Abstract
Pancreatic neuroendocrine tumors are rare and the majority of patients present in the advanced stage. Over the past few decades, treatment for patients with metastatic well- or moderately differentiated pancreatic neuroendocrine tumors have not significantly impeded tumor progression nor improved survival. However, recent mapping of intracellular signaling pathways promoting tumor proliferation, growth, and angiogenesis has presented mammalian target of rapamycin (mTOR) as a potential target within the phosphatidylinositol 3-kinase-Akt pathway. With the development of the new-generation mTOR inhibitor everolimus, a series of clinical trials over the last 5 years have demonstrated significant benefit in delaying tumor progression. This review focuses on the mechanism of mTOR inhibition and traces the development of clinical evidence for the use of mTOR inhibitors in well- to moderately differentiated advanced pancreatic neuroendocrine tumors.
Keywords: everolimus, mTOR, neuroendocrine, pancreatic, signaling, targeted
Introduction
Over two-thirds of neuroendocrine tumors (NET) occur in the gastrointestinal tract. Those which arise from the pancreas are known as islet cell tumors, or pancreatic NET (PNET), and constitute around 1% of all pancreatic cancers.1 Tumors may be nonfunctional or secretory and classified according to cellular origin (insulinoma, gastrinoma, glucagonoma, VIPoma, and somatostatinoma). They are further divided into well-,moderately, or poorly differentiated tumors based on mitotic and Ki-67 indices. Around two-thirds present in the advanced stage with unresectable tumors or metastases. In patients with metastatic disease, median survival is around 24–27 months.1,2 According to the Surveillance, Epidemiology, and End Results (SEER) registries, the annual incidence has increased from 1.09 per 100,000 in 1973 to 5.25 per 100,000 in 2004.1 Over the past few decades, there has been little impact on improving clinical outcome. For patients with unresectable and progressive disease, systemic treatments including interferon and chemotherapy entail severe side effects and have not made a significant impact on impeding slow growing, well-differentiated tumors. However, recent greater understanding of biological mechanisms driving tumor growth has identified new therapeutic opportunities. Although an array of targeted therapies have been investigated, recent milestone Phase III clinical trials have demonstrated forerunners sunitinib (multitarget tyrosine kinase inhibitor) and everolimus (mammalian target of rapamycin [mTOR] inhibitor) could significantly improve progression-free survival (PFS).3–7 The Food and Drug Administration granted approval for the use of everolimus in patients with progressive well- or moderately differentiated, unresectable localized or metastatic PNET in May 2011. This review focuses on the emerging role of everolimus in the treatment of PNET.
Cell signaling and the role of mTOR within the phosphatidylinositol 3-kinase (PI3K)-Akt pathway
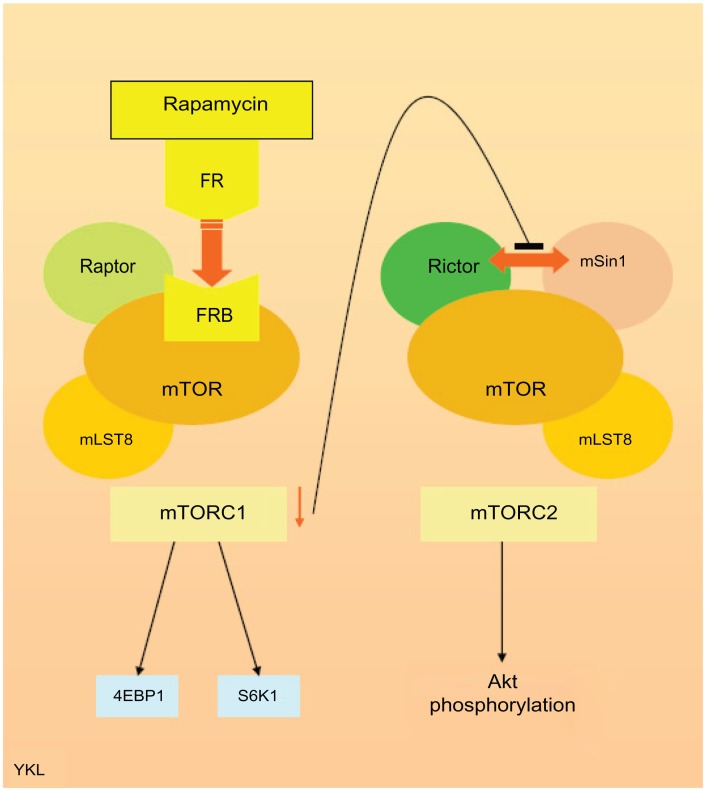
mTOR is a serine/threonine kinase intermediary within the PI3K-Akt pathway regulating cell growth, proliferation, and apoptosis.8 Its mode of action was studied via the macrolide rapamycin (sirolimus), which was originally used as an antifungal treatment approved by the Food and Drug Administration in 1999. However, due to its immunosuppressive properties, early use of mTOR inhibitors such as tacrolimus was for prevention of organ transplant rejection before being investigated as an anticancer therapy over the last decade with later drugs such as temsirolimus and everolimus. mTOR is a complex molecule comprising mTOR complex- 1 (mTORC1) and mTOR complex-2 (mTORC2), which regulate cellular function including proliferation, survival, and angiogenesis. mTORC1 consists of mTOR associated with two proteins: regulatory-associated protein of mTOR (raptor) and target of rapamycin complex subunit LST8. mTORC2 consists of mTOR and associated proteins: target of rapamycin complex subunit LST8, rapamycin-insensitive companion of mTOR (rictor), and mitogen-activated protein kinase-associated protein-1. mTORC1 regulates cellular proliferation via downstream regulators of translation: eukaryotic translation initiation factor 4E binding protein-1 and ribosomal S6 kinase-1 (S6K1). The role of mTORC2 is less well defined, but is known to directly phosphorylate Akt in the PI3K-Akt pathway. S6K1 from mTORC1 inhibits the PI3K-Akt pathway via suppression of insulin receptor substrate-1; thus, the mTORC1/mTORC2 complex is both an upstream and downstream regulator of cellular function.9,10 The intracellular receptor of rapamycin FKBP12 has been shown to bind directly to the corresponding FKBP12-rapamycin binding domain in mTORC1, suppressing downstream phosphorylation of S6K1 and eukaryotic translation initiation factor 4E binding protein-1. The FKBP12-rapamycin complex cannot bind directly to mTORC2. However, the inhibition of mTORC1 by this route then interferes with the binding of rapamycin-insensitive companion of mTOR and mitogen-activated protein kinase-associated protein-1 within the mTORC2 complex, subsequently leading to a reduction in downstream Akt signaling (Figure 1).
Figure 1.
Simplified diagram illustrating the mammalian target of rapamycin complex and mechanism of mammalian target of rapamycin inhibition by rapamycin.
Abbreviations: 4EBP1, eukaryotic translation initiation factor 4E binding protein-1; FR, FKBP12-rapamycin; FRB, FKBP12-rapamycin binding; mLST8, target of rapamycin complex subunit LST8; mSin1, mitogen-activated protein kinase-associated protein-1; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex-1; mTORC2, mammalian target of rapamycin complex-2; raptor, regulatory-associated protein of mammalian target of rapamycin; rictor, rapamycin-insensitive companion of mammalian target of rapamycin; S6K1, ribosomal protein S6 kinase-1.
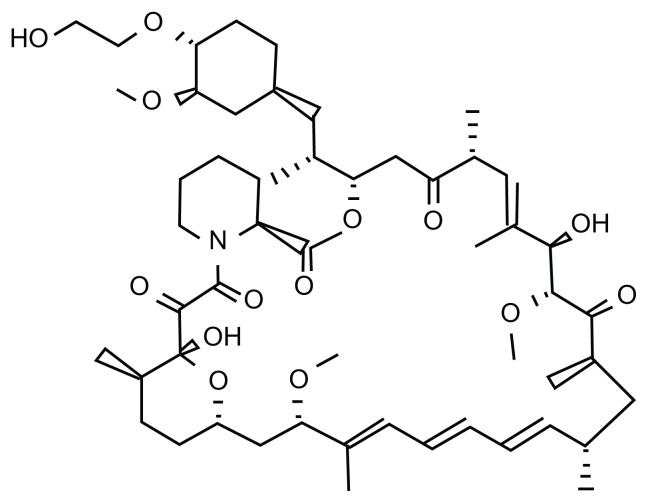
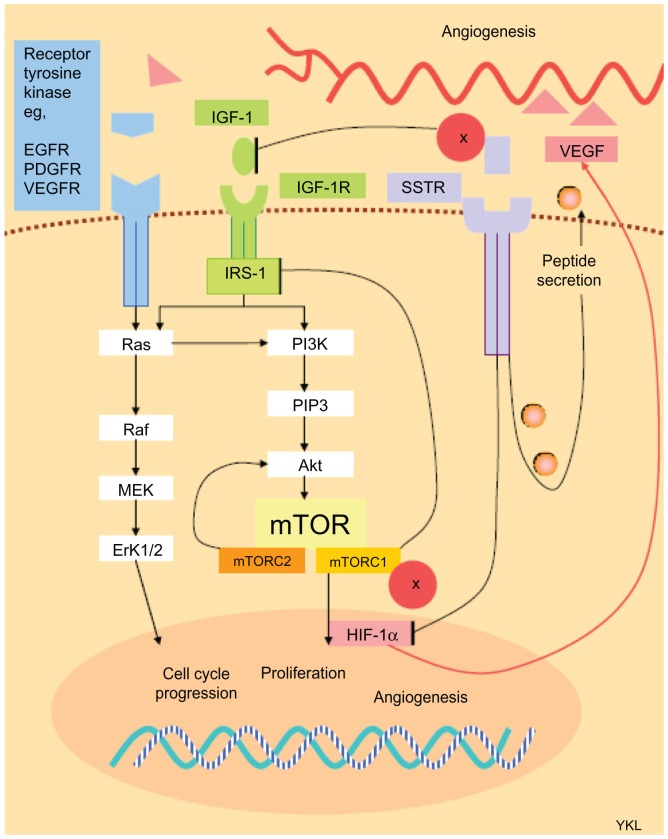
The PI3K-Akt-mTOR pathway has been implicated in the development and progression of NET.11–15 Initial use of temsirolimus produced modest results in 2006. Already granted Food and Drug Administration approval for the treatment of advanced renal carcinoma in 2009, the arrival of everolimus demonstrated clinical benefit in a series of important clinical trials in PNET over the past 5 years. Everolimus (RAD-001) is the 40-O-(2-hydroxyethyl) derivative of the macrolide rapamycin (Figure 2). However, unlike rapamycin, which is a universal inhibitor of mTORC1 and a cell type-specific inhibitor for mTORC2, everolimus is selective for mTORC1. Proof of concept was demonstrated in rodent-derived insulinoma (INS-1) cell lines when exposure to octreotide and everolimus produced downstream signal inhibition of S6K1.16 Similar results were also seen with human carcinoid cell lines.17 However, the effect on Akt regulation after mTOR inhibition remains unclear. mTOR inhibition in different tumor cell lines may promote apoptosis;18,19 or conversely, encourage cell survival. 20 It is postulated that the differential switching between suppression of the PI3K-Akt pathway by mTORC1 and phosphorylation of Akt by mTORC2 could dictate the fate of cell survival or death.9 In NET cell lines, treatment with octreotide and everolimus was shown to block cellular proliferation via the Akt-mTOR-S6K pathway.16 Nevertheless, clinical studies have corroborated the benefit of mTOR suppression in PNET, and it is probable that the overall effect of mTOR inhibition in PNET is tumor downregulation. However, the net effect of mTOR inhibition on collateral intracellular signaling networks is not straightforward because the regulation of the PI3K-Akt pathway in PNET is known to be further elaborated by other pathways including cross signaling from the insulin-like growth factor-1 receptor (IGF-1R) axis21,22 (Figure 3).
Figure 2.
Molecular structure of everolimus.
Figure 3.
Simplified diagram of the signaling network between surface receptors and intracellular pathways involved in tumor progression and angiogenesis in pancreatic neuroendocrine tumors, and interaction with mammalian target of rapamycin inhibition and somatostatin analog therapy.
Abbreviations: EGFR, epidermal growth factor receptor; ERK1/2, extracellular signal-regulated protein kinase 1/2; HIF-1α, hypoxia-inducible factor-1α; IGF-1 insulin-like growth factor-1; IGF-1R, insulin-like growth factor-1 receptor; IRS-1, insulin receptor substrate-1; MEK, mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; MTORC1, mammalian target of rapamycin complex-1; mTORC2, mammalian target of rapamycin complex-2; PDGFR, platelet-derived growth factor receptor; PI3K, phosphatidylinositol 3-kinase; PIP3, phosphatidylinositol (3,4,5)-triphosphate; SSTR, somatostatin receptor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Everolimus is available as oral medication which is absorbed rapidly, achieving peak concentration after 1.8 hours and reaching steady state after 7 days. Its pharmacokinetic properties are dose-dependent but not influenced by age or body weight.23 Reported side effects include stomatitis, rash, diarrhea, fatigue, nausea, headache, vomiting, anorexia, hyperglycemia, pruritus, thrombocytopenia, neutropenia, anemia, and pneumonitis. The developing role of everolimus through clinical trials is discussed in the following section.
Clinical data for mTOR inhibition in NET (Table 1)
Table 1.
Summary of neuroendocrine tumor clinical trials using mammalian target of rapamycin inhibitors
| Study author | Tumor type | n | Treatment | PR (%) | SD (%) | Outcome |
|---|---|---|---|---|---|---|
| Duran et al24 | NET | 36 (15 PNET) | Temsirolimus | 6 | 60 | mTTP 6 months |
| Yao et al4 | NET | 60 (29 PNET) | Everolimus 5 mg once daily vs 10 mg once daily with octreotide | 22 | 70 | Improved mPFS with 10-mg once-daily dose |
| Yao et al5 RADIANT 1 |
PNET | 160 | Everolimus vs everolimus + octreotide | 9.6 vs 4.4 | 67.8 vs 80 | 9.7 months vs 16.7 months |
| Yao et al32 | NET | 39 | Everolimus with bevacizumab | 26 | 69 | mPFS 14.4 months |
| Yao et al6 RADIANT 3 |
PNET | 410 | Everolimus vs placebo | 5 vs 2 | 73 vs 51 | mPFS 11 months vs 4.6 months |
Abbreviations: mPFS, median progression-free survival; mTTP, median time-to-progression; NET, neuroendocrine tumor; PNET, pancreatic neuroendocrine tumor; PR, partial response; RADIANT, RAD001 In Advanced Neuroendocrine Tumors trial; SD, stable disease; vs, versus.
Temsirolimus was an early mTOR inhibitor which demonstrated clinical benefit in a nonrandomized Phase II trial that consisted of 36 patients with progressive PNET (n = 15) or carcinoid tumors (n = 21) receiving intravenous temsirolimus at a weekly dose of 25 mg.24 Confirmed partial response (PR) was observed in two patients with an overall confirmed response rate (RR) of 6% out of the 33 evaluable patients (6.7% in the PNET group). In intention-to-treat analysis, overall RR was 5.6% (95% confidence interval [CI]: 0.6%–18.7%) and tumor control rate (stable disease + PR) was 63.9% (95% CI: 46.2%–79.2%). Overall disease stabilization was achieved in 60% (20/33) with duration of response lasting more than 2 months. Nevertheless, nearly a third progressed on treatment. Median time-to-progression was 6 months (95% CI: 3.7 months–upper limit not reached), with a 6-month PFS rate of 48.1% (95% CI: 33.0%–70.1%) and a 1-year PFS rate of 40.1% (95% CI: 23.8%–67.4%).
With the introduction of everolimus, an open-label, Phase II, dose-finding, pilot study evaluated 60 patients with low- to intermediate-grade NET (including 29 patients with PNET) comparing 5 mg/day versus 10 mg/day combined with octreotide long-acting repeatable (LAR) 30 mg every 28 days until disease progression or minimum of twelve cycles.4 The drug was well tolerated, with 6% of the patients on 10 mg/day experiencing grade 3–4 leukopenia, hyperglycemia (9%), and hypophosphatemia (11%). Three patients (9%) experienced grade 2 pneumonitis and one patient experienced grade 3 pneumonitis.
Results were encouraging with an overall confirmed PR rate of 22% and 70% disease stabilization. When the subgroup of PNET was analyzed, the confirmed PR rate was higher (27% and 60% had stable disease). The higher dose correlated with a better PR rate (30% versus 13%), lower risk of progression (3% versus 13%), and longer median PFS (72 weeks [95% CI: 60–83 weeks] versus 50 weeks [95% CI: 23–78 weeks]). Responses were delayed; the best effect was seen after 12 months of therapy. Biochemical response and reduction in Ki-67 were also reported. Although the study was not powered for prospective survival analysis, encouraging results meant that the 10-mg dose was adopted as the standard in follow-on studies by the investigators.
RADIANT 1 (RAD001 In Advanced Neuroendocrine Tumors 1) was a dedicated multicenter Phase II PNET study for patients with progressive well to moderately differentiated unresectable or metastatic disease.5 In order to stratify for the effect of somatostatin analog treatment, patients were divided into those who were naive to octreotide (n = 115) to receive everolimus at a dose of 10 mg/day; and those who had previously progressed on octreotide prior to entry (n = 45) to continue with octreotide LAR in combination with everolimus at 10 mg/day. The primary endpoint of the study was objective RR (ORR) in the everolimus only group. Secondary endpoints included ORR in the combination group, overall PFS, duration of response, overall survival, and safety.
In the everolimus group, according to Response Evaluation Criteria in Solid Tumors at central review, ORR was 9.6% (95% CI: 4.9%–16.5%), stable disease was 67.8%, PFS was 9.7 months (95% CI: 8.3–13.3 months), and overall survival was 24.9 months (95% CI: 20.2–27.1 months). In the combination group, ORR was 4.4% (95% CI: 0.5%–15.1%), stable disease was 80%, PFS was 16.7 months (95% CI: 11.1 months–upper limit not available), and overall survival was not reached after a follow-up period of over 16 months. Although this trial did not aim to assess any superiority of everolimus in combination with octreotide LAR, the authors attributed improved PFS to the additional stabilizing effect observed with octreotide.25–27 Although RR was lower than previously observed in the pilot trial (9.6% versus 27%), this was attributed to a smaller cohort and less stringent entry criteria not requiring evidence of progression in the earlier study.
Combination treatment was well tolerated and side effects most frequently requiring dosage adjustment or interruption included hyperglycemia (7.8%), stomatitis (7.0%), diarrhea (5.2%), and pyrexia (4.3%) in the monotherapy group, and thrombocytopenia (11.1%), pyrexia (11.1%) and stomatitis (8.9%) in the combination group. Pneumonitis was grade 2 or lower, and manageable with dose reduction or interruption of treatment.
The follow-on multicenter, randomized, double-blinded, placebo-controlled, Phase III trial (RADIANT 3) allocated 410 patients with progressive well- or moderately differentiated PNET to best supportive care with everolimus 10 mg/day or placebo.6 The primary endpoint was PFS based on Response Evaluation Criteria in Solid Tumors. Patients were treated until progression or development of unacceptable side effects, and crossover to everolimus was allowed on progression. After a median follow-up period of 17 months, the authors reported a significant improvement in PFS in the everolimus group compared to placebo (11 months versus 4.6 months), leading to a significant reduction in risk of progression or death (hazard ratio 0.35 [95% CI: 0.27–0.45]; P < 0.001) through stabilization of disease. Benefit was irrespective of age, gender, race, World Health Organization performance status, prior treatment (chemotherapy or somatostatin analog), or tumor grade.
Treatment with everolimus also led to a statistically significant increase in tumor response and disease stabilization (PR 5% versus 2% and 73% versus 51%, respectively; P < 0.001). Most frequent grade 3–4 toxicities in the everolimus group compared to placebo included stomatitis (7% versus 0%), anemia (6% versus 0%), and hyperglycemia (5% versus 2%). Although more deaths were seen in the everolimus group (6% versus 2%), only one was considered to be drug related. The lack of overall survival benefit was attributed to 73% of the patients who were initially allocated placebo crossing over to everolimus at progression.
Potential biomarkers
Dose- and time-dependent rise in lactate dehydrogenase with everolimus treatment has been associated with better PFS. This is postulated to be related to tumor hypoxia from mTOR inhibition.4 Biochemical responses in chromogranin A and neuron-specific enolase have also been shown to correlate with response to chemotherapy and improved PFS.5
In one report, microarray analysis of 72 PNET with seven matched metastatic lesions compared to normal pancreatic tissue identified several potential prognostic biomarkers. Shorter survival was correlated with downregulated tuberous sclerosis-2, downregulated phosphatase and tensin homolog, and low expression of somatostatin receptor-2 (SSTR2). There was also increased likelihood of liver metastases in tumors overexpressing the fibroblast growth factor-13 (FGF13) gene.12
Multitargeted therapy in combination with everolimus and future studies
mTOR inhibition plus bevacizumab
Hypoxia-inducible factor-1α is a downstream effector in the PI3K-Akt pathway which promotes angiogenesis (Figure 3). mTOR inhibition has been shown to target tumor vessel proliferation and metastasis.28–30 Moreover, the antiangiogenic mechanism of mTOR inhibition has been found to be distinct from that derived from vascular endothelial growth factor (VEGF) receptor inhibition;31 therefore, the combination of mTOR inhibition with an anti-VEGF antibody would seem a logical therapeutic option. Dual inhibition with everolimus and bevacizumab was evaluated in a small study of 39 patients with low- to intermediate-grade NET ≥ 3 cm using functional computed tomography.31 Patients were treated with either everolimus or bevacizumab for a 21-day initial cycle before receiving a combination of both drugs. Functional imaging at multiple time points demonstrated treatment with bevacizumab alone resulted in a significant (32%; P < 0.01) decrease in tumor blood flow and treatment with everolimus alone resulted in a significant (13%; P = 0.02) increase in mean blood transit time. However, combination treatment showed synergistic antitumor activity where a further decrease in blood flow and increase in mean transit time was observed. When functional imaging results were compared to Response Evaluation Criteria in Solid Tumors for response, bidimensional tumor shrinkage correlated with functional imaging markers including higher baseline permeability, increased posttreatment mean blood transit time, and reduced tumor blood flow and blood volume. Thus, with these encouraging results, a Phase II study aiming to randomize 138 patients with unresectable locally advanced or metastatic PNET to everolimus with or without bevacizumab is underway, with PFS as the primary endpoint.
Synergistic effect of somatostatin analog treatment with everolimus
Somatostatin analog therapy has been traditionally administered for controlling carcinoid symptoms. Its direct effect is to block peptide secretion by binding to somatostatin cell surface receptors. However, there is evidence of a secondary antiproliferative effect in well-differentiated NET irrespective of the functional status of the tumors.25,26,33 It is postulated that somatostatin analog therapy attenuates intracellular signaling via the IGF-1R axis,34 and it has been shown that stimulation of IGF-1R causes crossactivation of the PI3K-Akt cascade.21,22 Therefore, inhibition of the IGF-1R channel could lead to a physiological downregulation of cellular progression via the PI3K-Akt pathway. However, targeted disruption of mTOR has been demonstrated to cause upstream positive feedback and increase Akt activity via the IGF-1R pathway, leading to the attenuation of an antiproliferative effect and contributing to a possible route for tumor resistance.18,21,22,35 Additionally, octreotide therapy also has an antiangiogenic effect via modulation of the intracellular levels of VEGF and hypoxia-inducible factor-1α;36 thus, dual inhibition could lead to improved antitumor activity. This could explain the possible synergistic effect of octreotide LAR when combined with everolimus.
Pasireotide (SOM 230) is a new-generation somatostatin analog which binds to more SSTR subtypes than octreotide (SSTR1, SSTR2a, SSTR2b, SSTR3, and SSTR5). It also exhibits antiangiogenic properties by inhibiting VEGF secretion37 and suppressing IGF-1.38 A pilot study showed symptomatic response in patients refractory to octreotide without any increase in the occurrence of side effects.39 Two trials are underway to evaluate the combination of everolimus and pasireotide: COOPERATE-1 (Efficacy of Everolimus Alone or in Combination with Pasireotide LAR in Advanced PNET-1) will assess safety and tolerability and COOPERATE-2 (Efficacy of Everolimus Alone or in Combination with Pasireotide LAR in Advanced PNET-2) aims to randomize 150 patients with advanced PNET to receive everolimus with or without pasireotide LAR, with PFS as the primary endpoint.
Further trials
Several current clinical trials are evaluating a range of targeted agents in addition to everolimus. In the Phase I SORAVE (Sorafenib and Everolimus in Solid Tumors) trial, multitarget tyrosine kinase inhibitor sorafenib (targets include receptors for VEGF and platelet-derived growth factor) is used in patients with refractory solid tumors. For patients with low- to intermediate-grade NET, epidermal growth factor receptor inhibitor erlotinib is combined with everolimus in a Phase II study assessing response as the primary endpoint; cixutumumab, a fully humanized antibody to IGF-1R, is to be evaluated in combination with everolimus in a Phase I study.
Conclusion
Cell line studies demonstrating the feasibility of mTOR inhibition have largely been translated into impressive outcomes in clinical studies in PNET. However, despite significant improvement in median PFS demonstrated in the largest clinical trial to date, tumor shrinkage has remained modest. In RADIANT 3, the ORR rate was 5% in the everolimus group. This is low in comparison to results in RADIANT 1 and the pilot study (a 9.6% and 22% PR rate, respectively). Accepting the heterogeneity of cohorts between the trials, mechanisms which impede treatment apoptosis should be addressed. For example, the effect of mTOR inhibition on upstream and downstream regulation within the PI3K-Akt and collateral signaling pathways has yet to be fully understood. The upregulation of mTORC2 and hyperactivation of Akt from selective mTORC1 inhibition by everolimus has been described as a possible route for tumor resistance. Further understanding of how mTOR inhibition influences the differential activation of upregulation versus downregulation of Akt by the mTORC1/mTORC2 complex could lead to the identification of genetic mutations and polymorphisms, development of tailored mTOR inhibitors, and more effective sequencing strategies for mTOR therapy.
The influence of parallel intracellular signaling pathways on the PI3K-Akt pathway is a complementary area of interest and suggests a potential benefit for multiple target inhibition in circumventing aberrant autocrine signaling and curbing potential escape mechanisms. Collateral pathways involved in tumor angiogenesis and cross triggering of the PI3K-Akt cascade by the IGF-1R axis is of current interest. Sequential and concurrent treatment with multitarget tyrosine kinase inhibitor sunitinib – which has also shown impressive results in a separate randomized, placebo-controlled, Phase III study – would be interesting to explore. Numerous clinical trials are underway to investigate a wide array of targeted therapies in combination with mTOR inhibition including antibodies to VEGF, epidermal growth factor receptor, IGF-1R, and new-generation somatostatin analogs. Building on recent milestones, further understanding of the complex interaction between intracellular signaling pathways is required to develop more effective future treatment strategies. Perhaps then could a better cytotoxic response be achieved in order to lead to the true benefit of improved overall survival.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid:” epidemiology of and prognostic factors for neuroen-docrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Solorzano CC, Lee JE, Pisters PW, et al. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery. 2001;130(6):1078–1085. doi: 10.1067/msy.2001.118367. [DOI] [PubMed] [Google Scholar]
- 3.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 4.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26(26):4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28(1):69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yim KL. Role of biological targeted therapies in gastroenteropancreatic neuroendocrine tumours. Endocrine. 2011;40(2):181–186. doi: 10.1007/s12020-011-9513-y. [DOI] [PubMed] [Google Scholar]
- 8.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16(4):525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 9.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 10.Smrz D, Kim MS, Zhang S, et al. mTORC1 and mTORC2 differentially regulate homeostasis of neoplastic and non-neoplastic human mast cells. Blood. 2011;118(26):6803–6813. doi: 10.1182/blood-2011-06-359984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah T, Hochhauser D, Frow R, Quaglia A, Dhillon AP, Caplin ME. Epidermal growth factor receptor expression and activation in neuroen-docrine tumours. J Neuroendocrinol. 2006;18(5):355–360. doi: 10.1111/j.1365-2826.2006.01425.x. [DOI] [PubMed] [Google Scholar]
- 12.Missiaglia E, Dalai I, Barbi S, et al. Pancreatic endocrine tumors: expression profiling evidences a role for Akt-mTOR pathway. J Clin Oncol. 2010;28(2):245–255. doi: 10.1200/JCO.2008.21.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verhoef S, van Diemen-Steenvoorde R, Akkersdijk WL, et al. Malignant pancreatic tumour within the spectrum of tuberous sclerosis complex in childhood. Eur J Pediatr. 1999;158(4):284–287. doi: 10.1007/s004310051073. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida A, Hatanaka S, Ohi Y, Umekita Y, Yoshida H. von Recklinghausen’s disease associated with somatostatin-rich duodenal carcinoid (somatostatinoma), medullary thyroid carcinoma and diffuse adrenal medullary hyperplasia. Acta Pathol Jpn. 1991;41(11):847–856. doi: 10.1111/j.1440-1827.1991.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 15.Yim KL, Cunningham D. Targeted drug therapies and cancer. Recent Results Cancer Res. 2011;185:159–171. doi: 10.1007/978-3-642-03503-6_10. [DOI] [PubMed] [Google Scholar]
- 16.Grozinsky-Glasberg S, Franchi G, Teng M, et al. Octreotide and the mTOR inhibitor RAD001 (everolimus) block proliferation and interact with the Akt-mTOR-p70S6K pathway in a neuro-endocrine tumour cell line. Neuroendocrinology. 2008;87(3):168–181. doi: 10.1159/000111501. [DOI] [PubMed] [Google Scholar]
- 17.Zitzmann K, De Toni EN, Brand S, et al. The novel mTOR inhibitor RAD001 (everolimus) induces antiproliferative effects in human pancreatic neuroendocrine tumor cells. Neuroendocrinology. 2007;85(1):54–60. doi: 10.1159/000100057. [DOI] [PubMed] [Google Scholar]
- 18.Thimmaiah KN, Easton J, Huang S, et al. Insulin-like growth factor I-mediated protection from rapamycin-induced apoptosis is independent of Ras-Erk1-Erk2 and phosphatidylinositol 3′-kinase-Akt signaling pathways. Cancer Res. 2003;63(2):364–374. [PubMed] [Google Scholar]
- 19.Beuvink I, Boulay A, Fumagalli S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120(6):747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Fumarola C, La Monica S, Alfieri RR, Borra E, Guidotti GG. Cell size reduction induced by inhibition of the mTOR/S6K-signaling pathway protects Jurkat cells from apoptosis. Cell Death Differ. 2005;12(10):1344–1357. doi: 10.1038/sj.cdd.4401660. [DOI] [PubMed] [Google Scholar]
- 21.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Wichert G, Jehle PM, Hoeflich A, et al. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000;60(16):4573–4581. [PubMed] [Google Scholar]
- 23.Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet. 2004;43(2):83–95. doi: 10.2165/00003088-200443020-00002. [DOI] [PubMed] [Google Scholar]
- 24.Duran I, Kortmansky J, Singh D, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95(9):1148–1154. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 26.Panzuto F, Di Fonzo M, Iannicelli E, et al. Long-term clinical outcome of somatostatin analogues for treatment of progressive, metastatic, well-differentiated entero-pancreatic endocrine carcinoma. Ann Oncol. 2006;17(3):461–466. doi: 10.1093/annonc/mdj113. [DOI] [PubMed] [Google Scholar]
- 27.Arnold R, Rinke A, Klose KJ, et al. Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol. 2005;3(8):761–771. doi: 10.1016/s1542-3565(05)00481-7. [DOI] [PubMed] [Google Scholar]
- 28.Bruns CJ, Koehl GE, Guba M, et al. Rapamycin-induced endothelial cell death and tumor vessel thrombosis potentiate cytotoxic therapy against pancreatic cancer. Clin Cancer Res. 2004;10(6):2109–2119. doi: 10.1158/1078-0432.ccr-03-0502. [DOI] [PubMed] [Google Scholar]
- 29.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 30.Seeliger H, Guba M, Kleespies A, Jauch KW, Bruns CJ. Role of mTOR in solid tumor systems: a therapeutical target against primary tumor growth, metastases, and angiogenesis. Cancer Metastasis Rev. 2007;26(3–4):611–621. doi: 10.1007/s10555-007-9077-8. [DOI] [PubMed] [Google Scholar]
- 31.Lane HA, Wood JM, McSheehy PM, et al. mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res. 2009;15(5):1612–1622. doi: 10.1158/1078-0432.CCR-08-2057. [DOI] [PubMed] [Google Scholar]
- 32.Yao JC, Phan AT, Fogleman D, et al. Randomized run-in study of bevacizumab (B) and everolimus (E) in low- to intermediate-grade neuroendocrine tumors (LGNETs) using perfusion CT as functional biomarker [abstract] J Clin Oncol. 2010;28(Suppl 15):4002. [Google Scholar]
- 33.Saltz L, Trochanowski B, Buckley M, et al. Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer. 1993;72(1):244–248. doi: 10.1002/1097-0142(19930701)72:1<244::aid-cncr2820720143>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 34.Pollak MN, Polychronakos C, Guyda H. Somatostatin analogue SMS 201–995 reduces serum IGF-I levels in patients with neoplasms potentially dependent on IGF-I. Anticancer Res. 1989;9(4):889–891. [PubMed] [Google Scholar]
- 35.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118(9):3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villaume K, Blanc M, Gouysse G, et al. VEGF secretion by neuroendocrine tumor cells is inhibited by octreotide and by inhibitors of the PI3K/Akt/mTOR pathway. Neuroendocrinology. 2010;91(3):268–278. doi: 10.1159/000289569. [DOI] [PubMed] [Google Scholar]
- 37.Zatelli MC, Piccin D, Vignali C, et al. Pasireotide, a multiple somatostatin receptor subtypes ligand, reduces cell viability in non-functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Endocr Relat Cancer. 2007;14(1):91–102. doi: 10.1677/ERC-06-0026. [DOI] [PubMed] [Google Scholar]
- 38.Petersenn S, Schopohl J, Barkan A, et al. Pasireotide (SOM230) demonstrates eff icacy and safety in patients with acromegaly: a randomized, multicenter, phase II trial. J Clin Endocrinol Metab. 2010;95(6):2781–2789. doi: 10.1210/jc.2009-2272. [DOI] [PubMed] [Google Scholar]
- 39.Kvols L, Wiedenmann B, Oberg K, et al. Safety and efficacy of pasireotide (SOM230) in patients with metastatic carcinoid tumors refractory or resistant to octreotide LAR: results of a phase II study [abstract] J Clin Oncol. 2006;24(Suppl 18):4082. [Google Scholar]