Abstract
Helicobacter cinaedi colonizes the colons of human and animals and can cause colitis, cellulitis, and sepsis in humans, with infections in immunocompromised patients being increasingly recognized. However, methods for analyzing the molecular epidemiology of H. cinaedi are not yet established. A genotyping method involving multilocus sequence typing (MLST) was developed and used to analyze 50 H. cinaedi isolates from Japanese hospitals in addition to 6 reference strains. Pulsed-field gel electrophoresis (PFGE) results were also compared with the MLST results. Based on the genomic information from strain CCUG18818, 21 housekeeping genes were selected as candidates for MLST and were observed to have high homology (96.5 to 100%) between isolates. Following a comparison of the 21 housekeeping genes from 8 H. cinaedi isolates, 7 genes were chosen for MLST, revealing 14 sequence types (STs). The isolates from 3 hospitals belonged to the same STs, but the isolates from the other 4 hospitals belonged to different STs. Isolates belonging to ST6 were analyzed by PFGE and showed similar, but not identical, patterns between isolates. Isolates belonging to ST9, ST10, and ST11, which belonged to the same clonal complex, had the same pattern. All isolates were found to contain mutations in GyrA and the 23S rRNA gene that confer ciprofloxacin and clarithromycin resistance, respectively, in H. cinaedi. These results raise concerns about the increase in H. cinaedi isolates resistant to clarithromycin and ciprofloxacin in Japan.
INTRODUCTION
Helicobacter cinaedi is a motile, Gram-negative, spiral bacterium that colonizes the colons of humans and animals; it is mainly isolated from blood and feces. The first H. cinaedi infection in humans was reported in 1984, after isolation from a homosexual man (32). Since then, H. cinaedi infections have usually been detected in immunocompromised patients (1, 12, 13, 15, 16, 20, 31), although in some cases, they may also occur in patients with normal immunity (9, 33). The clinical manifestations of H. cinaedi include enteritis, proctocolitis, cellulitis, arthritis, and bacteremia. Septicemia and meningitis resulting from an H. cinaedi infection have also been observed in a neonate (21).
The prevalence of H. cinaedi in humans and other animals has not been well investigated, and the mechanism and timing of H. cinaedi infection remain controversial. Since H. cinaedi has been detected in the feces of normal animals, including hamsters and monkeys, these animals may be natural reservoirs for H. cinaedi (5, 7). However, as the growth of H. cinaedi is slower than that of other bacteria present in the colon, the presence of H. cinaedi in feces may be undetected in some cases. In addition, the atmospheric conditions required for the growth of H. cinaedi are not commonly available in most laboratories. Indeed, the number of reports of H. cinaedi infection have increased as the knowledge of Helicobacter spp. has expanded, and the possible nosocomial spread of H. cinaedi was recently reported (18).
To identify the routes of H. cinaedi transmission, molecular epidemiologic analyses are required; however, a method is not yet available to classify H. cinaedi isolates. Many molecular epidemiologic tools, such as pulsed-field gel electrophoresis (PFGE), ribotyping, restriction fragment length polymorphism, and arbitrary-primer PCR, have been developed for use in bacteria. However, multilocus sequence typing (MLST) is increasingly becoming one of the gold standards by which isolates can be classified and identified as a result of the progressive improvements in DNA sequencing techniques (4, 25). PFGE is a highly discriminatory typing method that has been used for epidemiological analysis of many pathogenic bacteria. However, it is difficult to obtain clear band patterns using PFGE in some cases, and a low typeability of H. pylori by this technique has been reported (2). In contrast, the genomic DNA sequences needed for MLST analysis can be obtained from any isolate, if the appropriate culture conditions are available. MLST can also be used to analyze the genetic interrelationship between isolates. Therefore, MLST would be useful for elucidating the route of H. cinaedi infection. In this study, we developed MLST for H. cinaedi and compared the results obtained with observed PFGE patterns. Since the antimicrobial susceptibilities of H. cinaedi isolates from Japan have not yet been reported, we also measured antimicrobial susceptibilities and analyzed the genes related to antimicrobial resistance.
MATERIALS AND METHODS
H. cinaedi isolates and culture.
Fifty H. cinaedi isolates were obtained from blood or fecal samples from patients treated in seven hospitals in Japan. Twelve isolates were obtained from Sapporo City General Hospital (hospital A) in Sapporo; the other 38 isolates were obtained from six hospitals in Tokyo: 2 isolates from the Social Insurance Chuo General Hospital (hospital B), 6 isolates from Toranomon Hospital (hospital C), 6 isolates from Teikyo University Hospital (hospital D), 3 isolates from Nihon University Itabashi Hospital (hospital E), 4 isolates from Toho University (hospital F), and 17 isolates from Surugadai Nihon University Hospital (hospital G). Details regarding the clinical manifestation of the patients were available only for the 26 isolates from hospitals A to D. These patients consisted of 7 women and 19 men, with a mean age of 61.3 years (age range, 28 to 88 years). Most of these patients were immunocompromised and had diseases such as malignant lymphoma, non-Hodgkin's lymphoma, and chronic renal failure. Four of the 26 isolates were obtained from feces, 1 was from a colon biopsy specimen, and the remaining isolates were recovered from patient blood samples. The 12 isolates from hospital A were isolated from the same ward between April and June 2008. Two isolates from hospital B were also isolated from the same ward in May 2005. Of the 6 isolates from hospital C, 2 were from same ward in October 2004, and the remaining 4 isolates were isolated sporadically from different wards in 2000 and 2008. Six isolates from hospital D were isolated sporadically between 2003 and 2008. The 6 reference strains used in this study—CCUG18818, CCUG18819, CCUG43521, MIT 99-5915, MIT 00-5434, and MIT 01-5002—were previously described by Taylor et al. (30). CCUG18818, CCUG18819, and MIT 99-5915 were human isolates from the United States recovered in 1986, 1986, and 1999, respectively. CCUG43521 was isolated from a human in Australia in 2000. MIT 00-5434 and MIT 00-5002 were isolated from rhesus monkeys in the United States in 1999 and 2000, respectively.
All isolates were subcultured on brucella agar (Becton, Dickinson, Franklin Lakes, NJ), with 5% horse blood, under microaerobic conditions with hydrogen obtained by the gas replacement method using an anaerobic gas mixture (H2, 10%; CO2, 10%; and N2, 80%) (5, 6). H. cinaedi isolates were identified by morphological analysis and by DNA sequencing of both the 16S rRNA and the 23S rRNA genes. The primers H276f (5′-CTATGACGGGTATCCGGC-3′) and C05R (5′-ACTTCACCCCAGTCGCTG-3′), reported by Riley et al. (22), were used for amplification and DNA sequencing of the 16S rRNA gene. For the amplification and DNA sequencing of the 23S rRNA gene, the same primers used for MLST (described below) were used.
Multilocus sequence typing.
Genomic information for CCUG18818 was obtained from the NCBI database (http://www.ncbi.nlm.nih.gov/sites/genome), and 21 genes, chosen mainly from genes used for MLST of H. pylori and Campylobacter spp., were selected as candidate genes for MLST (see Table S1 in the supplemental material). H. cinaedi genomic DNA was obtained by the classical phenol-chloroform DNA extraction method, as described by Stauffer et al. (26). Primers for each gene were designed using Primer3 software (http://frodo.wi.mit.edu/primer3/) to amplify approximately 700 bp of each gene. Amplification was performed using Ex Taq polymerase (TaKaRa Bio Inc., Shiga, Japan) under the following conditions: initial denaturation at 94°C for 2 min, followed by 35 cycles of 94°C for 30 s, 58°C for 20 s, and 72°C for 30 s, and a final extension step at 72°C for 5 min. PCR products were analyzed by DNA sequencing using the same primers used for amplification. Eight isolates from 4 hospitals (2 from each hospital) were analyzed, and the sequences were compared to select the appropriate genes for MLST. Genes with high homology between isolates were excluded; the 7 genes listed in Table 1 were ultimately used for MLST analysis. Both strands of DNA from each isolate were sequenced, and each gene was analyzed in all the isolates to determine its allele number. Alignment was performed by ATGC version 6 software (Genetyx Corporation, Tokyo Japan). For each gene, the alleles of CCUG18818 were assigned allele number 1, and the alleles from number 2 onward were assigned for each gene, according to the order in which the genes were encountered. The sequence type (ST) was defined by the allelic profile determined based on the combination of the 7 alleles. A clonal complex (CC) was defined as a group of STs in which every ST shared 6 alleles with at least 1 other member of the group, using the eBURST software program (http://eburst.mlst.net/). The phylogeny for the 56 isolates was estimated using concatenated sequences, comprising the 7 loci, via the neighbor-joining method with the maximum composite likelihood model using MEGA (version 5.05) software (27).
Table 1.
Genes and primers used for MLST analysis of H. cinaedi
| Gene | ID | Putative gene product | Primer (5′–3′) |
Product size (bp) | |
|---|---|---|---|---|---|
| Forward | Reverse | ||||
| 23S rRNA | AY596254 | 23S rRNA | GAAGGACGTACTAAGCTGCGAT | GTTTGGCCTTTCACCCCTAT | 762 |
| ppa | HCCG_01345 | Inorganic pyrophosphatase | TCTCTCAAAAGTATCAGTAGGCGA | GCCCTTGTAGGCTTTGATTG | 514 |
| aspA | HCCG_00537 | Aspartate ammonia-lyase | GGCGGCTCTAGCAAATAATG | CCGTATCTTGTGTCGCTTCA | 650 |
| aroE | HCCG_00648 | Shikimate 5-dehydrogenase | CGCACATTCTAAATCCCCAC | TAAGGCTAGGGCTGCTTGAT | 688 |
| atpA | HCCG_02167 | ATP synthase subunit alpha | TGTGGTTGGACGCGTTATTA | TGGCAATGCTGTAAGTGAGC | 646 |
| tkt | HCCG_01495 | Transketolase | AATCTGCTTCACTAGCCGGA | CTGTGGAAAATCGCCTTCAT | 665 |
| cdtB | HCCG_01069 | Cytolethal distending toxin B subunit | GGTGTAGCATTTGGTGCGAT | TCAAGTATGCCTCCGCTTCT | 635 |
Pulsed-field gel electrophoresis.
The PFGE method for Helicobacter hepaticus, reported previously (24), was modified and used in this study. Organisms cultured for 3 days were harvested and suspended in a solution containing 1 M NaCl and 10 mM EDTA [pH 8.0] and then embedded in 1% low-melting-point agarose with plug molds (Bio-Rad, Hercules, CA). The plugs were treated with lysozyme (1 mg/ml) in a solution containing 1 M NaCl, 0.1 M EDTA [pH 8.0], 10 mM Tris-HCl, 0.2% sodium deoxycholate, and 0.5% sodium N-lauryl-sarcosine at 37°C for 4 h, followed by a 1-mg/ml solution of proteinase K in 0.25 M EDTA [pH 8.0], and 1% sodium N-lauryl-sarcosine at 50°C for 24 h. The plugs were washed four times with TE buffer (10 mM Tris-HCl and 1 mM EDTA [pH 8.0]). The DNA plugs were washed with a solution containing 10 mM Tris-HCl and 1 mM EDTA [pH 8.0] and then washed with restriction enzyme buffer (New England BioLabs, Ipswich, MA) before digestion. The plugs were digested with 10 U of XhoI (New England BioLabs) or 10 U of SpeI (New England BioLabs) per plug in fresh restriction enzyme buffer at 37°C overnight.
PFGE of the digested plugs was carried out by the CHEF Mapper system (Bio-Rad) using 1% PFGE-grade agarose in 0.5× TBE (45 mM Tris base, 45 mM boric acid, 1 mM EDTA [pH 8]) with the following autoalgorithm for XhoI-digested DNA: 20- to 600-kb range, 6 V/cm, 120° included angle, an initial switch time of 2.98 s, and a final switch time of 54.17 s, with a linear switch time ramp for 27 h at 14°C. For SpeI-digested DNA, an autoalgorithm with a 20- to 200-kb range, an initial switch time of 2.98 s, and a final switch time of 17.33 s was used. A lambda ladder (Bio-Rad) was used as the molecular mass standard. After electrophoresis, the gel was stained in ethidium bromide (0.5 μg/ml) for 1 h, and DNA bands were visualized under UV light. Unfortunately, due to the inability to reculture 32 of the 50 Japanese H. cinaedi isolates from the stocks stored at −80°C, PFGE could be performed for only 18 isolates, of which 11 were from hospital A, 3 were from hospital F, and 4 were from hospital G. The 6 reference strains were also analyzed by PFGE.
Antimicrobial susceptibilities.
Since H. cinaedi causes bacteremia and sepsis, the susceptibilities of the isolates to antimicrobial agents used to treat systemic infections were measured using the agar dilution method. The susceptibilities of the previously mentioned 18 isolates from Japan and the 6 reference strains against the following antimicrobial agents were determined: amoxicillin-clavulanic acid, imipenem, clarithromycin, ciprofloxacin, minocycline, and gentamicin—drugs that might commonly be used to treat such infections. In brief, H. cinaedi cells were suspended in saline to achieve a turbidity equivalent to that of a McFarland 2.0 standard, and approximately 5 μl of the inoculum was spotted onto Muller Hinton agar (Becton, Dickinson) containing 5% horse blood and various concentrations of the antimicrobial agents. Concentrations of the antimicrobial agents ranged from 0.008 μg/ml to 128 μg/ml. The plates were incubated under microaerobic conditions for 2 days. The MIC was defined as the minimum concentration of the antimicrobial agent that inhibited the growth of H. cinaedi.
DNA sequencing of the 23S rRNA and gyrA genes of H. cinaedi.
The 23S rRNA gene and the gyrA gene of H. cinaedi were amplified using primer pairs of 23S-F2 (5′-CGGTGCTCGAAGGTTAAGAG-3′) and 23S-R2 (5′-TTCAGCGGTTATCACATCCA-3′), and gyrA F (5′-TCTCACACACGGAGCAAAAG-3′) and gyrA R (5′-CCTGCATTACCAAGGGCTAA-3′), respectively. These primers were also used for the DNA sequencing of the PCR products. The 23S rRNA sequences and GyrA amino acid sequences of all isolates were compared with those of CCUG18818 to identify mutations in the isolates used in this study.
RESULTS
Similarity of the 16S rRNA gene and the 23S rRNA gene between H. cinaedi isolates.
A comparison of a 1,022-bp section of the 16S rRNA gene, corresponding to the region from position 329 to 1350 of the 16S rRNA gene of CCUG18818 (GenBank accession number AB275317) indicated that the 50 H. cinaedi isolates had 98.8 to 99.9% similarity with the CCUG18818 strain. A 658-bp region of the 23S rRNA gene, corresponding to the region from 75 to 732 of the 23S rRNA gene of CCUG18818 (GenBank accession number AY596254), was also compared among the 50 isolates and 6 reference strains; the results indicated 99.5 to 100% similarity, confirming that all the isolates used in this study were H. cinaedi.
Diversity of 21 housekeeping genes in H. cinaedi.
To identify genes suitable for MLST, 21 candidate genes from 8 isolates, collected from 4 hospitals, were selected and amplified. The sequence comparisons of the isolates revealed low diversity in housekeeping genes among the H. cinaedi isolates. In fact, the majority of the genes tested in this preliminary experiment had high homology. Of the 21 genes investigated, 5 genes were found to have 100% homology between the 8 isolates, while only 1 polymorphism site was detected in 4 genes. For example, the allele of the yphC gene, which encodes the GTP-binding protein, was found to be identical in all isolates. Of the 21 genes examined from the 8 H. cinaedi isolates, 7 genes were found to have relatively high diversity and were selected for use in the MLST analysis (Table 1).
Characteristics of MLST genes in H. cinaedi isolates.
Genes from all of the clinical isolates and the 6 reference strains were successfully amplified and analyzed in this study. The characteristics of the 7 loci identified as being suitable for MLST are described in Table 2. These loci have lengths ranging between 411 and 658 bp, with a total composite length of 3,806 bp. The number of variable sites present in each allele ranged from 3 (tkt) to 21 (aroE), and the allele number for each gene ranged from 3 (cdtB) to 6 (aroE).
Table 2.
Characteristics and allelic profiles of seven loci used for H. cinaedi MLST
| Locus | Size (bp) | No (%) of polymorphic sites | Allelic profilea |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC1 |
CC4 |
CC7 |
CC8 |
CC9 |
CC12 |
CC13 |
CC14 |
|||||||||
| ST1 | ST2 | ST3 | ST4 | ST5 | ST6 | ST7 | ST8 | ST9 | ST10 | ST11 | ST12 | ST13 | ST14 | |||
| 23S rRNA | 658 | 4 (0.61) | 1 | 1 | 1 | 3 | 3 | 3 | 4 | 3 | 3 | 4 | 2 | 5 | 3 | 3 |
| ppa | 411 | 13 (3.16) | 1 | 1 | 1 | 3 | 3 | 2 | 2 | 4 | 2 | 2 | 2 | 5 | 4 | 4 |
| aspA | 532 | 6 (1.13) | 1 | 1 | 1 | 3 | 3 | 3 | 5 | 4 | 2 | 2 | 2 | 2 | 2 | 2 |
| aroE | 572 | 21 (3.67) | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 3 | 2 | 2 | 2 | 5 | 4 | 6 |
| atpA | 536 | 5 (0.93) | 1 | 1 | 1 | 2 | 4 | 2 | 3 | 2 | 2 | 2 | 2 | 5 | 2 | 2 |
| tkt | 562 | 3 (0.53) | 1 | 4 | 4 | 2 | 2 | 2 | 4 | 3 | 1 | 1 | 1 | 1 | 4 | 1 |
| cdtB | 535 | 9 (1.68) | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 3 | 1 | 2 |
ST, sequence type; CC, clonal complex.
Sequence types of H. cinaedi.
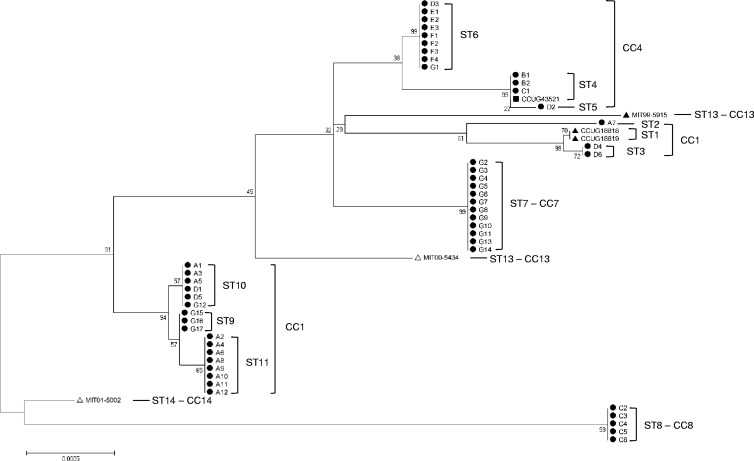
The 50 clinical isolates and the 6 reference strains were classified into 14 STs using the MLST method described in this study. The allelic profiles of the STs are presented in Table 2. Following eBURST analysis, ST1, ST2, and ST3 were defined as CC1. Similarly, ST4, ST5, and ST6 were assigned to CC4, while ST9, ST10, and ST11 formed CC9. Accordingly, the 56 isolates and reference strains were classified into 8 CCs. The phylogenetic tree for these strains is shown in Fig. 1. The phylogeny was estimated using concatenated sequences comprising the 7 loci (23S rRNA, ppa, aspA, aroE, atpA, tkt, and cdtB) with statistical support for the nodes being assessed via the bootstrap resampling method with 1,000 resamplings.
Fig 1.
Genetic relationship of sequence types among 50 H. cinaedi isolates from 7 hospitals in Japan and 6 reference strains. The phylogenic tree was constructed using the neighbor-joining method with MEGA (version 5.05) (27) software and the maximum composite likelihood model, with statistical support for the nodes being assessed via the bootstrap resampling method with 1,000 resamplings. The letter in each isolate's identifier indicates the hospital at which the strain was isolated (e.g., A1 to A12 were isolated from hospital A). ●, human isolates from Japan; ▲, human isolates from the United States; ■, human isolates from Australia; △, rhesus monkeys isolates from the United States; ST, sequence type; CC, clonal complex.
Relationship between the sequence types of H. cinaedi isolates and the hospitals from which they were isolated, including clinical manifestations.
H. cinaedi isolates were collected from 7 hospitals in Japan, and the distribution of STs in each hospital was compared (Table 3). Of the 12 isolates from hospital A, obtained over 3 months within the same ward, 11 isolates were CC9 [ST10 (n = 3), ST11 (n = 8)] and 1 isolate was CC1 (ST2). Both isolates from hospital B, isolated in the same ward over a 1-month period, were demonstrated to belong to the same ST, CC4 (ST4). Six isolates from hospital D, obtained sporadically between 2003 and 2008, revealed CC1 [ST3 (n = 2)], CC4 [ST5 (n = 1), ST6 (n = 1)], and CC9 [ST10 (n = 2)]. Clinical manifestations were available for 26 isolates and no relationships were identified between clonal complexes (STs) and patient age, diseases, and H. cinaedi-isolated clinical materials. The 3 isolates belonging to CC1 [ST2 (n = 1), ST3 (n = 2)] were isolated from female patients; however, CCUG18818 and CCUG18819 belonged to CC1 (ST1) and were reportedly isolated from male patients. All other isolates, belonging to CC4, CC8, and CC9, were isolated more frequently from male patients (the male/female ratios were 4/1, 5/0, and 10/3 in isolates belonging to CC4, CC8, and CC9, respectively). The 6 reference strains revealed different STs, compared to the Japanese isolates, except for CCUG43521 (from Australia), which was classified as CC4 (ST4), the same ST as the isolates from hospital B in Japan.
Table 3.
Prevalence of STs among H. cinaedi isolates from 7 hospitals in Japan
| Strains | Hospital or host | City or country | No. of isolates | Clonal complex [sequence type (no. of isolates)] |
|---|---|---|---|---|
| Clinical isolates from Japan (n = 18) | A | Sapporo | 12 | CC1 [ST2 (1)], CC9 [ST10 (3), ST11 (8)] |
| B | Tokyo | 2 | CC4 [ST4 (2)] | |
| C | Tokyo | 6 | CC4 [ST4 (1)], CC8 [ST8 (5)] | |
| D | Tokyo | 6 | CC1 [ST3 (2)], CC4 [ST5 (1), ST6 (1)], CC9 [ST10 (2)] | |
| E | Tokyo | 3 | CC4 [ST6 (3)] | |
| F | Tokyo | 4 | CC4 [ST6 (4)] | |
| G | Tokyo | 17 | CC4 [ST6 (1)], CC7 [ST7 (12)], CC9 [ST9 (3), ST10 (1)] | |
| Reference strains | ||||
| CCUG18818 | Human | USA | 1 | CC1 [ST1] |
| CCUG18819 | Human | USA | 1 | CC1 [ST1] |
| CCUG43521 | Human | Australia | 1 | CC4 [ST4] |
| MIT 99–5914 | Human | USA | 1 | CC12 [ST12] |
| MIT 00–5433 | Rhesus monkey | USA | 1 | CC13 [ST13] |
| MIT 01–5001 | Rhesus monkey | USA | 1 | CC14 [ST14] |
PFGE patterns of H. cinaedi and relationship with sequence types.
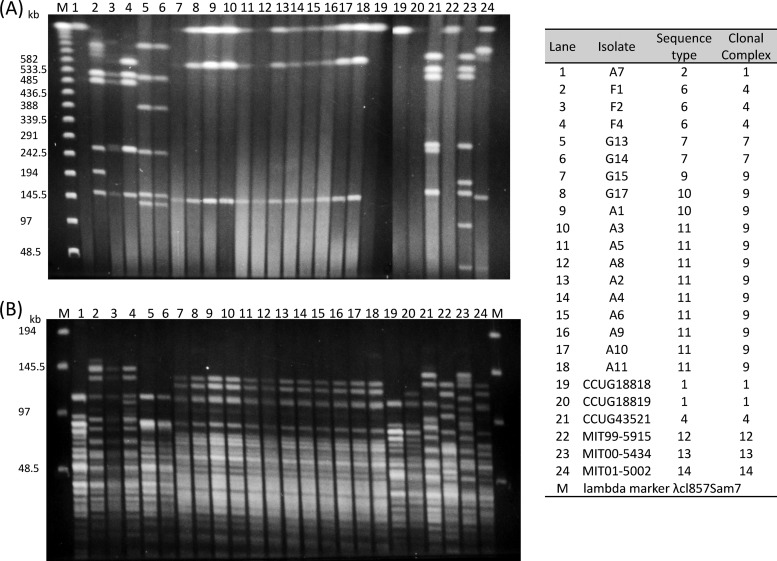
PFGE was performed on the 18 culturable clinical isolates and the 6 reference strains (Fig. 2). Based on the genomic information for CCUG18818 (available in the NCBI database), XhoI was chosen as the restriction enzyme because it cleaves chromosomal DNA less frequently. Seventeen of 18 isolates and 3 reference strains (CCUG43521, MIT 00-5434, and MIT 01-5002) were typed by XhoI digestion. Isolate A7, the CC1 [ST2] isolate obtained from hospital A, was not digested by XhoI, and similarly, CCUG18818 [CC1 (ST1)], CCUG18819 [CC1 (ST1)] and MIT 99-5915 [CC13 (ST13)] were nontypeable by XhoI digestion. SpeI digestion, which has been used for DNA digestion for PFGE analysis of H. cinaedi (13), was also tested, and all isolates were digested, though many small bands appeared.
Fig 2.
Pulsed-field gel electrophoresis patterns of XhoI-digested (A) and SpeI-digested (B) H. cinaedi isolates from Japan as well as the six reference strains. The letter in each isolate's identifier indicates the hospital at which the strain was isolated (e.g., A1 to A12 were isolated from hospital A).
Of the 18 Japanese isolates (11 from hospital A, 3 from hospital F, and 4 from hospital G), 10 isolates from hospital A and 2 isolates from hospital G revealed identical PFGE patterns. These 12 isolates belonged to CC9 [ST9 (n = 2), ST10 (n = 3), ST11 (n = 7)]. Another 2 isolates from hospital G, belonging to CC7 (ST7), also revealed identical PFGE patterns. Three isolates from hospital F, belonging to CC4 (ST6), revealed similar, but not identical, patterns between isolates. Isolate A7, which belonged to CC1 (ST2), revealed a pattern similar to that of CCUG18818 and CCUG18819, which belong to CC1 (ST1). CCUG43521, which belongs to CC4 (ST4), revealed a pattern similar to that of isolates F1, F2, and F4, which belong to CC4 (ST6).
Antimicrobial susceptibilities and antimicrobial resistance genes in H. cinaedi isolates.
The susceptibilities of 18 isolates of the H. cinaedi isolates from Japan as well as the 6 reference strains to the antimicrobial agents were measured. The MICs of each antimicrobial agent are shown in Table 4. Since significant differences were not observed between the MICs of amoxicillin and those of amoxicillin plus clavulanic acid, the MICs of amoxicillin are not shown in Table 4. Although the MICs of clarithromycin and ciprofloxacin were high for all isolates tested in this study (the MIC90s were >128 mg/liter and 128 mg/liter for clarithromycin and ciprofloxacin, respectively), the MICs of imipenem, minocycline, and gentamicin were relatively low (the MIC90s for imipenem, minocycline, and gentamicin were 0.125 mg/liter, 0.125 mg/liter, and 0.5 mg/liter, respectively). Although the breakpoints for H. cinaedi have yet to be determined, these isolates were obviously resistant to clarithromycin and ciprofloxacin (3, 17). To identify the mechanism of this resistance, the 23S rRNA and gyrA genes of all isolates were sequenced. The isolates from Japan revealed a mutation from adenine to guanine at position 2018 in the H. cinaedi 23S rRNA; the numbering is according to the DNA sequence of the 23S rRNA gene of CCUG18818 (accession no. AY596254). Three of the six reference strains, CCUG18818, MIT00-5434, and MIT01-5002, did not demonstrate a mutation at position 2018, and clarithromycin MICs ranged from 0.008 to 0.031 mg/liter. However, CCUG18819, CCUG43521, and MIT99-5915 revealed an adenine-to-guanine mutation at position 2018, and their MICs ranged from 4 to >128 mg/liter. MIT99-5915 (MIC, >128 mg/liter) revealed an additional adenine-to-cytosine mutation at position 2017. DNA sequencing of the gyrA genes of ciprofloxacin-resistant H. cinaedi isolates and the reference strains further indicated that all isolates had a threonine to isoleucine mutation at position 84 of GyrA, the numbering is according to the amino acid sequence of the CCUG18818 GyrA protein (accession no. ZP_07806036). Three isolates were also found to have an additional mutation at position 88, involving a mutation from aspartic acid to glycine, histidine, or asparagine.
Table 4.
Antimicrobial susceptibilities of 18 H. cinaedi isolates from Japan and 6 reference strains
| Strain | Host, country, yr of isolation | MIC (μg/μl) ofa: |
|||||
|---|---|---|---|---|---|---|---|
| Amoxicillin + clavulanic acid | Imipenem | Clarithromycin | Ciprofloxacin | Minocycline | Gentamicin | ||
| Clinical isolates (n = 50) | Human, Japan, 2003–2008 | 0.5–8 (8) | 0.031–0.125 (0.125) | 16–>128 (>128) | 16–128 (128) | 0.016–0.25 (0.125) | 0.125–1 (0.5) |
| CCUG18818 | Human, USA, 1986 | 8 | 0.063 | 0.008 | 0.25 | 0.25 | 0.25 |
| CCUG18819 | Human, USA, 1986 | 4 | 0.031 | 4 | 0.125 | 0.125 | 0.5 |
| CCUG43521 | Human, Australia, 2000 | 4 | 0.031 | 128 | 0.125 | 0.125 | 0.5 |
| MIT 99–5915 | Human, USA, 1999 | 0.5 | 0.016 | >128 | 0.063 | 0.031 | 0.125 |
| MIT00–5434 | Rhesus monkey, USA, 2000 | 4 | 0.031 | 0.031 | 16 | 0.063 | 0.5 |
| MIT 01–5002 | Rhesus monkey, USA, 2000 | 4 | 0.031 | 0.031 | 8 | 0.031 | 0.5 |
Values for the Japanese clinical isolates are ranges (MIC90s).
DISCUSSION
This study attempted to identify the genes that may be used to classify H. cinaedi by MLST. However, the housekeeping genes of H. cinaedi were found to have low diversity. For example, although data obtained using the MLST analysis method developed in this study suggested that 8 isolates, which were used for the identification of suitable genes for MLST, should belong to different STs, the yphC gene, encoding GTP-binding protein, was found to be identical between the 8 isolates. This is unlike the situation with H. pylori, where yphC has been used for MLST analysis and has revealed a high degree of diversity among H. pylori isolates. A similar phenomenon was observed for the mutY and trpC genes, which were found to be unsuitable for H. cinaedi MLST, despite their use in MLST for H. pylori. Of the 7 genes used for MLST in H. pylori, only the ppa and atpA genes were suitable for use in the H. cinaedi analysis. These results indicate that, whereas H. pylori is a genetically diverse bacterial species, the housekeeping genes of H. cinaedi are highly conserved. Therefore, we analyzed other housekeeping genes that have been used for MLST analysis in Campylobacter spp. and other bacterial species. Of the 7 genes used for MLST in C. jejuni, aspA and tkt were suitable for H. cinaedi analysis.
A previous comparison of the 23S rRNA gene sequences of the isolates from hospital A indicated that there was a high possibility of nosocomial transmission of H. cinaedi between patients (18). Furthermore, 11/12 (91.7%) of the isolates identified in hospital A were found to belong to CC9 in this study, and the PFGE patterns of the isolates belonging to CC9 were identical. These results indicated that CC9 may have been prevalent in patients from hospital A. Furthermore, 12 of the 17 isolates from hospital G were ST7, and although only 4 of those isolates were available for PFGE, 2 of the isolates belonging to ST7 showed an identical pattern, and it was different from the patterns of 2 other isolates that belonged to ST9. Additionally, both isolates from hospital B, which were isolated from the same ward within a single month, revealed the same ST, CC4 [ST4]; these results suggested that nosocomial transmission of H. cinaedi is possible and that this organism may colonize individuals in the hospital environment.
H. pylori infection is believed to occur rarely in adults; instead, the infection is thought to be established during childhood, likely by oral transmission from an infected mother or contaminated water source (8). In contrast, the results of this study indicate that H. cinaedi infection may be transmissible between adults and that the possibility of nosocomial transmission between individuals should be considered, particularly among immunocompromised patients. Similarly, the isolates from hospital D represented three CCs: CC1, CC4, and CC9. These results are consistent with the fact that these isolates were obtained between 2003 and 2008 and that H. cinaedi infections had occurred sporadically throughout that period. When and how these patients became infected with H. cinaedi remains unknown; it is possible, as well, that H. cinaedi may have been present in a latent form in these patients and may have induced symptoms when the immune system was weakened.
Two isolates from rhesus monkeys and four human isolates from countries other than Japan were also analyzed, and all belonged to different STs than the isolates from Japan, except CCUG43521, which belonged to same ST (ST4) as the isolates from hospital B. A comparison of the CCs and STs with the PFGE patterns of the isolates indicated that the MLST results were consistent with the PFGE patterns. PFGE is a useful technique for typing the isolates, even though some isolates were nontypeable by XhoI. Generally, restriction enzymes that cleave chromosomal DNA less frequently should be selected for PFGE. HincII, which also cleaves chromosomal DNA less frequently, similar to XhoI, was also tried and was found to digest the DNA from a few isolates. All isolates were successfully digested by SpeI; however, SpeI cleaves chromosomal DNA more frequently than XhoI and HincII and is not the best restriction enzyme for PFGE. Given the limitations of PFGE analysis in H. cinaedi, further investigations are required, but the MLST method developed in this study may be useful for typing H. cinaedi isolates and for elucidating their route(s) of transmission.
The susceptibilities of multiple H. cinaedi isolates to various antimicrobial agents were also examined in this study. Susceptibilities to amoxicillin-clavulanic acid, imipenem, gentamicin, and minocycline showed no significant differences between the H. cinaedi isolates from Japan and the 6 reference strains. On the other hand, the MICs of clarithromycin for the human isolates from Japan were very high, compared to those for CCUG 18818 and the two H. cinaedi strains from rhesus monkeys. The mutation from adenine to guanine, identified at position 2018 in the H. cinaedi 23S rRNA sequence, corresponds to mutations at positions 2143 in H. pylori and 2059 in E. coli; these mutations have been proven to confer resistance to clarithromycin in these strains by removing the adenine required for macrolides to inhibit protein synthesis (29). Similarly, Kuijper et al. analyzed the 23S rRNA gene of erythromycin-susceptible and erythromycin-resistant H. cinaedi isolates and showed that erythromycin-resistant H. cinaedi strains possessed this mutation at position 2018, while no mutation was present in erythromycin-susceptible H. cinaedi (14). In the current study, the mutations at position 2018 corresponded to the clarithromycin resistance in all analyzed strains, and all isolates from Japan had this mutation. These results indicate that the isolates used in this study were all highly resistant to clarithromycin.
H. cinaedi isolates from humans, prior to 2000, revealed low MICs of ciprofloxacin, while MICs of ciprofloxacin were high not only for isolates from Japan but also for isolates from rhesus monkeys. DNA sequencing of the gyrA genes of ciprofloxacin-resistant H. cinaedi isolates indicated that all of the isolates also had a mutation from threonine to isoleucine at position 84 of GyrA. This mutation, which corresponds to positions 87 in H. pylori and 83 in E. coli, has been shown to confer fluoroquinolone resistance on many bacterial species (10). Furthermore, 3 isolates had an additional mutation at position 88, corresponding to positions 91 and 87 in H. pylori and E. coli, respectively, which has also been shown to confer resistance. Double mutations have usually been found to confer high-level resistance to fluoroquinolone upon bacteria (10, 19, 28). Unfortunately, because of the inability to reculture 32 of the 50 H. cinaedi isolates from the stocks stored at −80°C, the susceptibilities of the isolates that possessed double mutations in GyrA were not measured; however, considering our data together with previous reports, the H. cinaedi isolates from Japan used in this study are resistant to fluoroquinolone, and in some cases the level of this resistance may be high. Finally, three isolates which possessed double mutations in GyrA in this study belonged to different STs (ST6, -10, and -11, respectively), suggesting that the relationship between STs and resistance may be weak. Further investigations using susceptible isolates are, therefore, needed. With regard to erythromycin and ciprofloxacin, Kiehlbauch et al. (11) have shown that H. cinaedi isolates from both humans and animals are susceptible to both of these antimicrobial agents, with MIC ranges for erythromycin and ciprofloxacin in animal-isolated H. cinaedi of <0.06 to 0.5 mg/liter and 0.12 to 1.0 mg/liter, respectively, and 0.06 to >128 mg/liter and 0.12 to 8.0 mg/liter in isolates from humans, respectively. Ciprofloxacin was also reported to successfully eradicate H. cinaedi infections in humans in 1991 and 2000 (15, 23). These results suggest that H. cinaedi was once susceptible to macrolides and fluoroquinolones, with the majority of H. cinaedi isolates gaining resistance in response to the increased use of these antimicrobials.
This study describes the use of molecular epidemiological analysis using MLST to genotype H. cinaedi isolates. We were able to classify 50 Japanese hospital isolates and 6 reference strains into 14 STs. The distribution of STs suggested the occurrence of nosocomial infection of H. cinaedi in hospitals, while in other cases, H. cinaedi infections occurred sporadically within a single hospital. Further analysis is needed to elucidate the epidemiology of H. cinaedi. All isolates had mutations in the 23S rRNA gene and GyrA, suggesting the seriousness of the increase in resistance of H. cinaedi isolates to clarithromycin and ciprofloxacin, in Japan.
Supplementary Material
ACKNOWLEDGMENTS
We thank the following individuals for providing the H. cinaedi isolates used in this study: Hiroyuki Nishiyama, Nihon University School of Medicine; Keizo Yamaguchi, Toho University; Michiko Yagoshi, Nihon University School of Medicine; Sayoko Kawakami, Teikyo University Hospital; Akiko Yoneyama, Toranomon Hospital; Shunji Takahashi, Sapporo City General Hospital; Masaya Mukai, Sapporo City General Hospital; Koichiro Minauchi, Sapporo City General Hospital. We also thank Kai Kumiko, Yoshie Taki, Yumiko Yoshimura, and Yumiko Hongo for their technical assistance.
This study was supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for Promotion of Science (no. 22590410), and a grant from the Ministry of Health, Labor and Welfare of Japan (H21-Shinkou-Ippan-008).
Footnotes
Published ahead of print 16 May 2012
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Burman WJ, Cohn DL, Reves RR, Wilson ML. 1995. Multifocal cellulitis and monoarticular arthritis as manifestations of Helicobacter cinaedi bacteremia. Clin. Infect. Dis. 20:564–570 [DOI] [PubMed] [Google Scholar]
- 2. Burucoa C, Lhomme V, Fauchere JL. 1999. Performance criteria of DNA fingerprinting methods for typing of Helicobacter pylori isolates: experimental results and meta-analysis. J. Clin. Microbiol. 37:4071–4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 9th ed CLSI, Wayne, PA [Google Scholar]
- 4. Falush D, et al. 2003. Traces of human migrations in Helicobacter pylori populations. Science 299:1582–1585 [DOI] [PubMed] [Google Scholar]
- 5. Fox JG, et al. 2001. Isolation of Helicobacter cinaedi from the colon, liver, and mesenteric lymph node of a rhesus monkey with chronic colitis and hepatitis. J. Clin. Microbiol. 39:1580–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox JG, et al. 2009. Chronic hepatitis, hepatic dysplasia, fibrosis, and biliary hyperplasia in hamsters naturally infected with a novel helicobacter classified in the H. bilis cluster. J. Clin. Microbiol. 47:3673–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gebhart CJ, Fennell CL, Murtaugh MP, Stamm WE. 1989. Campylobacter cinaedi is normal intestinal flora in hamsters. J. Clin. Microbiol. 27:1692–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goh KL, Chan WK, Shiota S, Yamaoka Y. 2011. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter 16(Suppl. 1):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holst H, et al. 2008. A case of Helicobacter cinaedi bacteraemia in a previously healthy person with cellulitis. Open Microbiol. J. 2:29–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacoby GA. 2005. Mechanisms of resistance to quinolones. Clin. Infect. Dis. 41(Suppl. 2):S120–126 [DOI] [PubMed] [Google Scholar]
- 11. Kiehlbauch JA, et al. 1995. Genotypic and phenotypic characterization of Helicobacter cinaedi and Helicobacter fennelliae strains isolated from humans and animals. J. Clin. Microbiol. 33:2940–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kiehlbauch JA, Tauxe RV, Baker CN, Wachsmuth IK. 1994. Helicobacter cinaedi-associated bacteremia and cellulitis in immunocompromised patients. Ann. Intern. Med. 121:90–93 [DOI] [PubMed] [Google Scholar]
- 13. Kitamura T, et al. 2007. Helicobacter cinaedi cellulitis and bacteremia in immunocompetent hosts after orthopedic surgery. J. Clin. Microbiol. 45:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuijper EJ, Stevens S, Imamura T, De Wever B, Claas EC. 2003. Genotypic identification of erythromycin-resistant campylobacter isolates as helicobacter species and analysis of resistance mechanism. J. Clin. Microbiol. 41:3732–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lasry S, et al. 2000. Helicobacter cinaedi septic arthritis and bacteremia in an immunocompetent patient. Clin. Infect. Dis. 31:201–202 [DOI] [PubMed] [Google Scholar]
- 16. Mammen MP, Jr, Aronson NE, Edenfield WJ, Endy TP. 1995. Recurrent Helicobacter cinaedi bacteremia in a patient infected with human immunodeficiency virus: case report. Clin. Infect. Dis. 21:1055. [DOI] [PubMed] [Google Scholar]
- 17. Megraud F, Lehours P. 2007. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin. Microbiol. Rev. 20:280–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Minauchi K, et al. 2010. The nosocomial transmission of Helicobacter cinaedi infections in immunocompromised patients. Intern. Med. 49:1733–1739 [DOI] [PubMed] [Google Scholar]
- 19. Miyachi H, et al. 2006. Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. Helicobacter 11:243–249 [DOI] [PubMed] [Google Scholar]
- 20. Murakami H, et al. 2003. Isolation of Helicobacter cinaedi from blood of an immunocompromised patient in Japan. J. Infect. Chemother. 9:344–347 [DOI] [PubMed] [Google Scholar]
- 21. Orlicek SL, Welch DF, Kuhls TL. 1993. Septicemia and meningitis caused by Helicobacter cinaedi in a neonate. J. Clin. Microbiol. 31:569–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riley LK, Franklin CL, Hook RR, Jr, Besch-Williford C. 1996. Identification of murine helicobacters by PCR and restriction enzyme analyses. J. Clin. Microbiol. 34:942–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sacks LV, Labriola AM, Gill VJ, Gordin FM. 1991. Use of ciprofloxacin for successful eradication of bacteremia due to Campylobacter cinaedi in a human immunodeficiency virus-infected person. Rev. Infect. Dis. 13:1066–1068 [DOI] [PubMed] [Google Scholar]
- 24. Saunders KE, McGovern KJ, Fox JG. 1997. Use of pulsed-field gel electrophoresis to determine genomic diversity in strains of Helicobacter hepaticus from geographically distant locations. J. Clin. Microbiol. 35:2859–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh A, Goering RV, Simjee S, Foley SL, Zervos MJ. 2006. Application of molecular techniques to the study of hospital infection. Clin. Microbiol. Rev. 19:512–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stauffer GV, Plamann MD, Stauffer LT. 1981. Construction and expression of hybrid plasmids containing the Escherichia coli glyA genes. Gene 14:63–72 [DOI] [PubMed] [Google Scholar]
- 27. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tankovic J, Lascols C, Sculo Q, Petit JC, Soussy CJ. 2003. Single and double mutations in gyrA but not in gyrB are associated with low- and high-level fluoroquinolone resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 47:3942–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor DE, Ge Z, Purych D, Lo T, Hiratsuka K. 1997. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob. Agents Chemother. 41:2621–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taylor NS, Ge Z, Shen Z, Dewhirst FE, Fox JG. 2003. Cytolethal distending toxin: a potential virulence factor for Helicobacter cinaedi. J. Infect. Dis. 188:1892–1897 [DOI] [PubMed] [Google Scholar]
- 31. Tee W, Street AC, Spelman D, Munckhof W, Mijch A. 1996. Helicobacter cinaedi bacteraemia: varied clinical manifestations in three homosexual males. Scand. J. Infect. Dis. 28:199–203 [DOI] [PubMed] [Google Scholar]
- 32. Totten PA, et al. 1985. Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): two new Campylobacter species associated with enteric disease in homosexual men. J. Infect. Dis. 151:131–139 [DOI] [PubMed] [Google Scholar]
- 33. Vandamme P, Falsen E, Pot B, Kersters K, De Ley J. 1990. Identification of Campylobacter cinaedi isolated from blood and feces of children and adult females. J. Clin. Microbiol. 28:1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.