valve switches (to add or remove test agents) were performed in the middle of the pump cycle. Data points were then acquired every 90 seconds. The peak effects during stimulation were expressed as percentage increases from average basal ECARs from five rate measurements prior to the application of the test agent(s).
ERK assays
ERK phosphorylation was assessed using a phosphorylation state-specific ERK antibody (Cell Signaling, Beverly, MA), which specifically recognizes threonine202 and tyrosine204-phosphorylated ERK as described previously.9–11 Cells were briefly cultured in 12-well plates, serum-starved for 24 hours, and stimulated with a vehicle or BK. After treatment, cells were scraped into a Laemmli buffer and subjected to SDS-PAGE using 4%–20% pre-cast gels (Life Technologies), and then semi-dry transferred to PVDF membranes (Millipore, Billerica, MA). The membranes were blocked with a BLOTTO buffer and incubated with the phospho-ERK antibody (at 1:1000 dilutions), followed by incubation with goat anti-rabbit alkaline phosphatase-conjugated IgG (Chemicon International, Inc, Temecula, CA). Immunoreactive bands were visualized by a chemiluminescent method (CDP StarTM; New England Biolabs, Ipswich, MA) using pre-flashed Kodak X-AR film, and quantified using ImageJ software (Image Processing and Analysis in Java, http://rsb.info.nih.gov/ij/index.html ; Bethesda, MA). The membranes were stripped using the Re-Blot Plus antibody stripping solution (Millipore) and reprobed with the control ERK antibody, which recognizes equally well phosphorylated and nonphosphorylated ERK, in order to quantify total ERK. The results are presented as intensities of phospho-ERK bands relative to total ERK bands and expressed as folds of basal phosphorylation (untreated cells).
Proliferation assays
A498 cell proliferation was assessed by a BrdU cell proliferation assay (Calbiochem). A498 cells were seeded (104 per well) in 96-well microplates and incubated in serum-free minimum essential medium for 36 hours. Next, cells were preincubated for 1 hour with 10 μM PD98059 (a MEK inhibitor), with 10 μM MIA (an Na+/H+ exchange inhibitor), or a vehicle before the addition of 100 nM BK or 20% fetal bovine serum (positive control) or a vehicle (negative control) for 24 hours. After incubation with a BrdU label for an additional 24 hours at 37°C, the assay was performed according to the manufacturer’s protocol, and the absorbance was measured using a SpectraMax M5/M5e Microplate Reader (Molecular Devices) at dual wavelengths of 450–540 nm.
Data analysis
ERK assays were performed in duplicate and repeated at least three times. Proliferation assays were performed in quadruplicate and repeated at least three times. Data are presented as mean ± standard error of the mean. Statistical evaluations of the data were performed using analyses of variance or Student’s t-tests as appropriate. Differences were considered significant at P < 0.05.
Results
A498 cells express endogenous BK B1 and B2 receptors
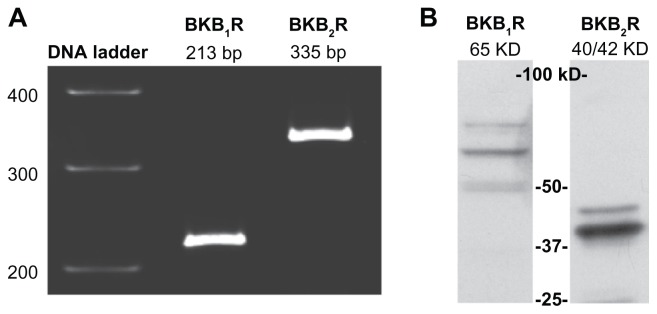
Total RNA extracted from A498 cells was subjected to RT-PCR using specific primers for BK B1 and BK B2 mRNA. As shown in Figure 1A, products with the expected sizes of 213 bp for BK B1 receptors and 335 bp for BK B2 receptors were detected. To confirm the expression of BK receptors at the protein level, we performed Western blotting of A498 lysates with BK B1 and BK B2 receptor antibodies. Western blot analyses showed a major band at 65 kDa that is immunoreactive for BK B1 receptors, as well as a duplet at 40/42 kDa that is immunoreactive for BK B2 (Figure 1B).
Figure 1.
A498 cells express BK B1 and BK B2 receptors.
Notes: (A) RT-PCR demonstrates the presence of BK B1 and BK B2 mRNA receptors in A498 cells. (B) Western blot analyses of A498 cell lysates (40 μg of total protein) with BK B1 and BK B2 receptor antibodies supporting the expression of BK receptors on a protein level. Antibodies were used at a 1:1000 dilution as per the manufacturer’s recommendations.
Abbreviations: BK, bradykinin; RT-PCR, reverse transcription-polymerase chain reaction.
Bradykinin induces elevations in intracellular Ca2+ in A498 cells
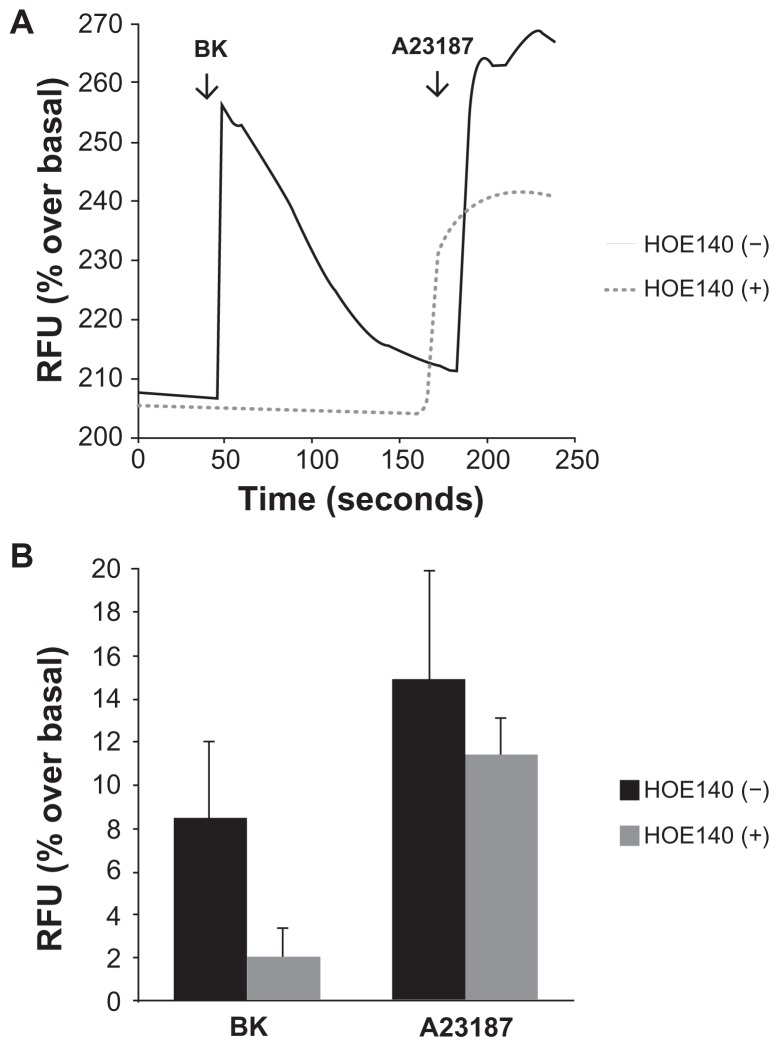
Cells grown on collagen-coated cover slips were incubated with the calcium-sensitive probe Fluo-4 AM, and the detection of calcium-dependent fluorescence was performed with a confocal microscope as described. Figure 2A shows representative raw data from a single experiment demonstrating that 100 nM of BK induced a rapid elevation of intracellular Ca2+ in A498 cells in the absence of HOE-140 (a BK B2 receptor antagonist). Pre-incubation with 1 μM of HOE-140 completely eliminated the Ca2+ signal (Figure 2B), whereas preincubation with the BK B1 receptor antagonist des-Arg10-HOE-140 (1 μM) had no effect (not shown), providing strong evidence that the effect is mediated by BK B2 (and not BK B1) receptors.
Figure 2.
Bradykinin induces elevations in intracellular Ca2+ in A498 cells.
Notes: Cells grown on collagen-coated cover slips were incubated with the calcium-sensitive probe Fluo-4AM, and the detection of calcium-dependent fluorescence was performed with a confocal microscope as described in the Materials and methods section. Changes in fluorescence (intracellular free calcium) were recorded as changes in gray-scale intensity. (A) Shown are representative tracings from two recordings in the presence or absence of the BK B2 receptor antagonist HOE140 (1 μM). (B) Average values of percentage increase over basal in grey-scale intensity subsequent to the addition of BK (100 nM) or the calcium ionophore A23187 (3 μM). Experiments were performed three times.
Abbreviation: BK, bradykinin; RFU, relative fluorescence units.
Bradykinin stimulates NHE activity in A498 cells
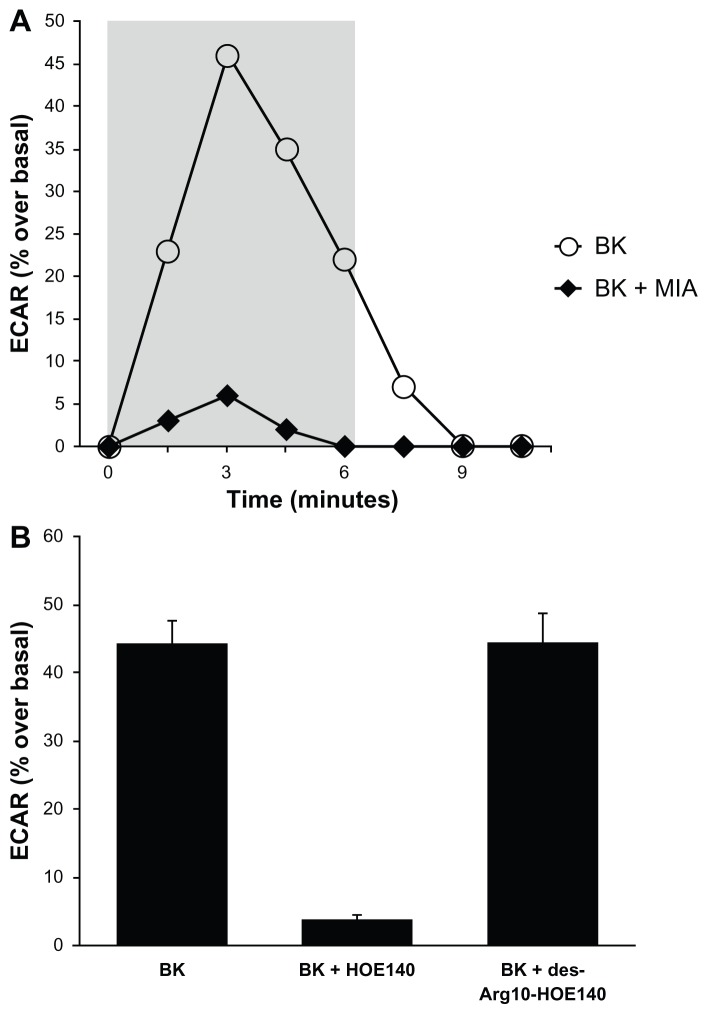
Proton microphysiometry was performed on quiescent A498 cell monolayers, as described in the Materials and methods section. ECARs were measured after 100 nM of BK was applied to the cells for four measurement cycles. Figure 3A shows that cells treated with 100 nM BK (open circles) had a rapid increase in extracellular acidification rates that did not occur when cells were exposed to BK after pre-incubation with 5 μM of MIA or NHE-1 and -2 inhibitors (black diamonds). BK-induced proton efflux was blocked by pre-incubation with 1 μM of HOE140, a BK B2 receptor antagonist, while a BK B1 receptor antagonist, des-Arg10-HOE140, did not change BK-induced ECARs (Figure 3B), supporting the involvement of the BK B2 receptor. Thus, Figure 3 presents evidence that BK B2 receptors in A498 cells activate proton efflux through NHE stimulation.
Figure 3.
Bradykinin stimulates NHE activity in A498 cells.
Notes: Proton microphysiometry was performed on quiescent A498 cell monolayers as described in the Methods section. Cells were exposed to a perfusate-containing drug (100 nM BK) during the time span encompassed by the gray box. (A) BK (white circles) stimulates ECAR, whereas pretreatment with 5 μM of the NHE-1 and -2 inhibitor 5-(N-methyl-N-isobutyl)-amiloride (MIA) for 30 minutes prior to BK stimulation blocked the effect (black diamonds). (B) BK-induced proton efflux was blocked by preincubation with 1 μM of HOE140, a BK B2 receptor antagonist. The BK B1 receptor antagonist, des-Arg10-HOE140, did not change BK-induced ECAR. Experiments were performed three times. Data are mean + SEM.
Abbreviations: BK, bradykinin; ECAR, extracellular acidification rate; NHE, Na+/H+ exchange; SEM, standard error of the mean.
Bradykinin stimulates ERK phosphorylation in A498 cells
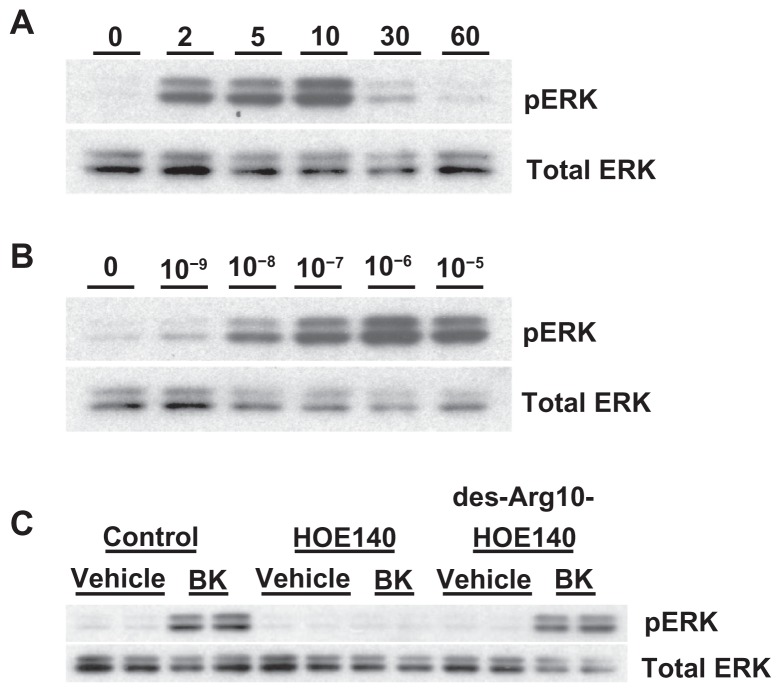
A498 cells were stimulated with 100 nM of BK for the indicated periods of time. Figure 4A shows that BK B2 receptors in A498 cells activate ERK in a time-dependent manner, with maximal stimulation at 10 minutes. Next, A498 cells were stimulated with the indicated concentrations of BK for 5 minutes. Figure 4B demonstrates that BK in A498 cells activates ERK in a concentration-dependent manner, with maximal activation at 1 μM. In subsequent experiments ERK assays were usually carried out with stimulation by 100 nM BK for 5 minutes, unless otherwise mentioned.
Figure 4.
Bradykinin stimulates ERK phosphorylation in A498 cells.
Notes: (A) Time course: A498 cells were stimulated with 100 nM of BK for the indicated periods of time. ERK phosphorylation was measured by immunoblotting with anti-phospho-ERK antibodies, as described in the Materials and methods section. (B) Concentration response: A498 cell were stimulated with the indicated concentrations of BK for 5 minutes. (C) BK-induced ERK phosphorylation is mediated by the B2 receptor: cells were pretreated for 30 minutes with a vehicle, with 1 μM of HOE140, or with 1 μM of des-Arg10-HOE140, then stimulated with 100 nM BK for 5 minutes. Representative phospho-ERK blots are shown. The same blots were stripped and reprobed with antibodies for total ERK to assure the equal loading of a protein sample on a gel (total ERK). Experiments were performed at least three times.
Abbreviations: BK, bradykinin; ERK, extracellular signal-regulated kinase.
BK stimulates ERK phosphorylation through a BK B2 receptor
A498 cells were pretreated for 30 minutes with the BK B1 receptor antagonist des-Arg10-HOE-140 (1 μM) or the BK B2 receptor antagonist HOE-140 (1 μM), and then stimulated with 100 nM of BK for 5 minutes. Figure 4C shows that pretreatment with HOE-140 completely prevented the BK-induced activation of ERK, whereas des-Arg10-HOE-140 was ineffectual. Thus, BK stimulates ERK activity via the BK B2 receptor.
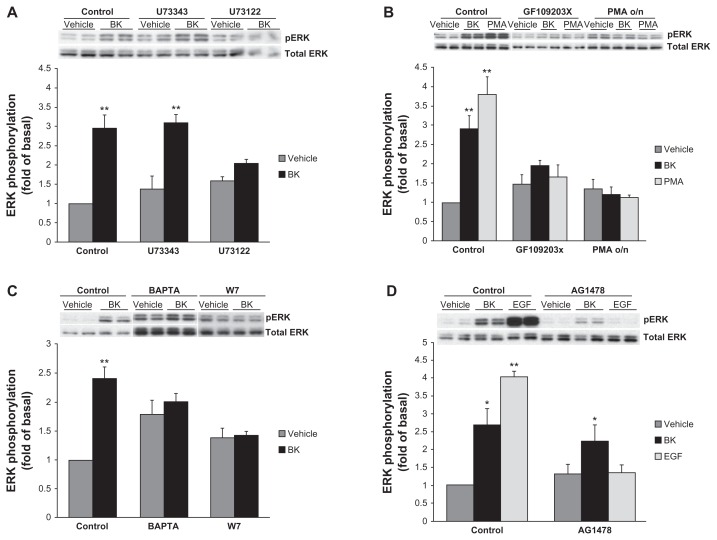
Bradykinin-induced ERK phosphorylation requires phospholipase C (PLC) and protein kinase C (PKC) activities and is Ca2+- and calmodulin-dependent
Because PKC-dependent and Ca2+/calmodulin (CaM)-dependent mechanisms of BK-induced ERK activation have been described previously in vascular smooth muscle cells (VSMC),27 we next tested if the BK B2 receptor employs the same pathway in our cell model. PLC inhibitors decreased BK-induced ERK activation, suggesting that PLC is involved in this process (Figure 5A). PKC inhibitors and PKC depletion by the prolonged treatment with phorbol 12-myristate 13-acetate (PMA) also effectively blocked BK-induced ERK activation, supporting a role for PKC (Figure 5B). Next, A498 cells were pretreated for 30 minutes with a vehicle, 10 μM of BAPTA (an intracellular Ca2+ chelator), or 50 μM of W7 (a CaM inhibitor), and then stimulated for 5 minutes with 100 nM BK. Both treatments caused an increase in basal ERK phosphorylation and impaired BK-induced signal, suggesting that Ca2+ and CaM are important for BK-induced ERK activation (Figure 5C).
Figure 5.
Effects of phospholipase C (PLC), protein kinase C (PKC), Ca2+-, and calmodulin (CaM) inhibitors on BK-induced ERK phosphorylation.
Notes: (A) Cells were preincubated with a vehicle, 10 μM U73122 (a PLC inhibitor), or 10 μM U73343 (negative control) for 30 minutes before stimulation with 100 nM BK for 5 minutes. (B) Cells were pretreated with either 2 μM GF109203X (a PKC inhibitor) for 30 minutes or with 160 nM PMA for 20 hours, followed by either BK 100 nM or PMA 1 μM stimulation for 5 minutes. (C) A498 cells were pretreated for 30 minutes with a vehicle, 10 μM of BAPTA (an intracellular Ca2+ chelator), or 50 μM of W7 (a CaM inhibitor), and then stimulated for 5 minutes with 100 nM BK. (D) BK-induced ERK phosphorylation does not depend on EGFR tyrosine kinase activity: cells were pretreated for 30 minutes with a vehicle, with 1 μM of AG1478 (an EGFR kinase inhibitor) before stimulation with 100 nM BK, or with 10 ng/mL EGF for 5 minutes. ERK phosphorylation was detected by immunoblotting with an anti-phospho-ERK antibody. Bars represent intensities of phospho-ERK bands relative to total ERK expressed as a fold of basal of control (cells treated with a vehicle). Insets show representative phospho-ERK blots. The same blots were stripped and reprobed with antibodies for total ERK to assure the equal loading of a protein sample on a gel (total ERK). Experiments were performed three times in duplicate. Data are presented as mean + SEM. Statistical probability in figures is expressed as *P < 0.05, and as **P < 0.01 versus vehicle-treated samples.
Abbreviations: BK, bradykinin; ERK, extracellular signal-regulated kinase; PMA, phorbol 12-myristate 13-acetate; SEM, standard error of the mean.
Bradykinin-induced ERK phosphorylation does not depend on EGFR tyrosine kinase activity
Because it has been reported that BK causes tyrosine phosphorylation of endogenous EGFR in several kidney cancer cell lines,19 and because in our previous work we demonstrated that BK-induced ERK activation in kidney cells was dependent on EGFR transactivation,9,10 in the next series of experiments we studied the role of EGFR in BK-induced ERK activation in A498 cells. Cells were pretreated for 30 minutes with a vehicle or with 1 μM of AG1478 (an EGFR kinase inhibitor) before stimulation with 100 nM BK or 10 ng/mL EGF for 5 minutes. Figure 5D shows that the inhibition of EGFR kinases effectively blocked EGF-induced ERK activation but did not alter BK-induced signals, indicating that EGFR is not involved in BK-induced ERK phosphorylation in A498 cells.
Bradykinin activates ERK through a pathway that requires NHE activity
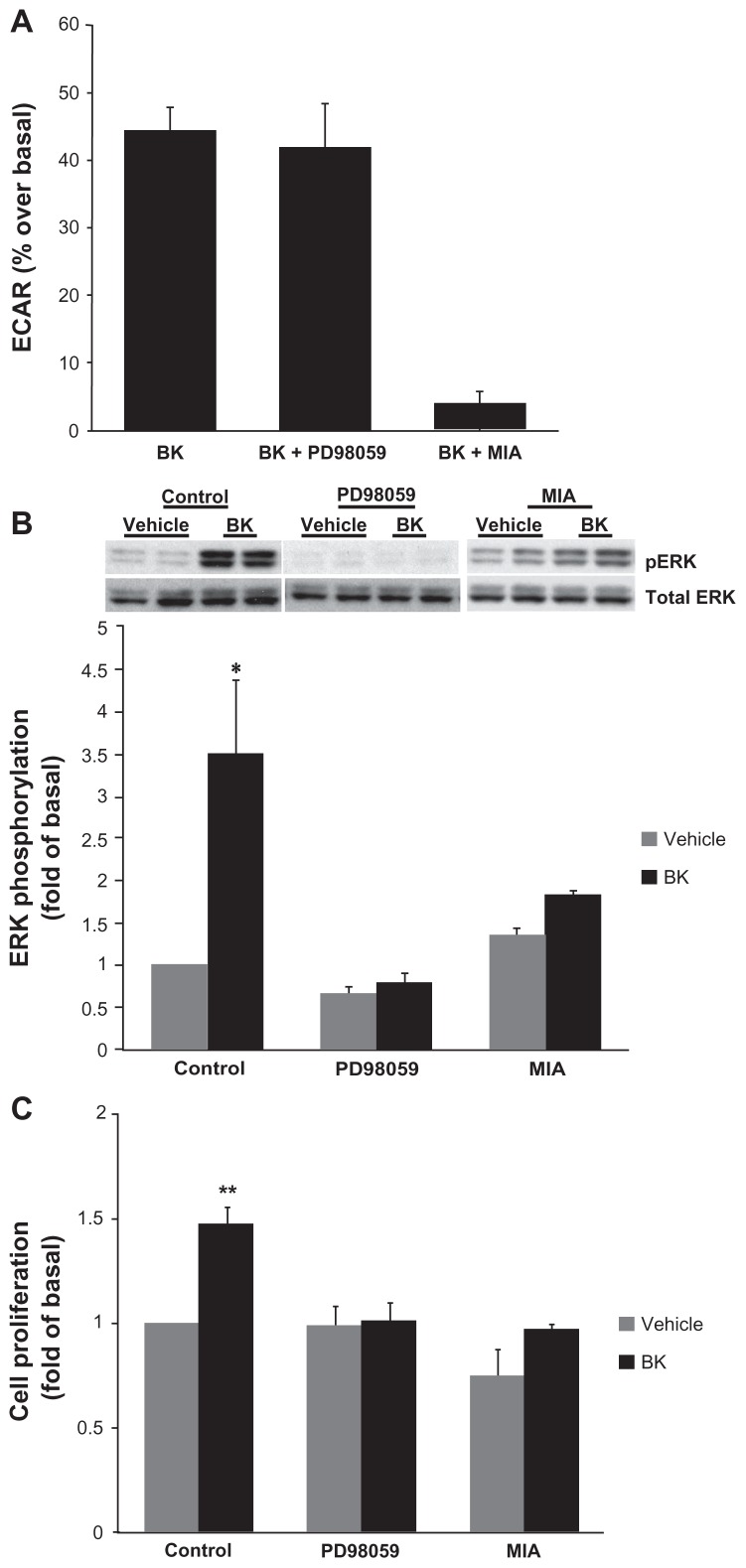
Because BK B2 receptor activated both NHE (Figure 3) and ERK (Figure 4) in A498 cells, in the next series of experiments we investigated the possible relationship between these two pathways. Cells were pretreated with either 10 μM PD98059 to block ERK activation or 5 μM MIA for 30 minutes prior to stimulation with 100 nM BK. NHE activity was assessed by microphysiometry, and ERK phosphorylation by Western blotting with phosphorylation state-specific antibodies. While the pretreatment of cells with 5 μM MIA as expected completely blocked BK-induced ECARs, preincubation with 10 μM of PD98059 did not change BK-induced ECARs, suggesting that ERK does not play a regulatory role in the activation of NHE in A498 cells (Figure 6A). At the same time, pretreatment with PD98059 completely abolished BK-induced ERK phosphorylation (Figure 6B), confirming that ERK phosphorylation in A498 cells proceeds through MEK. Interestingly, pretreatment with MIA—which blocks NHE activity in A498 cells (Figure 6A)—attenuated ERK phosphorylation induced by BK, suggesting that NHE activity is required for ERK activation (Figure 6B).
Figure 6.
NHE activity is required for bradykinin-induced ERK activation.
Notes: (A) BK-induced NHE activity does not require ERK activation. Cells were pretreated with either 10 μM of an MEK inhibitor (PD98059) or with 5 μM of an NHE inhibitor (MIA) for 30 minutes prior to the application of 100 nM of BK for four measurement cycles, and ECAR was assessed by proton microphysiometry as described in the Materials and methods section. Experiments were performed at least three times. The data are means + SEM. (B) BK-induced ERK phosphorylation is NHE-dependent. Cells were pretreated with 10 μM PD98059 or with 5 μM MIA for 30 minutes prior to stimulation with 100 nM BK for 5 minutes. Bars represent the intensities of phospho-ERK bands relative to the total ERK expressed as a fold of control (cells treated with a vehicle). Experiments were performed three times in duplicate. Data are presented as means + SEM. (C) Bradykinin stimulates the proliferation of A498 cells. A498 cells were preincubated for 1 hour with a vehicle, or with 10 μM PD98059 or 5 μM MIA before the addition of 100 nM BK or 20% fetal bovine serum (positive control) or a vehicle (negative control) for 24 hours. After incubation with a BrdU label for an additional 24 hours at 37°C, the BrdU cell proliferation assay was performed according to the manufacturer’s protocol. Experiments were performed at least three times in triplicate. Data are presented as means + SEM. Statistical probability in figures is expressed as *P < 0.05, and as **P < 0.01 versus vehicle-treated samples.
Abbreviations: BK, bradykinin; ECAR, extracellular acidification rate; ERK, extracellular signal-regulated kinase; MEK, mitogen- and extracellular signal-regulated kinase; MIA, 5-(N-methyl-N-isobutyl)-amiloride; NHE, Na+/H+ exchange; SEM, standard error of the mean.
Bradykinin stimulates the proliferation of A498 cells
The mitogenic effect of BK was evaluated by measuring the proliferation of A498 cells by a BrdU cell proliferation assay. BK caused a significant increase in BrdU-positive cells (48% ± 8% over control values; P < 0.05, n = 4). MEK inhibitors completely blocked this increase without changing the basal proliferation rate. NHE inhibitors decreased both the basal level of proliferation and BK-induced BrdU incorporation (Figure 6C). These findings demonstrate that BK stimulates the proliferation of A498 cells, and implicate roles for ERK and NHE in BK mitogenic activity.
Discussion
In this study we found the presence of BK B1 and BK B2 receptor mRNAs in renal carcinoma A498 cells, and demonstrated the presence of these receptors at the protein level by Western blotting (Figure 1). In order to investigate whether these receptors are functional, we tested three signaling pathways known to be stimulated by BK in other cell types. The BK B2 receptor usually couples to the GTP-binding protein Gq and stimulates phospholipase C activity, which leads to the generation of inositol triphosphate and to the subsequent mobilization of intracellular Ca2+ from the endoplasmic reticulum.28 We demonstrated that BK causes an elevation of intracellular Ca2+ in A498 cells, which was blocked by the BK B2 receptor antagonist HOE-140, but not by the BK B1 receptor antagonist des-Arg10-HOE-140, indicating a role for the BK B2 receptor (Figure 2). Similar BK-induced intracellular Ca2+ mobilization has been described in several kidney cell lines, including rat mesangial cells,29 the mIMCD-3 murine inner medullary collecting duct cell line,25 Kirsten murine sarcoma-virus transformed rat kidney KNRK cells,30 the mouse proximal tubule epithelial cell line,31 and in the human embryonic kidney (HEK293) cell line.26
Because BK B2 receptors have also been shown to activate sodium-proton exchanges in mIMCD-3 cells,25 HEK293 cells,26 KNRK cells,30 and renal tubular epithelial cells,32 we tested potential roles for BK receptors in the regulation of sodium-proton exchanges in A498 cells. A498 cells treated with 100 nM BK demonstrated a rapid increase in ECARs as measured by microphysiometry (Figure 3), which was blocked by preincubation with 5 μM MIA, an inhibitor of NHE-1 and NHE-2. This response to BK was blocked by the BK B2 receptor antagonist HOE-140, but not by the BK B1 receptor antagonist des-Arg10-HOE-140, supporting a role for the BK B2 receptor.
The activation of ERK by BK in kidney cells has been demonstrated in cultured mesangial cells,5,6 rabbit cortical collecting duct cells,33 mIMCD-3 cells,9,10,11 and HEK293 cells.26 In the current study we showed that, in A498 cells, BK induces time- and concentration-dependent threonine/tyrosine phosphorylation of ERK, which peaked at 10 minutes after BK application, and was blocked by a BK B2 receptor antagonist, indicating that BK activates ERK via a BK B2 receptor (Figure 4). It has been described previously that BK B2 receptors employ PKC- and Ca2+/CaM-dependent mechanisms of ERK activation in VSMC27 and COS-7 cells, transiently transfected with BK B2 receptor.34 PKC-dependent mechanisms of BK-induced ERK activation have been also reported in multiple tumor cell lines.35 In our experiments, the inhibition of PLC or PKC altered ERK activation by BK, suggesting that these proteins are involved in the signal transduction pathway (Figures 5A and B). We also demonstrated that Ca2+ and CaM are important for the BK stimulation of ERK in A498 cells (Figure 5C). Therefore, mapping studies with chemical inhibitors of candidate molecules revealed that BK-induced ERK activation in A498 cells was dependent on PLC, PKC, and Ca2+/CaM activities (Figure 7). In our previous work we established that the BK B2 receptor stimulates early mitogenic signals associated with the activation of ERK in kidney mIMCD-3 cells, and demonstrated that BK-induced cell proliferation was dependent on EGFR activation.9 We hypothesized that BK uses a similar pathway to activate ERK in A498 cells. It appears, however, that the inhibition of EGFR tyrosine kinase activity did not alter ERK activation by BK, suggesting that EGFR transactivation is not necessary in this signal transduction pathway (Figure 5D). Interestingly, in A431 epidermoid carcinoma cells,36 and in head and neck squamous cell carcinomas,37 BK-induced ERK activation also did not require EGFR tyrosine kinase activity. Thus, the pathway of the B2 receptor-induced ERK activation in renal carcinoma A498 cells was different from the one described for the kidney mIMCD-3 cells,9,10 and similar to the one reported in tumor cell lines.35–37
Figure 7.
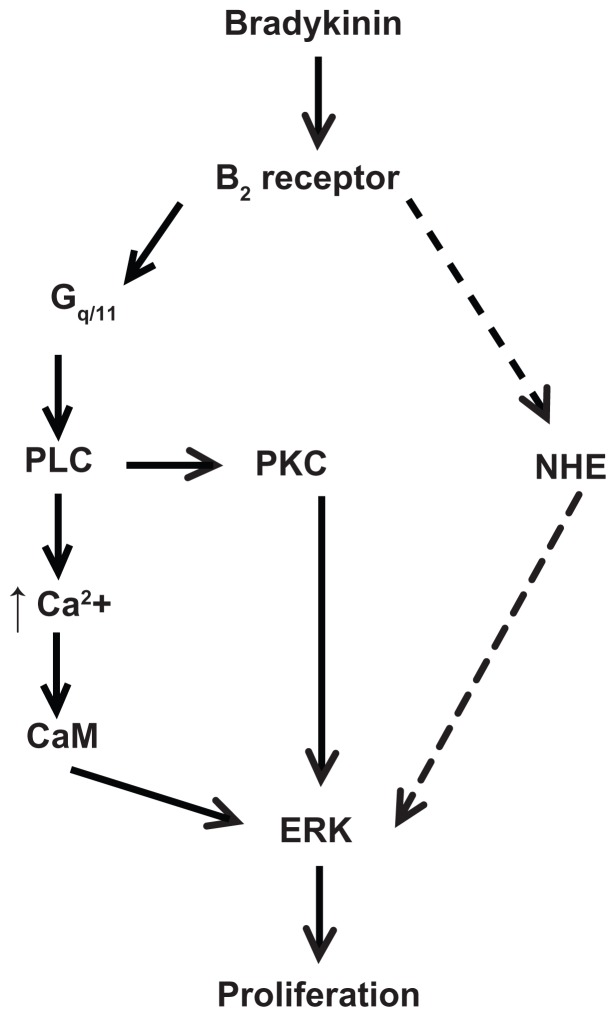
Scheme of proposed signaling pathway used by bradykinin to stimulate proliferation in A498 cells. This scheme is described in the Discussion section.
Notes: BK stimulates ERK through the B2 receptor-Gq/11 pathway, which includes the activation of phospholipase C (PLC) and protein kinase C (PKC), PLC-dependent Ca2+ mobilization, and calmodulin (CaM) activity. BK B2 receptors stimulate NHE1 activity, which is necessary for the ERK-dependent proliferation of A498 cells. The dashed line indicates a putative pathway used by BK to activate NHE upstream of ERK.
Abbreviations: BK, bradykinin; ERK, extracellular acidification rate; NHE, Na+/H+ exchange.
Our data also support the presence of mRNA for the BK B1 receptor, but do not support a role for BK B1 receptors in the stimulation of the tested pathways. Therefore, the functions of the BK B1 receptor in A498 cells remain unclear. One possible function of the BK B1 receptor in A498 cells could be cross-talk between BK B1 and BK B2 receptors. In that regard, the formation of a complex relationship between BK B1 and BK B2 receptors has been described in prostate cancer PC3 cells.38
Another interesting fact is that the NHE1 inhibitor MIA decreased BK-induced levels of ERK phosphorylation in A498 cells (Figure 6B), suggesting that NHE1 may act upstream of ERK in the BK-induced signaling pathway in renal carcinoma cells. Because NHE and ERK have both been implicated as key mediators of growth signals,39 the relationships between these pathways are very important although not completely understood. It has been well established that NHE1 could be regulated by ERK,40,41 although our group and others were unable to demonstrate any role of ERK in the regulation of NHE1 in several cell types.42,43 In addition, there have been several reports suggesting that NHE1 might play a role in regulating ERK activation.44 For example, in human colon cancer epithelial cells, NHE1 inhibition suppressed ERK activation and led to decreased production of interleukin-8 in response to inflammatory signals.45 It also has been shown that an antagonist of NHE1 inhibits the activation of ERK under osmotic shrinkage in Ehrlich–Lettre ascites cells44 and glucose-induced ERK activation in a high-glucose model of cardiomyocyte hypertrophy.46 Therefore, such NHE1-dependent regulation of ERK in most cases has been described in cells stimulated by mechanical stretching, osmotic shrinkage, hypertrophy, and inflammatory mediators.44–46 Little is known about GPCR-induced NHE1-dependent ERK regulation. One report suggests that NHE1 is not a regulator for LPA-induced ERK activation in C6 glioma cells,47 though, our group showed that NHE1 activity plays a necessary role in the activation of ERK by angiotensin II AT1 and serotonin 5-HT2A receptors in VSMC.48 The results of the current study demonstrate that BK B2 receptor-induced ERK activation in A498 cells depends on NHE activity, suggesting that the critical role of NHE1 in GPCR-induced ERK activation is not restricted to one specific cell type or receptor. Figure 7 depicts one possible scheme, which can account for our findings. Based on previous studies from our group25 and others,32 which described BK-induced NHE1 activation in renal cell lines, we hypothesize that BK-induced pathways that lead to NHE1 activation in A498 cells may involve PLC and elevated intracellular Ca2+ and CaM; however, detailed mapping studies of this pathway were outside of the scope of this study.
In the next series of experiments we evaluated the physiological significance of BK in A498 cells and assessed BK-induced proliferation by a BrdU cell proliferation assay. The results presented in Figure 6C show that BK exerts mitogenic effects in A498 cells, and that BK-induced proliferation is mediated by ERK. Another interesting observation is that the NHE1 inhibitor MIA decreased BK-induced levels of proliferation in A498 cells (Figure 6C), suggesting that NHE1 may be upstream of ERK in the signaling pathway, which leads to the increased proliferation of renal carcinoma cells.
Although the role of NHE1 has been well established in the myocardial remodeling and heart failure process,49 its role in renal function is less known. Mice with a spontaneous point mutation that results in truncation between the eleventh and twelfth NHE1 transmembrane domains and a loss of NHE1 function50 do not present a visible renal phenotype, consistent with the concept that NHE1 “housekeeping” activity under normal conditions is not required. Previous studies from our laboratory demonstrated that the activation of the BK B2 receptor leads to ERK-dependent proliferation in normal renal (mIMCD-3) cells, 9 and also described BK-induced activation of NHE1 in the same cells through the pathway, which involved PLC, elevated intracellular Ca2+, CaM, and Janus kinase 2.25 However, pretreatment with NHE1 inhibitors did not change the levels of BK-induced ERK phosphorylation in mIMCD-3 cells, suggesting that NHE1 activity does not play a necessary role in the activation of ERK by BK in this model. Thus, an observation that NHE1 acts upstream of ERK in the signaling pathway, which leads to the increased proliferation of A498 cells, suggests that this role of NHE1 is specific to A498 renal cancer cells.
Previously, NHE1-mediated intracellular alkalinization has been proposed to play a role in cancer cell growth because it has been shown that an increased pHi of tumor cells is associated with increased in vivo tumor growth, DNA synthesis, and cell-cycle progression, suggesting that an overexpression of NHE1 contributes to the transformed phenotype of multiple cancer cells.49 The cellular alkalinization of tumor cells induced by hyperactivation of NHE1 has been shown to be directly related to increased protein synthesis and tumor cell growth.49,50 It has been suggested that the mechanism of NHE1-mediated tumor cell growth and metastasis does not depend on its ion-transporting activities, but rather employs NHE1 as a scaffolding protein to directly regulate cytoskeletal dynamics.49 Further it has been shown that NHE1 antisense genes suppress cell growth, induce cell apoptosis, and partially reverse the malignant phenotypes of human gastric carcinoma cells.51 Similarly, the silencing of the NHE1 gene by siRNA interference and/or the inhibition of NHE1 activity by amiloride analogs effectively blocked the invasiveness of human hepatocellular carcinoma cells.52 The results of the current study demonstrate that NHE also plays a role in the proliferation A498 cells, suggesting that the inhibition of NHE1 might result in an antiproliferative effect.
Conclusion
In summary, we demonstrated that BK exerts mitogenic effects in A498 cells via an endogenously expressed BK B2 receptor, which is functionally coupled to PLC-dependent Ca2+ mobilization, NHE activation, and ERK phosphorylation. Our results demonstrate that NHE1 is involved in BK-induced ERK activation and the proliferation of A498 cells, suggesting that NHE1 may be a potential target for chemotherapeutics for the treatment of renal carcinoma.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs (Merit Awards to MNG and JRR), the AHA (GIA 0655445U to MNG), and the National Institutes of Health (DK52448 and GM63909 to JRR).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cáceres W, Cruz-Chacón A. Renal cell carcinoma: molecularly targeted therapy. P R Health Sci J. 2011;30(2):73–77. [PubMed] [Google Scholar]
- 2.Choueiri M, Jonasch E. Have molecularly targeted therapies improved overall survival in renal cell carcinoma? Curr Oncol Reps. 2011;13(3):153–156. doi: 10.1007/s11912-011-0167-y. [DOI] [PubMed] [Google Scholar]
- 3.Mukai H, Fitzgibbon WR, Bozeman G, Margolius HS, Ploth DW. Bradykinin B2 receptor antagonist increases chloride and water absorption in rat medullary collecting duct. Am J Physiol. 1996;271(2 Pt 2):R352–R360. doi: 10.1152/ajpregu.1996.271.2.R352. [DOI] [PubMed] [Google Scholar]
- 4.Bagaté K, Grima M, Imbs JL, Jong WD, Helwig JJ, Barthelmebs M. Signal transduction pathways involved in kinin B(2) receptor-mediated vasodilation in the rat isolated perfused kidney. Br J Pharmacol. 2001;132(8):1735–1752. doi: 10.1038/sj.bjp.0704027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Dahr SS, Dipp S, Baricos WH. Bradykinin stimulates the ERK--> Elk-1--> Fos/AP-1 pathway in mesangial cells. Am J Physiol. 1998;275(3 Pt 2):F343–F352. doi: 10.1152/ajprenal.1998.275.3.F343. [DOI] [PubMed] [Google Scholar]
- 6.Jaffa AA, Miller BS, Rosenzweig SA, Naidu PS, Velarde V, Mayfield RK. Bradykinin induces tubulin phosphorylation and nuclear translocation of MAP kinase in mesangial cells. Am J Physiol. 1997;273(6 Pt 2):F916–F924. doi: 10.1152/ajprenal.1997.273.6.F916. [DOI] [PubMed] [Google Scholar]
- 7.Hess JF, Borkowski JA, Young GS, Strader CD, Ransom RW. Cloning and pharmacological characterization of a human bradykinin (BK-2) receptor. Biochem Biophys Res Commun. 1992;184(1):260–268. doi: 10.1016/0006-291x(92)91187-u. [DOI] [PubMed] [Google Scholar]
- 8.Menke JG, Borkowski JA, Bierilo KK, et al. Expression cloning of a human B1 bradykinin receptor. J Biol Chem. 1994;269(34):21583–21586. [PubMed] [Google Scholar]
- 9.Mukhin YV, Garnovsky EA, Ullian ME, Garnovskaya MN. Bradykinin B2 receptor activates extracellular signal-regulated protein kinase in mIMCD-3 cells via epidermal growth factor receptor transactivation. J Pharmacol Exp Ther. 2003;304(3):968–977. doi: 10.1124/jpet.102.043943. [DOI] [PubMed] [Google Scholar]
- 10.Mukhin YV, Gooz M, Raymond JR, Garnovskaya MN. Collagenase-2 and -3 mediate epidermal growth factor receptor transactivation by bradykinin B2 receptor in kidney cells. J Pharmacol Exp Ther. 2006;318(3):1033–1043. doi: 10.1124/jpet.106.104000. [DOI] [PubMed] [Google Scholar]
- 11.Kramarenko II, Bunni MA, Raymond JR, Garnovskaya MN. Bradykinin B2 receptor interacts with integrin α5/β1 to transactivate epidermal growth factor receptor in kidney cells. Mol Pharmacol. 2010;78(1):126–134. doi: 10.1124/mol.110.064840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhoola K, Ramsaroop R, Plendl J, Cassim B, Dlamini Z, Naicker S. Kallikrein and kinin receptor expression in inflammation and cancer. Biol Chem. 2001;382(1):77–89. doi: 10.1515/BC.2001.013. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Akaike T, Hayashida K, et al. Identification of bradykinin receptors in clinical cancer specimens and murine tumor tissues. Int J Cancer. 2002;98(1):29–35. doi: 10.1002/ijc.10142. [DOI] [PubMed] [Google Scholar]
- 14.Dlamini Z, Bhoola KD. Upregulation of tissue kallikrein, kinin B1 receptor, and kinin B2 receptor in mast and giant cells infiltrating oesophageal squamous cell carcinoma. J Clin Pathol. 2005;58(9):915–922. doi: 10.1136/jcp.2004.021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chee J, Naran A, Misso NL, Thompson PJ, Bhoola KD. Expression of tissue and plasma kallikreins and kinin B1 and B2 receptors in lung cancer. Biol Chem. 2008;389(9):1225–1233. doi: 10.1515/BC.2008.139. [DOI] [PubMed] [Google Scholar]
- 16.Greco S, Elia MG, Muscella A, Romano S, Storelli C, Marsigliante S. Bradykinin stimulates cell proliferation through an extracellular signal-regulated protein kinase 1 and 2- dependent mechanism in breast cancer cells in primary culture. J Endocrinol. 2005;186(2):291–301. doi: 10.1677/joe.1.06052. [DOI] [PubMed] [Google Scholar]
- 17.Greco S, Storelli C, Marsigliante S. Protein kinase C (PKC)-δ/-ɛ mediate the PKC/Akt-dependent phosphorylation of extracellular signal-regulated protein kinases 1 and 2 in MCF-7 cells stimulated by bradykinin. J Endocrinol. 2006;188(1):79–89. doi: 10.1677/joe.1.06433. [DOI] [PubMed] [Google Scholar]
- 18.Chan D, Gera L, Stewart J, et al. Bradykinin antagonist dimer, CU201, inhibits the growth of human lung cancer cell lines by a “biased agonist” mechanism. Proc Natl Acad Sci U S A. 2002;99(7):4608–4613. doi: 10.1073/pnas.072077299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schäfer B, Gschwind A, Ullrich A. Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene. 2004;23(4):991–999. doi: 10.1038/sj.onc.1207278. [DOI] [PubMed] [Google Scholar]
- 20.Moodley R, Snyman C, Odhav B, Bhoola KD. Visualisation of transforming growth factor-β 1, tissue kallikrein, and kinin and transforming growth factor-β receptors on human clear-cell renal carcinoma cells. Biol Chem. 2005;386(4):375–382. doi: 10.1515/BC.2005.045. [DOI] [PubMed] [Google Scholar]
- 21.Giard DJ, Aaronson SA, Todaro GJ, et al. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 22.Wu SY, Pan SL, Chen TH, et al. YC-1 induces apoptosis of human renal carcinoma A498 cells in vitro and in vivo through activation of the JNK pathway. Br J Pharmacol. 2008;155(4):505–513. doi: 10.1038/bjp.2008.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F, Shang D, Zhang Y, Tian Y. Interleukin-22 suppresses the growth of A498 renal cell carcinoma cells via regulation of STAT1 pathway. PLoS One. 2011;6(5):e20382. doi: 10.1371/journal.pone.0020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertram CM, Baltic S, Misso NL, et al. Expression of kinin B1 and B2 receptors in immature, monocyte-derived dendritic cells and bradykinin-mediated increase in intracellular Ca2+ and cell migration. J Leukoc Biol. 2007;81(6):1445–1454. doi: 10.1189/jlb.0106055. [DOI] [PubMed] [Google Scholar]
- 25.Mukhin YV, Vlasova T, Jaffa AA, et al. Bradykinin B2 receptors activate Na+/H+ exchange in mIMCD-3 cells via Janus kinase 2 and Ca2+/calmodulin. J Biol Chem. 2001;276(20):17339–17346. doi: 10.1074/jbc.M010834200. [DOI] [PubMed] [Google Scholar]
- 26.Kramarenko II, Bunni MA, Morinelli TA, Raymond JR, Garnovskaya MN. Identification of functional bradykinin B2 receptors endogenously expressed in HEK293 cells. Biochem Pharmacol. 2009;77(2):269–276. doi: 10.1016/j.bcp.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velarde V, Ullian ME, Morinelli TA, Mayfield RK, Jaffa AA. Mechanisms of MAPK activation by bradykinin in vascular smooth muscle cells. Am J Physiol. 1999;277(2 Pt 1):C253–C261. doi: 10.1152/ajpcell.1999.277.2.C253. [DOI] [PubMed] [Google Scholar]
- 28.Wiernas TK, Davis TL, Griffin BW, Sharif NA. Effects of bradykinin on signal transduction, cell proliferation, and cytokine, prostaglandin E2 and collagenase-1 release from human corneal epithelial cells. Br J Pharmacol. 1998;123(6):1127–1137. doi: 10.1038/sj.bjp.0701700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bascands JL, Emond C, Pecher C, Regoli D, Girolami JP. Bradykinin stimulates production of inositol (1,4,5) triphosphate in cultured mesangial cells of the rat via a B2 receptor. Br J Pharmacol. 1991;102(4):962–966. doi: 10.1111/j.1476-5381.1991.tb12284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefler D, Mukhin YV, Pettus T, Leeb-Lundberd LM, Garnovskaya MN, Raymond JR. Jak2 and Ca2+/calmodulin are key intermediates for bradykinin B2 receptor-mediated activation of Na+/H+ exchange in KNRK and CHO cells. Assay Drug Dev Technol. 2003;1(2):281–289. doi: 10.1089/15406580360545099. [DOI] [PubMed] [Google Scholar]
- 31.Tiwari MM, Prather PL, Mayeux PR. Mechanism of bradykinin-induced Ca2+ mobilization in murine proximal tubule epithelial cells. J Pharmacol Exp Ther. 2005;313(2):798–805. doi: 10.1124/jpet.104.080408. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura K, Singer WD, Cano A, Miller RT. G alpha q and G alpha13 regulate NHE-1 and intracellular calcium in epithelial cells. Am J Physiol. 1995;268(1 Pt 1):C101–C110. doi: 10.1152/ajpcell.1995.268.1.C101. [DOI] [PubMed] [Google Scholar]
- 33.Lal MA, Proulx PR, Hébert RL. A role for PKC epsilon and MAP kinase in bradykinin-induced arachidonic acid release in rabbit CCD cells. Am J Physiol. 1998;274(4 Pt 2):F728–F735. doi: 10.1152/ajprenal.1998.274.4.F728. [DOI] [PubMed] [Google Scholar]
- 34.Adomeit A, Graness A, Gross S, Seedorf K, Wetzker R, Liebmann C. Bradykinin B(2) receptor-mediated mitogen-activated protein kinase activation in COS-7 cells requires dual signaling via both protein kinase C pathway and epidermal growth factor receptor transactivation. Mol Cell Biol. 1999;19(8):5289–5297. doi: 10.1128/mcb.19.8.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liebmann C. Regulation of MAP kinase activity by peptide receptor signalling pathway: paradigms of multiplicity. Cell Signal. 2001;13(11):777–785. doi: 10.1016/s0898-6568(01)00192-9. [DOI] [PubMed] [Google Scholar]
- 36.Graness A, Hanke S, Boehmer FD, Presek P, Liebmann C. Protein-tyrosine-phosphatase-mediated epidermal growth factor (EGF) receptor transinactivation and EGF receptor-independent stimulation of mitogen-activated protein kinase by bradykinin in A431 cells. Biochem J. 2000;347(Pt 2):441–447. doi: 10.1042/0264-6021:3470441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas SM, Bhola NE, Zhang Q, et al. Cross-talk between G protein-coupled receptor and epidermal growth factor receptor signaling pathways contributes to growth and invasion of head and neck squamous cell carcinoma. Cancer Res. 2006;66(24):11831–11839. doi: 10.1158/0008-5472.CAN-06-2876. [DOI] [PubMed] [Google Scholar]
- 38.Barki-Harrington L, Bookout AL, Wang G, Lamb ME, Leeb-Lundberg LM, Daaka Y. Requirement for direct cross-talk between B1 and B2 kinin receptors for the proliferation of androgen-insensitive prostate cancer PC3 cells. Biochem J. 2003;371(Pt 2):581–587. doi: 10.1042/BJ20021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noël J, Pouysségur J. Hormonal regulation, pharmacology, and membrane sorting of vertebrate Na+/H+ exchanger isoforms. Am J Physiol. 1995;268(2 Pt 1):C283–C296. doi: 10.1152/ajpcell.1995.268.2.C283. [DOI] [PubMed] [Google Scholar]
- 40.Bianchini L, L’Allemain G, Pouysségur J. The p42/p44 mitogen-activated protein kinase cascade is determinant in mediating activation of the Na+/H+ exchanger (NHE-1 isoform) in response to growth factors. J Biol Chem. 1997;272(1):271–279. doi: 10.1074/jbc.272.1.271. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Silva N, Lucchesi PA, et al. Phosphorylation and regulation of the Na+/H+ exchanger through mitogen-activated protein kinase. Biochemistry. 1997;36(30):9151–9158. doi: 10.1021/bi970802f. [DOI] [PubMed] [Google Scholar]
- 42.Pederson SF, Varming C, Christensen ST, Hoffmann EK. Mechanisms of activation of NHE by cell shrinkage and by calyculin A in Ehrlich ascites tumor cells. J Membr Biol. 2002;189(1):67–81. doi: 10.1007/s00232-001-0190-2. [DOI] [PubMed] [Google Scholar]
- 43.Garnovskaya MN, Mukhin Y, Raymond JR. Rapid activation of sodium-proton exchange and extracellular signal-regulated protein kinase in fibroblasts by G protein-coupled 5-HT1A receptor involves distinct signalling cascades. Biochem J. 1998;330(Pt 1):489–495. doi: 10.1042/bj3300489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen SF, Darborg BV, Rentsch ML, Rasmussen M. Regulation of mitogen-activated protein kinase pathways by the plasma membrane Na+/H+ exchanger, NHE1. Arch Biochem Biophys. 2007;462(2):195–201. doi: 10.1016/j.abb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Németh ZH, Deitch EA, Szabó C, et al. Na+/H+ blockade inhibits enterocyte inflammatory response and protects against colitis. Am J Physiol Gastrointest Liver Physiol. 2002;283(1):G122–G132. doi: 10.1152/ajpgi.00015.2002. [DOI] [PubMed] [Google Scholar]
- 46.Chen S, Khan ZA, Karmazyn M, Chakrabarti S. Role of endothelin-1, sodium hydrogen exchanger-1 and mitogen activated protein kinase (MAPK) activation in glucose-induced cardiomyocyte hypertrophy. Diabetes Metab Res Rev. 2007;23(5):356–367. doi: 10.1002/dmrr.689. [DOI] [PubMed] [Google Scholar]
- 47.Cechin SR, Dunkley PR, Rodnight R. Signal transduction mechanisms involved in the proliferation of C6 glioma cells induced by lysophosphatidic acid. Neurochem Res. 2005;30(5):603–611. doi: 10.1007/s11064-005-2747-4. [DOI] [PubMed] [Google Scholar]
- 48.Mukhin YV, Garnovskaya MN, Ullian ME, Raymond JR. ERK is regulated by sodium-proton exchanger in rat aortic vascular smooth muscle cells. J Biol Chem. 2004;279(3):1845–1852. doi: 10.1074/jbc.M304907200. [DOI] [PubMed] [Google Scholar]
- 49.Karmazyn M, Kilić A, Javadov S. The role of NHE-1 in myocardial hypertrophy and remodelling. J Mol Cell Cardiol. 2008;44(4):647–653. doi: 10.1016/j.yjmcc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Cox GA, Lutz CM, Yang CL, et al. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell. 1997;91(1):139–148. doi: 10.1016/s0092-8674(01)80016-7. [DOI] [PubMed] [Google Scholar]
- 51.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005;5(10):786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 52.Harguindey S, Orive G, Luis Pedraz J, Paradiso A, Reshkin SJ. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin – one single nature. Biochim Biophys Acta. 2005;1756(1):1–24. doi: 10.1016/j.bbcan.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Liu HF, Teng XC, Zheng JC, Chen G, Wang X-W. Effect of NHE1 antisense gene transfection on the biological behavior of SGC-7901 human gastric carcinoma cells. World J Gastroenterol. 2008;14(14):2162–2167. doi: 10.3748/wjg.14.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang X, Wang D, Dong W, Song Z, Dou K. Suppression of Na+/H+ exchanger 1 by RNA interference or amiloride inhibits human hepatoma cell line SMMC-7721 cell invasion. Med Oncol. 2011;28(1):385–390. doi: 10.1007/s12032-010-9447-x. [DOI] [PubMed] [Google Scholar]