Abstract
A single-step multiplex PCR (here referred to as a duplex PCR) has been developed for simultaneous detection and diagnosis of Fasciola hepatica and F. gigantica. These species overlap in distribution in many countries of North and East Africa and Central and Southeast Asia and are similar in egg morphology, making identification from fecal samples difficult. Based on a comparative alignment of mitochondrial DNA (mtDNA) spanning the region of cox1-trnT-rrnL, two species-specific forward primers were designed, FHF (for F. hepatica) and FGF (for F. gigantica), and a single reverse primer, FHGR (common for both species). Conventional PCR followed by sequencing was applied using species-specific primer pairs to verify the specificity of primers and the identity of Fasciola DNA templates. Duplex PCR (using three primers) was used for testing with the DNA extracted from adult worms, miracidia, and eggs, producing amplicons of 1,031 bp for F. hepatica and 615 bp for F. gigantica. The duplex PCR failed to amplify from DNA of other common liver and intestinal trematodes, including two opisthorchiids, three heterophyids, an echinostomid, another fasciolid, and a taeniid cestode. The sensitivity assay showed that the duplex PCR limit of detection for each Fasciola species was between 0.012 ng and 0.006 ng DNA. Evaluation using DNA templates from 32 Fasciola samples (28 adults and 4 eggs) and from 25 field-collected stools of ruminants and humans revealed specific bands of the correct size and the presence of Fasciola species. This novel mtDNA duplex PCR is a sensitive and fast tool for accurate identification of Fasciola species in areas of distributional and zonal overlap.
INTRODUCTION
Two species of Fasciolidae (Trematoda; Platyhelminthes), i.e., Fasciola hepatica Linnaeus 1758 (mostly distributed in temperate zones) and Fasciola gigantica Cobbold 1856 (commonly in tropical regions), are the parasitic causative agents of fascioliasis, a common cosmopolitan disease which primarily occurs in domestic ruminant animals, including cattle, buffaloes, sheep, and goats. In recent years, an increasing number of human cases have been reported every year, particularly in the tropical developing countries, confirming its severe zoonotic transmission and emerging/reemerging parasitic status (7, 22, 25, 29, 30, 36, 37). Both species coexist in some countries of North and East Africa such as Egypt, Ethiopia, Niger, Kenya, and Tanzania and in countries of Central/Southeast Asia such as Pakistan, Iran, and China (6, 8, 9, 25, 30, 38). Eating raw or improperly cooked vegetables/plants contaminated with Fasciola metacercariae can lead to fascioliasis, with potentially fatal injuries to the liver and biliary tract (30, 48).
Shed eggs in fecal samples can scarcely be distinguished between the two Fasciola species, and they are easily confused with those of other trematodes (14, 30). Sensitive and accurate techniques for early diagnosis of neglected tropical diseases, including fascioliasis, are urgently required (17). To date, various diagnostic techniques for the identification and discrimination of Fasciola spp. have been developed, including the traditional detection of eggs in feces directly or after Kato-Katz based sedimentation (19, 43), the morphological examination of adults (38), the lateral flow immunoassay for serodiagnosis (28), and the enzyme-linked immunosorbent assay (indirect ELISA) (12, 13, 32, 36).
While often very sensitive, serological methods do not track cure very well and may not provide an accurate indication of active infection: infected individuals can remain seropositive for considerable lengths of time after treatment (44). Molecular methods targeting eggs in stool samples will be useful for this. An earlier study (42) found that eggs were absent from stool samples of almost all patients with patent fascioliasis 14 days after treatment with triclabendazole.
A number of molecular approaches targeting genomic DNA for identification/discrimination of every life stage of Fasciola in definitive and intermediate hosts have also been developed. These include different types of conventional PCR (31, 40, 1), real-time PCR (5, 10), loop-mediated isothermal amplification (3), and multiplex PCR (26, 27). The multiplex system simultaneously uses multiple specific primers in a single tube, detecting more than one target, and is therefore material and time saving, precise, efficient, and cost-effective (4). This is a suitable approach for the differential analysis of DNA templates from samples that may contain a mixture of pathogens, including trematodes (23, 41, 46) and some Fasciola spp. (10, 18, 26, 27, 45). In these multiplex reactions, nuclear regions, represented by two copies in a diploid genome, were chosen as the target rather than mitochondrial DNA (mtDNA). However, a single cell may contain hundreds of mitochondria, providing much more of a target DNA template for PCR (4, 24). The mtDNA genome of platyhelminths is a double-stranded DNA circle of 13.5 to 20 kb in size, containing 2 rRNAs, 22 tRNAs, and 12 protein-coding genes and noncoding regions (21). Complete or near-complete mtDNA genomes for various zoonotic parasitic pathogens (see http://gobase.bcm.umontreal.ca/) are available for designing multiplex primers of any desired level of specificity (see reference 24).
The objective of the present study was to develop and evaluate a sensitive and specific mitochondrial duplex PCR method for rapid, reliable, and simultaneous detection and differentiation of F. hepatica and F. gigantica that is applicable to all life cycle stages, including, importantly, eggs from fecal samples.
MATERIALS AND METHODS
Samples of parasites.
Table 1 presents a total of 32 individual Fasciola samples used in this study. Of 32 samples or DNA of Fasciola, 27 represented Fasciola gigantica from Vietnam and Thailand (23 adults, 1 miracidium, and 3 egg samples squeezed from the uteri of individual worms) and 5 F. hepatica (4 adults and 1 squeezed egg sample). Fasciola gigantica samples were collected from humans, cattle, buffaloes, goats, and sheep from Vietnam in the northern, central, and southern parts of the country, and an F. gigantica adult sample (FgigTL) was made available by Chalobol Wongsawad, Chiang Mai University, Thailand. Samples of F. hepatica were provided from Australia, Ecuador, Ethiopia, and Belgium. The majority of the samples have been morphologically and molecularly identified as described in our previous studies (22, 37).
Table 1.
Fasciola and platyhelminth species providing DNA template used in the assessment of the specificity and sensitivity for duplex PCR of F. hepatica and F. gigantica
| Sample no. | Sample name | Country where collecteda | Species | Life formb | Host | Reference |
|---|---|---|---|---|---|---|
| 1 | FspHU | Vietnam (HU) | F. gigantica | Adult | Human | This study |
| 2 | FgHaTiB | Vietnam (HT) | F. gigantica | Adult | Cattle | 37 |
| 3 | FspCB1 | Vietnam (CB) | F. gigantica | Adult | Cattle | 22 |
| 4 | FgNBD | Vietnam (NB) | F. gigantica | Adult | Goat | 37 |
| 5 | FhAUS | Australia | F. hepatica | Adult | Cattle | 22 |
| 6 | FspPT | Vietnam (PT) | F. gigantica | Adult | Buffalo | This study |
| 7 | FspBD | Vietnam (BD) | F. gigantica | Adult | Buffalo | This study |
| 8 | FspYB | Vietnam (YB) | F. gigantica | Adult | Goat | 37 |
| 9 | FspPY | Vietnam (PY) | F. gigantica | Adult | Cattle | 22 |
| 10 | FCBB | Vietnam (CB) | F. gigantica | Adult | Cattle | 37 |
| 11 | FspEQB | Ecuador | F. hepatica | Adult | Cattle | This study |
| 12 | FspX | Vietnam (SG) | F. gigantica | Miracidium | Human | 22 |
| 13 | FhETB | Ethiopia | F. hepatica | Adult | Cattle | This study |
| 14 | FNTD2 | Vietnam (NT) | F. gigantica | Adult | Goat | 37 |
| 15 | FNTO1 | Vietnam (NT) | F. gigantica | Adult | Sheep | 37 |
| 16 | FspB3 | Vietnam (HU) | F. gigantica | Adult | Cattle | 22 |
| 17 | FNTO2 | Vietnam (NT) | F. gigantica | Adult | Sheep | 37 |
| 18 | FNTD4 | Vietnam (NT) | F. gigantica | Adult | Goat | This study |
| 19 | FNTD6 | Vietnam (NT) | F. gigantica | Adult | Goat | 37 |
| 20 | FNTD8 | Vietnam (NT) | F. gigantica | Adult | Goat | 37 |
| 21 | FNTS8 | Vietnam (NT) | F. gigantica | Adult | Sheep | 37 |
| 22 | FNTD9 | Vietnam (NT) | F. gigantica | Adult | Goat | 37 |
| 23 | FspT4 | Vietnam (HU) | F. gigantica | Adult | Buffalo | 22 |
| 24 | FhBe | Belgium | F. hepatica | Adult | Cattle | 37 |
| 25 | FNTD7 | Vietnam (NT) | F. gigantica | Adult | Goat | This study |
| 26 | FspBD1 | Vietnam (BD) | F. gigantica | Adult | Human | 22 |
| 27 | FgQN2 | Vietnam (QN) | F. gigantica | Adult | Cattle | This study |
| 28 | FgigTL | Thailand | F. gigantica | Adult | Cattle | This study |
| 29 | FhAUS | Australia | F. hepatica | Eggs | This study | |
| 30 | FspCB1 | Vietnam (NB) | F. gigantica | Eggs | This study | |
| 31 | FspT4 | Vietnam (HU) | F. gigantica | Eggs | This study | |
| 32 | FgigTL | Thailand | F. gigantica | Eggs | This study | |
| 33 | CsND | Vietnam (ND) | Clonorchis sinensis | Adult | Human | 23 |
| 34 | OvBD | Vietnam (BD) | Opisthorchis viverrini | Adult | Human | 23 |
| 35 | HpMcND | Vietnam (ND) | Haplorchis pumilio | Adult | Human | This study |
| 36 | HTA1 | Vietnam (ND) | Haplorchis taichui | Adult | Human | This study |
| 37 | SfQN1 | Vietnam (QN) | Stellantchasmus falcatus | Adult | Human | This study |
| 38 | EcPT | Vietnam (PT) | Echinochasmus japonicus | Adult | Human | This study |
| 39 | FbN | Vietnam (HT) | Faciolopsis buski | Adult | Human | 24 |
| 40 | TsoVN | Vietnam (BN) | Taenia solium | Adult | Human | This study |
Capital letters in parentheses indicate provinces or cities in Vietnam where samples were isolated: HU, Hue city; HT, Ha Tay; CB, Cao Bang; NB, Ninh Binh; PT, Phu Tho; YB, Yen Bai; PY, Phu Yen; SG, Ho Chi Minh City; NT, Ninh Thuan; BD, Binh Dinh; QN, Quang Nam; ND, Nam Dinh; BN, Bac Ninh.
All eggs were squeezed from the uterus of an adult fluke.
The eggs from adult worms were obtained by laying the individual flukes on a glass slide and pressing them with forceps to squeeze the eggs out of the uterus (see reference 43). Eggs of each sample were washed with sterile water (0.5 ml water added to each egg sample) and then centrifuged (12,000 rpm for 10 min). The supernatant was removed and the washing and centrifugation repeated, leaving eggs at the bottom of the tube from which they could be obtained after decanting the supernatant.
For obtaining miracidia, eggs morphologically identified as being of a Fasciola sp. were recovered from feces of a human patient. A small number of eggs were allowed to develop and hatch in water (1 to 2 weeks), and 10 to 20 miracidia were collected from each patient. From these, a single miracidium was separated by microscopy for subsequent DNA extraction as previously described (22).
Samples of another eight platyhelminth species, used as controls for specificity, were collected by us in Vietnam and are listed toward the bottom of Table 1. All materials were preserved in 70% ethanol and kept at −20°C until being used for extraction of genomic DNA.
Genomic DNA extraction.
Total genomic DNA was extracted from the majority of samples listed in Table 1 using the commercial QIAamp DNA extraction kit (Qiagen Inc.) according to the manufacturer's instructions, briefly described in reference 23. Samples recently collected for this study were extracted by an AccuPrep genomic DNA extraction kit (Bioneer, Daejeon, South Korea). In the case of large adult worms, such as Fasciola and a few other species, only a piece of a single specimen was used in DNA extraction. For heterophyids, the whole individual fluke was used.
Additionally, for eggs squeezed from flukes or recovered from stools, an AccuPrep stool DNA extraction kit (Bioneer, South Korea) was used to obtain total genomic DNA, as previously described (24). The concentration of DNA samples was estimated using a GBC UV/visible 911A spectrophotometer (GBC Scientific Equipment Pty. Ltd., Australia).
Genomic DNA extracted from adult worms and squeezed eggs was diluted to a working concentration of 50 ng/μl and DNA from a single miracidium to 5 ng/μl; 1 μl of each DNA was used as the template in a PCR of 25 μl in volume.
Design of primers for semimultiplex PCR.
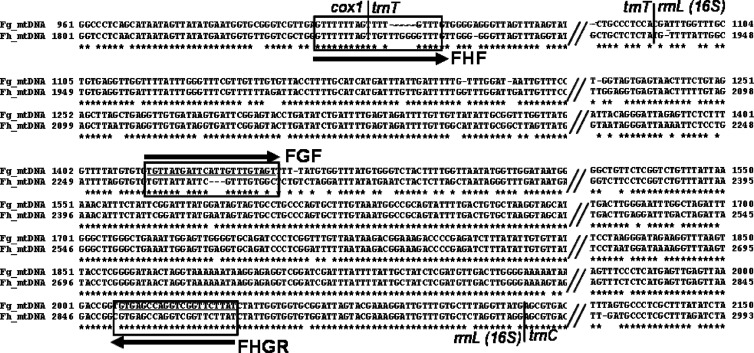
Mitochondrial nucleotide sequences spanning the 3′ region of the protein coding cox1 gene, all of the transfer RNAs for cysteine (trnC) and threonine (trnT), and most of rrnL (16S rRNA) of F. hepatica (20) and F. gigantica (unpublished data) were aligned (Fig. 1). Three primers, FHF (forward primer specific for F. hepatica), FGF (forward primer specific for F. gigantica), and FHGR (common reverse primer for both species), listed in Table 2, were designed. The reverse primer FHGR was based on regions conserved in the two species. A PCR product amplified by primer pair FHF/FHGR should be 1,031 bp for F. hepatica, and that amplified by FGF/FHGR should be 615 bp for F. gigantica (Table 2 and Fig. 1). The duplex PCR using the three-primer set could amplify products with about a 0.4-kb difference between the two species when they are clearly visualized on a 1% agarose gel stained with ethidium bromide and viewed under UV light.
Fig 1.
Comparative alignment of the mitochondrial genome spanning cox1-trnT-rrnL sequences of F. gigantica and F. hepatica for their variability between two species in regions chosen (boxed) for the species primers of multiplex PCR (here referred to as duplex PCR). The upper line in each block is the nucleotide sequence of F. gigantica (FspT4, Vietnam) and the lower is that of F. hepatica (FhAUS, Australia). Alignment gaps are indicated by hyphens and omitted regions by double slashes. Borders between genes are indicated. The horizontal arrows and names indicate direction (sense and antisense) of the primers (underlined), including FHF (forward F. hepatica primer), FGF (forward F. gigantica primer), and FHGR (common reverse primer).
Table 2.
Sequences of primers for duplex PCR of Fasciola spp.a
| Primer | Specific for | Sequence (5′–3′) | Length (bp) |
|---|---|---|---|
| FHF | F. hepatica | GTTTTTTAGTTGTTTGGGGTTTG | 23 |
| FGF | F. gigantica | TGTTATGATTCATTGTTTGTAG | 22 |
| FHGR | Both species | ATAAGAACCGACCTGGCTCAC | 21 |
The amplicon yielded by the FHF/FHGR primer pair is 1,031 bp, and that yielded by FGF/FHGR is 615 bp.
Uniplex and duplex PCR assays for confirmation of specificity of primers.
To test specificity, uniplex PCR using the species primers was performed on each DNA template of F. hepatica and F. gigantica, separately, and duplex PCR was performed using the three-primer set on mixed DNA of the two species. The duplex PCR assay was done with a range of pure genomic DNA from adults and that from squeezed eggs listed in Table 1. All the PCR products (10 μl of each) were examined on a 1% agarose gel, stained with ethidium bromide, and visualized under UV light (Wealtec, USA).
Uniplex PCR was carried out in a final volume of 25 μl, containing 12.5 μl PCR master mix from Promega, 1 μl of each primer (10 pmol/μl), 1.25 μl dimethyl sulfoxide (DMSO), 8.25 μl pure water, and 1 μl template (50 ng/μl). The addition of 5% DMSO to a PCR mixture greatly improves the reaction quality (16). A negative (no-DNA) control was included. The PCRs were carried out in an MJ PTC-100 thermal cycler (MJ Research, USA) with initiation at 95°C for 3 min, then 35 cycles, including denaturation at 95°C for 30 s, annealing at 52°C for 30 s, and extension at 72°C for 2 min, and then a final cycle of 7 min at 72°C to complete the amplification.
The duplex PCR mixture contained 2 μl of the three-primer set (10 pmol FHF, 10 pmol FGF, 20 pmol FHGR), 2 μl of DNA template mix (1 μl of 50 ng of F. hepatica and 1 μl of 50 ng of F. gigantica), and the remaining PCR components as described above.
The specificity of the primers was also determined by performing duplex PCR using DNA from adult Platyhelminthes species listed in Table 1, i.e., Opisthorchis viverrini, Clonorchis sinensis, Fasciolopsis buski, Taenia solium, Haplorchis pumilio, H. taichui, Echinochasmus japonicus, and Stellantchasmus falcatus.
Duplex PCR assay for template sensitivity.
The sensitivity of the duplex PCR was also assayed to establish the detection limit from a serially diluted DNA template mix, from F. hepatica (FhAU, Australian strain) and F. gigantica (FgT4, Vietnamese strain), as described above. A 2-fold serial dilution was started with 2 μl of this DNA mix (i.e., 2−1; 2−2…2−13, 2−14), providing genomic DNA template as 25, 12.5, 6.25, 3.13, 1.56, 0.78, 0.39…0.012, 0.006 ng, from each species in equal quantity in each reaction mixture. The assay was performed with 2 μl of the diluted template in each case. A negative (no-DNA) control was included. The PCR products (10 μl of each) were examined on a 1% agarose gel, stained with ethidium bromide, and visualized under UV light (Wealtec, USA).
Duplex PCR assay using template of reference Fasciola and eggs from stools of ruminants and humans. (i) Using DNA of the reference Fasciola template.
The duplex PCR was assayed using DNA extracted from 32 adult/miracidium/squeezed-egg samples of Fasciola spp., of which 20 were used as reference species and 12 as newly collected templates in this study (Table 1). The duplex assay was applied under optimized PCR conditions with the primer set, template, and components and thermal cycling as described above. FhAU (F. hepatica) and FgT4 (F. gigantica) were used as positive species-specific amplifications.
(ii) Using DNA template extracted from stools of ruminants and humans.
A total of 12 stool samples were freshly collected from ruminant animals (4 cattle, 8 water buffaloes) and 13 inhabitants (ranging from 18 to 45 years old) from different households in villages of Northern Vietnam (Ninh Binh Province) where Fasciola infection is endemic. The samples were kept on ice during transport to the laboratory. An AccuPrep stool DNA extraction kit (Bioneer, South Korea) was used to extract total genomic DNA from 100 mg of each stool sample. Duplex PCR compositions, conditions, controls, and evaluation were as described above.
For all duplex PCR tests, positive DNA (F. hepatica and F. gigantica, respectively) and negative (no-DNA) controls were included. Ten microliters of each amplicon of the tested reactions was examined on a 1% agarose gel, stained with ethidium bromide, and visualized under UV light (Wealtec).
RESULTS
Confirmation of primers and the Fasciola template by PCR.
DNA extracted from adult F. hepatica (FhBe, from Belgium) and F. gigantica (FspT4, from Vietnam) (22, 37) and their squeezed eggs were used to confirm the identities of the Fasciola templates. The PCR was set in a single reaction with template and single-species-specific primer pairs, and in a duplex form with mixed template and primers, for both adult and egg DNA of separate or mixed F. hepatica and F. gigantica (data not shown). Each PCR yielded a DNA product of the expected size. The nucleotide sequences from DNA products of each species were confirmed by sequencing (data not shown). This confirmed the specificity of primers for each Fasciola template, under single or duplex PCR conditions.
Specificity of the multiplex PCR.
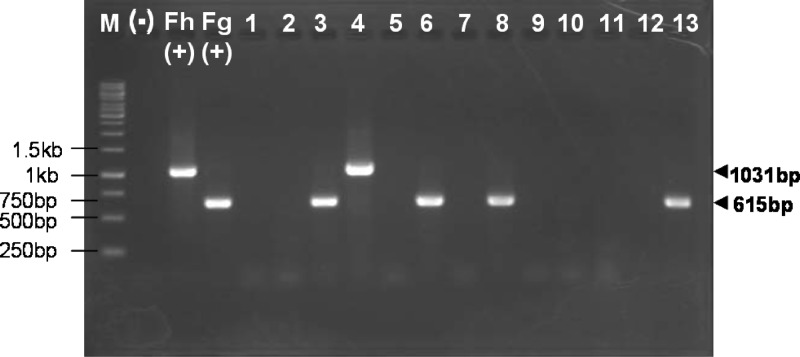
To determine the specificity of the multiplexing PCR performance (i.e., mixed primers), genomic DNA samples from Belgian F. hepatica, Vietnamese worms, including F. gigantica, and other platyhelminths that might yield eggs in fecal samples, such as C. sinensis, O. viverrini, Fasciolopsis buski, H. pumilio, H. taichui, Taenia solium, Stellantchasmus falcatus, and Echinochasmus japonicus, were used. After inspection on 1% agarose stained with ethidium bromide under UV light, PCR products were amplified only from DNA samples of F. hepatica or F. gigantica and from the egg templates of each of these two species. No cross-amplification occurred from eight other platyhelminth samples (Fig. 2).
Fig 2.
Specificity assay for assessment of duplex PCR using DNA template from different species, visualized on 1% agarose stained with ethidium bromide. M, 1-kb ladder marker; (−), negative control (no DNA); Fh (+) and Fg (+), F. hepatica and F. gigantica positive controls (100 ng DNA template in each case). Lanes: 1, C. sinensis (CsND); 2, O. viverrini (OvBD); 3, F. gigantica (FspT4); 4, F. hepatica (FhBe); 5, H. pumilio (HpMcND); 6, F. gigantica (FspCB1); 7, H. taichui (HTA1); 8, F. gigantica (FgigTL, adult); 9, Stellantchasmus falcatus (SfQN1); 10, Echinochasmus japonicus (EcPT); 11, Fasciolopsis buski (FbL2); 12, Taenia solium (TsoVN); 13, F. gigantica (FgigTL, eggs).
Reaction sensitivity as determined by the diluted template mix of F. hepatica and F. gigantica.
Twofold serial dilutions of a DNA template mix of two Fasciola strains (FhAU and FgT4) were used to assay the analytical sensitivity of the duplex PCR. For both species, the lower limit of detection was between 0.024 and 0.012 ng of mixed template, or 0.012 and 0.006 ng for each (data not shown). No DNA product was visualized in the negative control.
Assay for testing the duplex PCR using reference Fasciola spp.
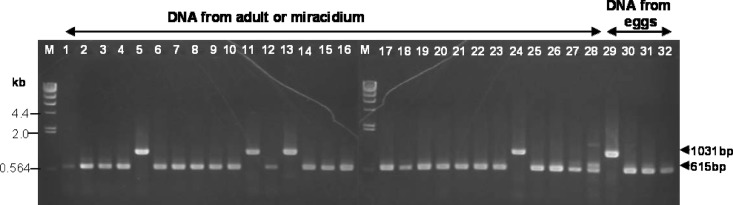
Genomic DNAs extracted from 27 adult, 1 miracidium, and 4 squeezed-egg samples of F. hepatica and F. gigantica, including 1 intermediate adult form (for species identification, see references 22 and 37), listed in Table 1, were used as templates in testing the duplex PCR assay. Amplicons of the anticipated sizes were obtained for these 32 samples (Fig. 3). Except for one sample (FspHU, adult, human [Fig. 3, lane 1]), the PCR amplicons yielded were good quality even from DNA template extracted from squeezed eggs. PCR products from DNA templates of 27 samples, including 8 new adult samples collected in Vietnam for this study (human, cattle, buffalo, goat), were 615 bp in length, indicative of F. gigantica mtDNA (Fig. 3). Four adult samples and one squeezed-egg sample of F. hepatica, regardless of geographical origins (i.e., Belgium, Australia, Ethiopia, and Equador), generated good-quality PCR products of 1,031 bp (Fig. 3).
Fig 3.
Testing of duplex PCR assay specificity using reference laboratory samples of adult, miracidium, and eggs squeezed from F. hepatica and F. gigantica. Lane M, molecular size marker (DNA of λ phage cut by HindIII); lanes 1 to 32, samples used as listed in Table 1, including eggs squeezed from each species.
Duplex PCR test with stool samples collected from ruminants and humans.
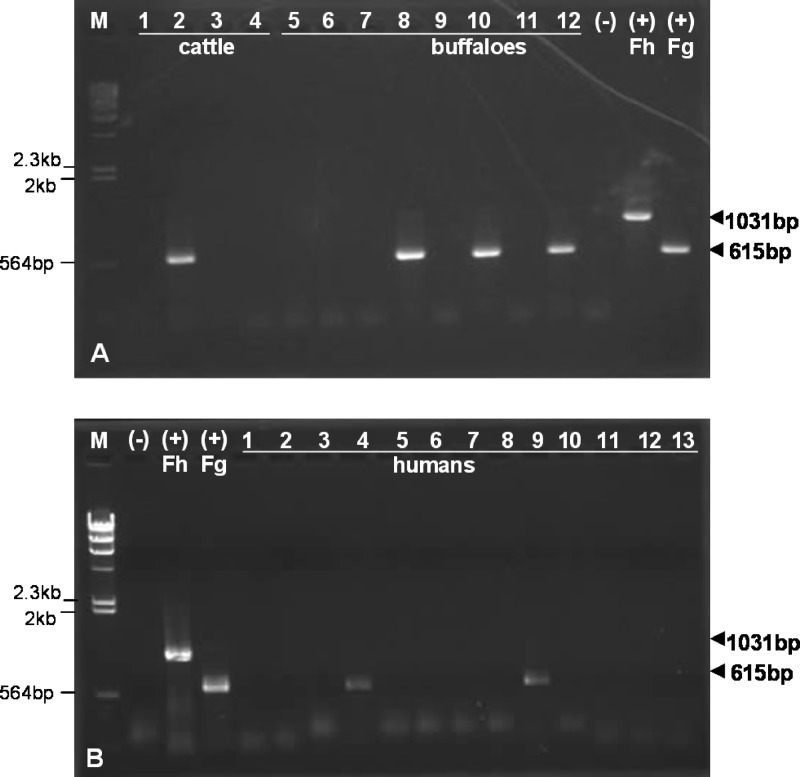
DNA template extracted from stool samples of ruminants (cattle and water buffaloes) and from humans in a province where Fasciola infection was endemic was tested with the duplex PCR. Results indicated that four DNA templates of 12 stools (one of four cattle; three of eight buffaloes) produced clear F. gigantica mtDNA products (Fig. 4A). None of them produced an F. hepatica mtDNA amplicon. Buffaloes are generally left to wander in wet meadows, where exposure to infection is likely. Cattle are usually penned up during the day.
Fig 4.
Specific duplex PCR assays for detection of F. hepatica or F. gigantica, using DNA material from eggs from feces of ruminants (A) and humans (B), collected in a province where infection with Fasciola sp. is endemic. Lanes: M, molecular size marker (DNA of λ phage cut by HindIII); (−), negative control (no DNA); (+) Fh and (+) Fg, F. hepatica and F. gigantica positive controls. Samples from humans in panel B are from each individual stool sample.
Of 13 human stools, 2 yielded amplicons of F. gigantica mtDNA (Fig. 4B). None of these samples produced PCR products of F. hepatica (Fig. 4A). Frequent, habitual consumption of incompletely washed raw vegetables is traditional in this area. The results of duplex PCR in ruminants and humans were in agreement with positive verification by microscopy of F. gigantica eggs in each case (data not shown). The number of eggs per gram of feces (EPG) was not calculated for any sample.
DISCUSSION
Vietnam is officially recognized as a country where Fasciola infection is endemic. Most human cases have been reported from Binh Dinh, Phu Yen, and Khanh Hoa Provinces in Central Vietnam, which are associated with local endemic animal fascioliasis (33, 34, 35, 47). Recent studies of cattle in Binh Dinh indicated an overall prevalence of 45.3% for Fasciola eggs using a sedimentation method (33, 34), 54.9% using a coprological approach, and 72.2% by serological analysis (35). Fascioliasis is clearly hyperendemic in cattle in Vietnam, with attendant risks for the human population.
The increasing number of human cases of Fasciola spp. (F. hepatica and F. gigantica) in humans and ruminants places a heavy burden on public health and veterinary services, particularly in countries of low development status (30). Because of their significance for public health and substantial economic loss caused in the livestock industry, effective methods for rapid and accurate detection of every life stage and identification of these two dangerous species are therefore needed for epidemiological surveys, clinical management, and infection control (39, 30, 4). A variety of morphological, immunological, molecular, and combined approaches have been developed, including conventional PCR and multiplex PCR methods. However, previously developed PCR/real-time/multiplex PCR methods (5, 26, 27, 45) used nuclear rather than mitochondrial targets. Mitochondrial DNA is probably a better choice for a multiplex PCR application, due to its stability and the likely higher copy number even in a single egg (24). We have successfully developed a multiplex system (i.e., a mitochondrial duplex PCR) for identification and discrimination of these two fasciolids. The mitochondrial DNA proved to be suitable target for this, distinguishing between F. hepatica and F. gigantica. The duplex PCR was assayed with 65 samples overall, comprising 40 laboratory samples (Table 1) and 25 fresh stools collected from ruminant animals and humans. The assay was specifically determined with a range of reference Fasciola life stages, including eggs (squeezed from adult worms and eggs in fecal samples), miracidia, and adults from different hosts (cattle, buffaloes, goats, sheep, and humans). The duplex PCR also reflected high species specificity among samples of different geographical origins, i.e., F. hepatica collected from Australia, Belgium, Ethiopia, and Ecuador and F. gigantica from Vietnam and Thailand.
The primer set (three primers) in the duplex reaction yielded amplicons specific in length for each Fasciola sp. These worked well in all templates tested, and they produced no amplicon from any other trematodes or from fecal samples containing eggs of other species. The duplex PCR assay in this study is highly sensitive, capable of producing amplicons visible in an agarose gel from as little as between 0.012 and 0.006 ng of each fasciolid in a mixed DNA template.
In this study, eggs squeezed from Fasciola adults and mixed eggs from fecal samples of ruminants and humans were used as a source of DNA. We have not yet tried our duplex PCR method using DNA from single eggs. However, Ai et al. (2) were able to amplify the nuclear ribosomal ITS2 region from a single egg of F. gigantica. In the absence of any other information, we assume that a single Fasciola egg contains about 1 to 6 pg of DNA, a small but probably adequate amount for yielding a PCR amplicon.
A number of diagnostic tools have been developed for single or simultaneous detection/discrimination of F. hepatica and F. gigantica, including morphological (using shape and size and morphometric features), immunodiagnostic (using monoclonal antibodies or copro-antigen (extracted from eggs in feces) and metacercarial/Fasciola-specific antigens for ELISA) and DNA-related loop-mediated isothermal amplification (LAMP), PCR (single or multiplex), restriction enzymatic, and sequencing methods (2, 3, 5, 11, 12, 13, 35). All the diagnostic methods developed so far have contributed to fast, accurate, and specific detection of Fasciola spp. Most have been coprological and serological methods, including antibody or antigen ELISA (Ab-ELISA or Ag-ELISA) and a couple of thioredoxin peroxidase- and saposin-like protein-2-based serodiagnoses (12, 49, 15). Fasciola spp. can elicit a specific antibody response which can be detected by Ab-ELISA as early as 1 to 2 weeks after infection (44), while shedding eggs are found in feces 10 to 12 weeks postinfection (30). Antibody detection tests are useful for determining seroprevalence in epidemiological studies but are not necessarily good indicators of active infection (44). A review (11) suggested that the most accurate, sensitive, and specific information could be determined easily and with low costs, making DNA-based tools available to investigate the epidemiology of the liver fluke in a laboratory with limited financial resources (11).
The multiplex approach developed here is highly sensitive and specific. It does not require very specific or expensive equipment and reagents, and it can make use of easily collected fecal samples. It is capable of distinguishing eggs of Fasciola species from those of other trematodes, and it also distinguishes between Fasciola species. The eggs shed in feces, normally persisting for a long period, can provide an easily accessed source of a DNA template for specific amplification, presenting a DNA multidisciplinary use for detection of contaminated trematodes and other intestinal parasites (11, 39). The presence of eggs in feces also can be evidence for the existence of live flukes in the host. Stools from a large number of patients could be collected easily. The duplex PCR assay developed in our study is an addition to the existing repertoire of molecular detection tools for Fasciola, with the utility of multiple-target DNA template use, time-saving performance, and cost-effectiveness. This combination of features makes it suitable for use in laboratories even in relatively poorly resourced areas. Cure can be assessed by serial testing of fecal samples for the presence of eggs. The identity of the species responsible for fascioliasis in areas where both species occur and the identity of species in agricultural and domestic animals will be valuable epidemiological information.
Our mitochondrial DNA-targeting duplex PCR assay is not able to discriminate between diploid and triploid Fasciola spp., which indeed, differ only in chromosomes, not in mitochondrial DNA. To solve this problem, it might be possible to perform karyotyping to see if sperm are present in the seminal vesicle, a common situation in triploids and parthenogenetic diploids (if live flukes are available), or to use an alternative molecular approach.
In conclusion, the duplex PCR method targeting mitochondrial DNA of F. hepatica and F. gigantica demonstrated successful detection of any life stage of these two zoonotic species. This is a useful tool in the clinical field for detection of F. hepatica and F. gigantica in diverse hosts where both species cooccur in areas where the infection is endemic.
ACKNOWLEDGMENTS
This work was supported by the National Foundation for Science and Technology Development (NAFOSTED) (grant no. 106.16-2010.60) and a small grant by Ministry of Health in Vietnam to Thanh Hoa Le.
We express thanks to colleagues of veterinary and public health provincial stations for their kind provision of materials used in this study. We extend our thanks to David Blair of School of Marine and Tropical Biology, James Cook University (Townsville, Australia), for the invaluable review of the manuscript.
Footnotes
Published ahead of print 12 June 2012
REFERENCES
- 1. Ai L, et al. 2011. Genetic characterization, species differentiation and detection of Fasciola spp. by molecular approaches. Parasit. Vectors 4:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ai L, et al. 2010. Specific PCR-based assays for the identification of Fasciola species: their development, evaluation and potential usefulness in prevalence surveys. Ann. Trop. Med. Parasitol. 104(1):65–72 [DOI] [PubMed] [Google Scholar]
- 3. Ai L, et al. 2010. Rapid identification and differentiation of Fasciola hepatica and Fasciola gigantica by a loop-mediated isothermal amplification (LAMP) assay. Vet. Parasitol. 174(3–4):228–233 [DOI] [PubMed] [Google Scholar]
- 4. Ai L, et al. 2011. Genetic diversity and relatedness of Fasciola spp. isolates from different hosts and geographic regions revealed by analysis of mitochondrial DNA sequences. Vet. Parasitol. 181(2–4):329–334 [DOI] [PubMed] [Google Scholar]
- 5. Alasaad S, et al. 2011. A TaqMan real-time PCR-based assay for the identification of Fasciola spp. Vet. Parasitol. 179(1–3):266–271 [DOI] [PubMed] [Google Scholar]
- 6. Ali H, et al. 2008. Genetic characterisation of Fasciola samples from different host species and geographical localities revealed the existence of F. hepatica and F. gigantica in Niger. Parasitol. Res. 102:1021–1024 [DOI] [PubMed] [Google Scholar]
- 7. Amer S, et al. 2011. Identification of Fasciola species isolated from Egypt based on sequence analysis of genomic (ITS1 and ITS2) and mitochondrial (NDI and COI) gene markers. Parasitol. Int. 60(1):5–12 [DOI] [PubMed] [Google Scholar]
- 8. Amor N, et al. 2011. Molecular characterization of Fasciola spp. from the endemic area of northern Iran based on nuclear ribosomal DNA sequences. Exp. Parasitol. 128(3):196–204 [DOI] [PubMed] [Google Scholar]
- 9. Ashrafi K, et al. 2006. Phenotypic analysis of adults of Fasciola hepatica, Fasciola gigantica and intermediate forms from the endemic region of Gilan, Iran. Parasitol. Int. 55:249–260 [DOI] [PubMed] [Google Scholar]
- 10. Caron Y, Righi S, Lempereur L, Saegerman C, Losson B. 2011. An optimized DNA extraction and multiplex PCR for the detection of Fasciola sp. in lymnaeid snails. Vet. Parasitol. 178(1–2):93–99 [DOI] [PubMed] [Google Scholar]
- 11. Caron Y, Rondelaud D, Losson B. 2008. The detection and quantification of a digenean infection in the snail host with special emphasis on Fasciola sp. Parasitol. Res. 103(4):735–744 [DOI] [PubMed] [Google Scholar]
- 12. Charlier J, De Meulemeester L, Claerebout E, Williams D, Vercruysse J. 2008. Qualitative and quantitative evaluation of coprological and serological techniques for the diagnosis of fasciolosis in cattle. Vet. Parasitol. 153(1–2):44–51 [DOI] [PubMed] [Google Scholar]
- 13. Demerdash ZA, et al. 2011. Diagnostic efficacy of monoclonal antibody based sandwich enzyme linked immunosorbent assay (ELISA) for detection of Fasciola gigantica excretory/secretory antigens in both serum and stool. Parasit. Vectors. 4:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Detwiler JT, Criscione CD. 2010. An infectious topic in reticulate evolution: introgression and hybridization in animal parasites. Genes 1:102–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Figueroa-Santiago O, Delgado B, Espino AM. 2011. Fasciola hepatica saposin-like protein-2-based ELISA for the serodiagnosis of chronic human fascioliasis. Diagn. Microbiol. Infect. Dis. 70(3):355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frackman S, Kobs G, Simpson D, Storts D. 1998. Betaine and DMSO: enhancing agents for PCR. Promega Notes. Promega Corp., Madison, WI [Google Scholar]
- 17. Johansen MV, Sithithaworn P, Bergquist R, Utzinger J. 2010. Towards improved diagnosis of zoonotic trematode infections in Southeast Asia. Adv. Parasitol. 73:171–195 [DOI] [PubMed] [Google Scholar]
- 18. Kaset C, Eursitthichai V, Vichasri-Grams S, Viyanant V, Grams R. 2010. Rapid identification of lymnaeid snails and their infection with Fasciola gigantica in Thailand. Exp. Parasitol. 126(4):482–488 [DOI] [PubMed] [Google Scholar]
- 19. Katz N, Chaves A, Pellegrino J. 1972. A simple device for quantitative stool thick smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. São Paulo 14:397–400 [PubMed] [Google Scholar]
- 20. Le TH, Blair D, McManus DP. 2001. Complete DNA sequence and gene organization of the mitochondrial genome of the liverfluke, Fasciola hepatica L. (Platyhelminthes; Trematoda). Parasitology 123(6):609–621 [DOI] [PubMed] [Google Scholar]
- 21. Le TH, Blair D, McManus DP. 2002. Mitochondrial genomes of parasitic flatworms. Trends Parasitol. 18:206–213 [DOI] [PubMed] [Google Scholar]
- 22. Le TH, et al. 2008. Human fascioliasis and the presence of hybrid/introgressed forms of Fasciola hepatica and Fasciola gigantica in Vietnam. Int. J. Parasitol. 38(6):725–730 [DOI] [PubMed] [Google Scholar]
- 23. Le TH, De NV, Blair D, Sithithaworn P, McManus DP. 2006. Clonorchis sinensis and Opisthorchis viverrini: development of a mitochondrial-based multiplex PCR for their identification and discrimination. Exp. Parasitol. 112(2):109–114 [DOI] [PubMed] [Google Scholar]
- 24. Le TH, Nguyen NT, Truong NH, De NV. 2012. Development of mitochondrial loop-mediated isothermal amplification (mito-LAMP) for detection of the small liver fluke Opisthorchis viverrini (Opisthorchiidae; Trematoda; Platyhelminthes). J. Clin. Microbiol. 50:1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lotfy WM, et al. 2008. Evolutionary origins, diversification, and biogeography of liver flukes (Digenea, Fasciolidae). Am. J. Trop. Med. Hyg. 79(2):248–255 [PMC free article] [PubMed] [Google Scholar]
- 26. Magalhães K. G., et al. 2008. Isolation and detection of Fasciola hepatica DNA in Lymnaea viatrix from formalin-fixed and paraffin-embedded tissues through multiplex-PCR. Vet. Parasitol. 152(3–4):333–338 [DOI] [PubMed] [Google Scholar]
- 27. Magalhães KG, Passos LKJ, dos Santos Carvalho O. 2004. Detection of Lymnaea columella infection by Fasciola hepatica through multiplex-PCR. Mem. Inst. Oswaldo Cruz 99(4):421–424 [DOI] [PubMed] [Google Scholar]
- 28. Martínez-Sernández V, et al. 2011. Development and evaluation of a new lateral flow immunoassay for serodiagnosis of human fasciolosis. PLoS Negl. Trop. Dis. 5(11):e1376 doi:10.1371/journal.pntd.0001376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mas-Coma S, Bargues MD, Valero MA. 2005. Fascioliasis and other plant-borne trematode zoonoses. Int. J. Parasitol. 35(11–12):1255–1278 [DOI] [PubMed] [Google Scholar]
- 30. Mas-Coma S, Valero MA, Bargues MD. 2009. Chapter 2. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasitol. 69:41–146 [DOI] [PubMed] [Google Scholar]
- 31. McGarry JW, Ortiz PL, Hodgkinson JE, Goreish I, Williams DJ. 2007. PCR-based differentiation of Fasciola species (Trematoda: Fasciolidae), using primers based on RAPD-derived sequences. Ann. Trop. Med. Parasitol. 101(5):415–421 [DOI] [PubMed] [Google Scholar]
- 32. Muiño L, et al. 2011. Molecular and immunological characterization of Fasciola antigens recognized by the MM3 monoclonal antibody. Mol. Biochem. Parasitol. 179(2):80–90 [DOI] [PubMed] [Google Scholar]
- 33. Nguyen ST, et al. 2012. Molecular identification of Fasciola spp. (Digenea: Platyhelminthes) in cattle from Vietnam. Parasite 19(1):85–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen ST, et al. Prevalence of Fasciola in cattle and of its intermediate host Lymnaea snails in central Vietnam. Trop. Anim. Health Prod., in press. doi:10.1007/s11250-012-0147-8 [DOI] [PubMed] [Google Scholar]
- 35. Nguyen TG, et al. 2011. Bovine fasciolosis in the human fasciolosis hyperendemic Binh Dinh province in Central Vietnam. Acta Trop. 117(1):19–22 [DOI] [PubMed] [Google Scholar]
- 36. Nguyen TG, et al. 2010. Assessment of a 27-kDa antigen in enzyme-linked immunosorbent assay for the diagnosis of fasciolosis in Vietnamese patients. Trop. Med. Int. Health 15(4):462–467 [DOI] [PubMed] [Google Scholar]
- 37. Nguyen TG, Van De N, Vercruysse J, Dorny P, Le TH. 2009. Genotypic characterization and species identification of Fasciola spp. with implications regarding the isolates infecting goats in Vietnam. Exp. Parasitol. 123(4):354–361 [DOI] [PubMed] [Google Scholar]
- 38. Periago MV, et al. 2008. First phenotypic description of Fasciola hepatica/Fasciola gigantica intermediate forms from the human endemic area of the Nile Delta, Egypt. Infect. Genet. Evol. 8(1):51–58 [DOI] [PubMed] [Google Scholar]
- 39. Robinson MW, Dalton JP. 2009. Zoonotic helminth infection with particular emphasis on fasciolosis and other trematodiases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364:2763–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rokni MB, et al. 2010. Identification and differentiation of Fasciola hepatica and Fasciola gigantica using a simple PCR-restriction enzyme method. Exp. Parasitol. 124(2):209–213 [DOI] [PubMed] [Google Scholar]
- 41. Sugiyama H, et al. 2006. Application of multiplex PCR for species discrimination using individual metacercariae of Paragonimus occurring in Thailand. Southeast Asian J. Trop. Med. Public Health 37(Suppl. 3):48–52 [PubMed] [Google Scholar]
- 42. Talaie H, et al. 2004. Randomized trial of a single, double and triple dose of 10 mg/kg of a human formulation of triclabendazole in patients with fascioliasis. Clin. Exp. Pharmacol. Physiol. 31(11):777–782 [DOI] [PubMed] [Google Scholar]
- 43. Valero MA, Perez-Crespo I, Periago MV, Khoubbane M, Mas-Coma S. 2009. Fluke egg characteristics for the diagnosis of human and animal fascioliasis by Fasciola hepatica and F. gigantica. Acta Trop. 111(2):150–159 [DOI] [PubMed] [Google Scholar]
- 44. Valero MA, et al. 2012. Assessing the validity of an ELISA test for the serological diagnosis of human fascioliasis in different epidemiological situations. Trop. Med. Int. Health. doi:10.1111/j.1365-3156.2012.02964.x [DOI] [PubMed] [Google Scholar]
- 45. Velusamy R, Singh BP, Raina OK. 2004. Detection of Fasciola gigantica infection in snails by polymerase chain reaction. Vet. Parasitol. 120(1–2):85–90 [DOI] [PubMed] [Google Scholar]
- 46. Webster BL, Rollinson D, Stothard JR, Huyse T. 2010. Rapid diagnostic multiplex PCR (RD-PCR) to discriminate Schistosoma haematobium and S. bovis. J. Helminthol. 84(1):107–114 [DOI] [PubMed] [Google Scholar]
- 47. WHO 2007. Report of a WHO informal meeting on the use of triclabendazole in fascioliasis control. World Health Organization, Geneva, Switzerland [Google Scholar]
- 48. Yen TJ, Hsiao CH, Hu RH, Liu KL, Chen CH. 2011. Education and imaging: hepatobiliary and pancreatic: chronic hepatic abscess associated with fascioliasis. J. Gastroenterol. Hepatol. 26(3):611. [DOI] [PubMed] [Google Scholar]
- 49. Zhang W, Rogniaux H, Huang W, Chauvin A, Moreau E. 2011. Analysis of thioredoxin peroxidase as a promising antigen for diagnosis of Fasciola gigantica infection: a preliminary study. Parasitol. Int. 60(2):206–208 [DOI] [PubMed] [Google Scholar]