Abstract
To reduce selective pressure for antimicrobial resistance, empirical use of antipseudomonal antibiotics is often reserved for patients with late-onset hospital-acquired infections. We examined the likelihood of isolating Pseudomonas aeruginosa as a function of time from hospital admission. We conducted a retrospective cohort study of all positive bacterial cultures in a tertiary-care hospital between March 2010 and November 2011. The primary outcome was the proportion of positive cultures yielding P. aeruginosa. Multivariable logistic regression was employed to assess the impact of time from admission on the likelihood of isolating P. aeruginosa, after adjusting for other important risk factors. A total of 7,668 positive cultures were obtained from 4,108 unique patients during the study interval, including 633 (8.3%) yielding P. aeruginosa. The probability of isolating P. aeruginosa increased linearly from 79/2,044 (3.9%) positive cultures obtained on admission to 153/664 (23%) in the 10th week of admission or beyond. The unadjusted odds ratio was 1.002/day (95% confidence interval [CI], 1.0016 to 1.0028; P < 0.0001); the adjusted odds ratio (aOR) was 1.0007/day (95% CI, 1.0001 to 1.0013; P = 0.02). Other important predictors of P. aeruginosa isolation included respiratory specimen type (aOR, 13.8; 95% CI, 9.1 to 21.1), recent hospital admission (aOR,1.8; 95% CI, 1.4 to 2.3), prior P. aeruginosa isolation during current admission (aOR, 4.9; 95% CI, 3.7 to 6.4), and prior antipseudomonal (aOR, 1.9; 95% CI, 1.4 to 2.5) or nonantipseudomonal (aOR, 1.8; 95% CI, 1.4 to 2.4) antibiotic exposure. It was determined that as time from admission increases, there is a linear increase in the likelihood of P. aeruginosa isolation. Any guidelines which distinguish early from late hospital-acquired infection must consider the implications of time point selection on the likelihood of inadequate P. aeruginosa empirical coverage.
INTRODUCTION
Pseudomonas aeruginosa is a prototypic nosocomial pathogen. It is among the most common causes of hospital-acquired infections (17), while it is infrequently responsible for community-acquired disease (20). P. aeruginosa is also a prototypic resistant pathogen, intrinsically resistant to most antibacterial agents and able to acquire almost all known mechanisms of antibacterial resistance (21). Very few available antibacterial agents offer reliable antipseudomonal activity. Therefore, as a general principle, use of these drugs is spared, if possible, to avoid driving further increases in resistance. However, minimization of empirical antimicrobials is challenging, because the adequacy of early antibiotic therapy (before microbiological results are available) is strongly associated with patient survival in critically ill patients (8, 11, 12). The stakes of failing to adequately cover P. aeruginosa during the empirical window may be particularly high (10), since this organism possesses a vast array of virulence factors (23) and is associated with higher clinical failure, relapse, and mortality rates than most other pathogens (3). In one recent study, 17% of patients with P. aeruginosa ventilator-associated pneumonia received inadequate initial therapy, and their mortality rate was 3-fold higher than that of those who received adequate coverage (10).
One practical means to optimize the use of empirical antipseudomonal therapy is to reserve these treatments for patients who have been admitted to a hospital for a sufficient duration to acquire this organism. For example, both the Canadian (18) and American (1) guidelines for hospital-acquired pneumonia list hospitalization of more than 5 days as a risk factor for P. aeruginosa and a reason to include antipseudomonal coverage when selecting empirical therapy for late-onset infections. These recommendations are based on prior research suggesting that P. aeruginosa is more commonly associated with late-onset than with early-onset infection (9, 24). However, prior research has dichotomized time of onset as either early or late (9, 22, 24), and so the full relationship between probability of P. aeruginosa infection and time from admission has not been clearly delineated. Meanwhile, there are theoretical and empirical data to suggest that acquisition of nosocomial antibiotic-resistant pathogens can be quite dispersed over time (2, 14, 15).
The objective of this study was to describe the relationship between the duration of admission and the probability of a positive clinical culture specimen yielding P. aeruginosa. Our hypothesis was that the risk of P. aeruginosa infection would increase consistently throughout the hospital stay, and thus, any arbitrary attempt to distinguish early from late infection must consider a probability threshold at which empirical antipseudomonal coverage is warranted.
MATERIALS AND METHODS
Study overview and setting.
We conducted a retrospective study of all bacterial culture and susceptibility testing between March 2010 and November 2011 at Sunnybrook Health Sciences Centre (SHSC), a university-affiliated, 700-bed tertiary-care hospital in Toronto, Ontario, Canada. The goal was to examine the impact of time from hospital admission on the likelihood of isolating the pathogen Pseudomonas aeruginosa.
Data source.
Study data were extracted from the Stewardship Program Integrated Resource Information Technology (SPIRIT) database. This relational database, described previously, is automatically populated by health level 7 (HL7) messages from SHSC microbiology, pharmacy, and electronic patient care databases for all admitted patients (6, 7). The database is stored on a secure server and includes information on all microbiology cultures, pharmacological treatments (including anti-infectives), and an array of other clinical, administrative, and laboratory variables.
Selection criteria.
We included all positive bacterial cultures obtained for all acute-care patients admitted to SHSC between March 2010 (inception of SPIRIT database) and November 2011 (most recently available data). We excluded emergency room cultures for patients that were not ultimately admitted to the hospital, cultures obtained in SHSC's affiliated long-term-care facility, cultures obtained for surveillance rather than clinical purposes, cultures that assessed for nonbacterial pathogens (e.g., fungal and viral cultures), culture tests not designed to detect P. aeruginosa (e.g., stool culture testing and throat swabs), and cultures with negative results. We also excluded patients admitted to obstetrical and psychiatry services, due to an expected low frequency of infections. It is usually standard procedure to delete all duplicate cultures per patient for deriving study cohorts or summarizing microbiology data in the form of antibiograms (4). However, repeat tests were not all deleted in this study, because this would preclude an analysis of the relationship between time from admission and P. aeruginosa prevalence. Instead, we deleted all duplicate cultures in which the same organism was isolated from the same patient within 72 h (regardless of specimen type), since these would most likely represent the same episode of infection. The final cohort consisted of unique culture-positive episodes of infection.
Primary predictor of interest (time from hospital admission).
The primary predictor of interest was the time from hospital admission to culture specimen collection (in days). Time from admission was analyzed as a continuous variable but for graphical plots was categorized by week of admission to ensure an adequate number of samples for each time point (on admission, 1st week of hospitalization, 2nd week of hospitalization, …, 12th week of hospitalization, >12 weeks of hospitalization).
Primary outcome.
The primary outcome of interest was the proportion of positive cultures that yielded P. aeruginosa. The numerator was the total number of culture tests which yielded P. aeruginosa at a given time point from admission; the denominator was the total number of positive cultures obtained at the same time point from admission. In a sensitivity analysis, the denominator was changed to include all culture testing (not limited to positive cultures).
Other covariates.
We examined a number of patient and microbiology covariates that might influence the likelihood of P. aeruginosa being implicated as a culprit pathogen. Recent hospitalization was defined as admission to SHSC within the preceding 60 days. The admitting service was categorized as medicine, surgery, or oncology; intensive care unit (ICU) exposure was categorized as current (at the date of culture collection), prior (between the date of admission and culture collection), or none (none during the admission prior to index culture collection). Prior antibiotic exposure was defined as receipt of antibiotics in the time window between hospital admission and culture collection. Current antibiotic regimens were further subclassified as antipseudomonal (if they included gentamicin, tobramycin, amikacin, ceftazidime, ciprofloxacin, imipenem, meropenem, piperacillin-tazobactam, or colistimethate) or nonantipseudomonal (if they included none of these agents). In addition, the specimen type was categorized as sterile (for blood cultures, bone or tissue biopsy specimens, and cerebrospinal or other fluid cultures) or nonsterile (for wound and miscellaneous cultures, urine cultures, and respiratory tract cultures). Neutropenia was defined as a neutrophil count nadir during admission of ≤0.5 109 cells/liter. Finally, prior P. aeruginosa infection was defined as isolation of this organism from the same patient from a preceding culture during the same admission.
Statistical analysis.
The primary analysis was a descriptive plot of the proportion of positive cultures yielding P. aeruginosa as a function of time from hospital admission. In secondary analyses, plots were constructed stratifying by other potential covariates, including intensive care unit exposure, prior antibiotic exposure, and body site of culture collection. Multivariable logistic regression was then performed to assess the impact of time from admission on the probability of a positive culture yielding P. aeruginosa, after accounting for other patient and microbiology covariates. Generalized estimating equations were used to account for clustering of culture results within unique patient admissions (25). Data extraction from SPIRIT was performed using Microsoft Access; statistical analyses were performed using SAS, version 9.3 (SAS, Cary, NC).
Sample size calculation.
To describe the proportion of positive cultures yielding P. aeruginosa at a given time point postadmission within 5% precision with 95% confidence, we determined that we would require 153 cultures (for 80% power), 211 cultures (for 90% power), or 266 cultures (for 95% power). To describe these proportions within ±10% precision with 95% confidence, we would require only 37 cultures (for 80% power), 40 cultures (for 90% power), or 63 cultures (for 95% power).
RESULTS
Numbers and types of patients and cultures.
During the study period, 53,798 bacterial culture specimens were collected from 49,488 hospital admissions of 38,581 unique patients. The study cohort included 7,668 unique culture-positive episodes of infection; these positive cultures were derived from 4,600 unique patient admissions of 4,108 unique patients (Table 1). Patients with positive cultures included roughly equal proportions of men (2,030, 49.4%) and women (2,078, 50.5%), spanned a wide age range (mean ± standard deviation, 69.4 ± 18.5; interquartile range [IQR], 58 to 84 years), and included patients on medical (2,967, 38.7%), surgical (3,695, 48.2%), and oncology (1,006, 13.1%) services. The median length of hospital stay for patients with positive cultures was 23.9 ± 52.4 days, and the in-hospital mortality rate was 5.7%. This mortality rate was higher among admissions during which at least one culture was positive for P. aeruginosa than among admissions during which only non-P. aeruginosa organisms were isolated (8.7% versus 5.5%, P = 0.01).
Table 1.
Derivation of the unique culture-positive episodes of infection
| Parameter | No. of: |
||
|---|---|---|---|
| Unique patients | Unique patient admissionsa | Unique episodes of infectionb | |
| Total patients and admission episodes | 38,581 | 49,488 | Not applicable |
| Total patients with positive bacterial cultures | 4,108 | 4,600 | 7,668 |
Multiple admissions can occur for the same patient within the study interval.
Multiple infection episodes can occur for the same patient within the same admission, but duplicate episodes with the same organism were deleted within 72 h as most likely indicating the same episode of infection.
Timing of culture collection.
The positive cultures were collected a mean of 26.9 ± 91.0 days postadmission. More than half of positive cultures were collected during the first week of admission (median was 6 days), but many were collected after much longer periods of admission (e.g., 75th percentile was 21 days and 90th percentile was 55 days).
Probability of isolating Pseudomonas aeruginosa.
P. aeruginosa was isolated from 633 (8.3%) of all positive bacterial cultures, including 40/1,500 (2.7%) blood cultures, 15/192 (7.8%) bone or tissue biopsy cultures, 8/309 (2.6%) other sterile fluid cultures, 268/1,082 (24.8%) respiratory cultures, 260/3,622 (7.2%) urine cultures, and 42/963 (4.4%) wound or miscellaneous cultures. P. aeruginosa was more frequently isolated from patients currently in ICUs (292/2,424, 12.1%) or with prior ICU exposure (113/1,088, 10.4%) than from those with no ICU exposure during the current admission (228/4,156, 5.5%) (P < 0.0001). The likelihood of isolating P. aeruginosa differed according to whether the patient had previously received antipseudomonal antibiotics (348/2,642, 13.2%), had previously received nonantipseudomonal antibiotics (141/1,889, 7.5%), or had no prior antibiotic treatment during the admission (144/3,137, 4.6%) (P < 0.0001).
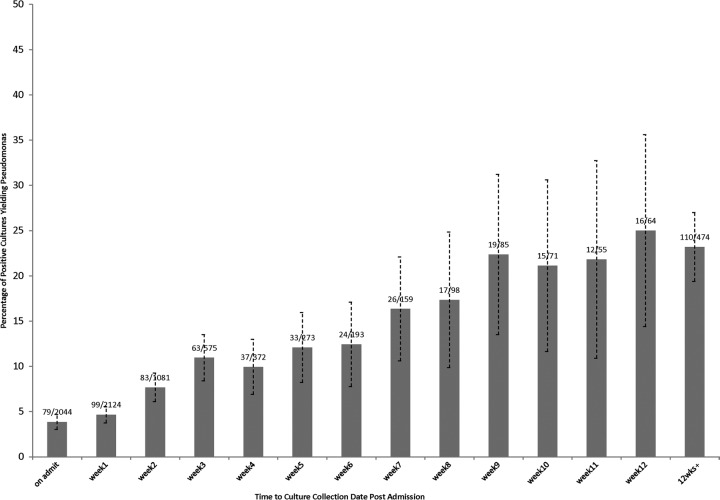
Overall probability of isolating Pseudomonas aeruginosa as a function of time from admission.
The probability of isolating P. aeruginosa from a positive bacterial culture increased from 79/2,044 (3.9%) positive cultures obtained on admission to 153/664 (23%) positive cultures obtained in the 10th week of admission or beyond. The increase in risk appeared linear over this time interval, with an increase of 1.4%/week of admission (Fig. 1). In a sensitivity analysis, the probability was retested with a denominator of all culture testing (positive and negative cultures). Although the total proportion of tests positive for P. aeruginosa was lower (890/53,798, 1.7%), the increase in risk remained linear during the hospital stay, from 94/17,545 (0.5%) cultures obtained on admission to 205/2,373 (8.6%) cultures obtained in the 10th week of admission or beyond (data not displayed).
Fig 1.
Proportion of positive bacterial cultures yielding Pseudomonas as a function of time between hospital admission and culture collection (in weeks).
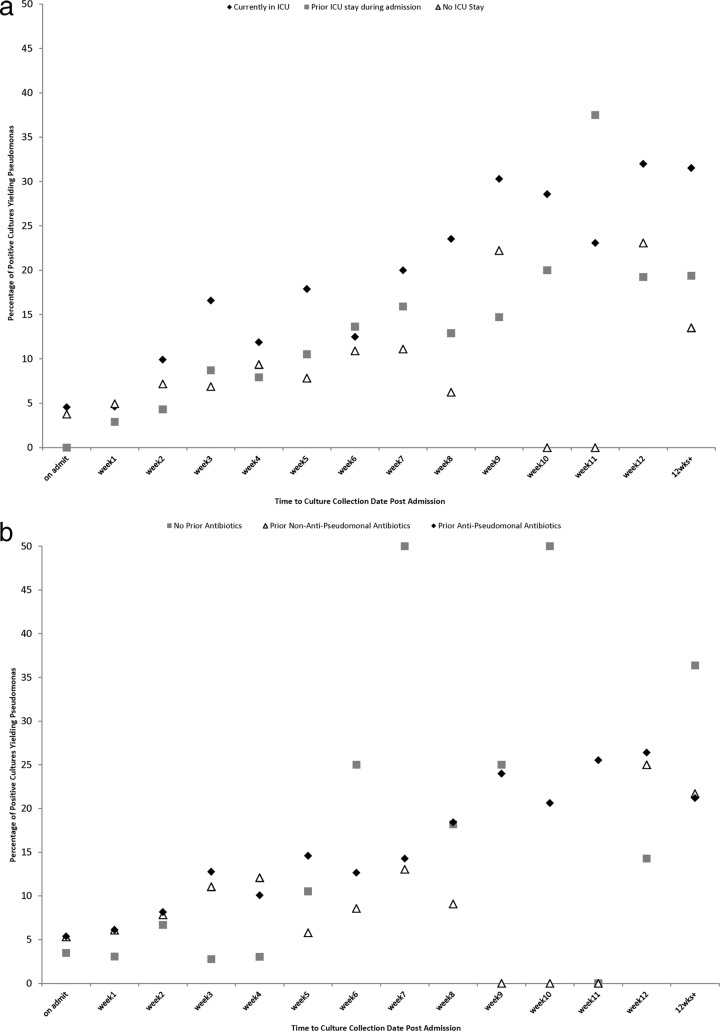
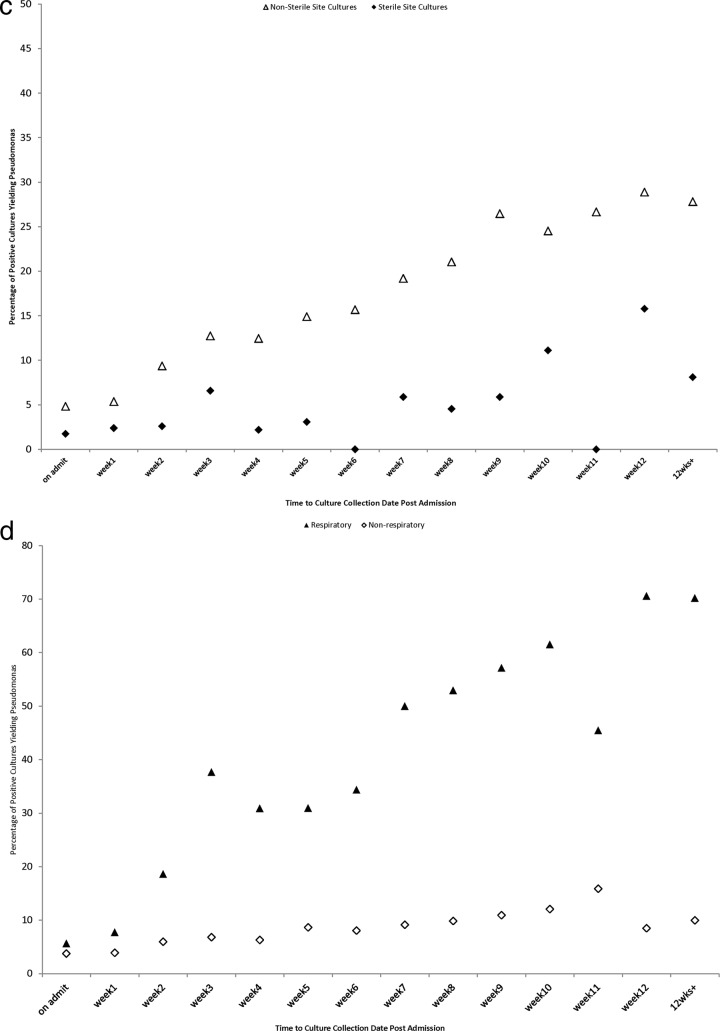
Subgroup analyses.
The relationship between the probability of isolating P. aeruginosa from a positive bacterial culture remained consistently linear across multiple prespecified subgroup analyses, including analyses stratified by ICU exposure (Fig. 2a), antibiotic exposure (Fig. 2b), sterile versus nonsterile cultures (Fig. 2c), and respiratory versus nonrespiratory cultures (Fig. 2d).
Fig 2.
(a) Proportion of positive bacterial cultures yielding Pseudomonas as a function of time of culture collection in relation to hospital admission (in weeks), stratified by intensive care unit exposure category. (b) Proportion of positive bacterial cultures yielding Pseudomonas as a function of time of culture collection in relation to hospital admission (in weeks), stratified by antibiotic exposure category. After the second week of admission, very few admitted patients have not received antibiotics, and so estimates for this group are based on very small sample sizes for subsequent time points. (c) Proportion of positive bacterial cultures yielding Pseudomonas as a function of time of culture collection in relation to hospital admission (in weeks), stratified by sterile versus nonsterile cultures. (d) Proportion of positive bacterial cultures yielding Pseudomonas as a function of time of culture collection in relation to hospital admission (in weeks), stratified by respiratory versus nonrespiratory cultures.
Multivariable analysis.
Logistic regression analysis (with generalized estimating equations to account for clustering of culture results within unique patient admissions) yielded an unadjusted odds ratio of 1.002 (95% confidence interval [CI], 1.0016 to 1.0028; P < 0.0001), suggesting a 0.2% absolute increased risk of isolating Pseudomonas aeruginosa per day of hospitalization (or 1.4% increased risk per week of hospitalization). The association of time from admission and P. aeruginosa isolation persisted after multivariable adjustment for admitting service, intensive care unit exposure, antibiotic exposure, and specimen type (adjusted odds ratio, 1.0007; 95% CI, 1.0001 to 1.0013; P = 0.016). The impact of other clinical and microbiological factors on the likelihood of P. aeruginosa is displayed in Table 2.
Table 2.
Multivariable predictors of Pseudomonas aeruginosa isolation from a positive bacterial culture
| Predictor | Adjusted odds ratio | 95% CI | P value |
|---|---|---|---|
| Time from admission (days) | 1.0007 | 1.0001–1.0013 | 0.014 |
| Specimen type | |||
| Respiratory | 13.79 | 9.10–20.99 | <0.0001 |
| Urine | 3.52 | 2.37–5.24 | <0.0001 |
| Bone or tissue biopsy | 3.38 | 1.75–6.50 | 0.0003 |
| Wound/miscellaneous | 1.89 | 1.16–3.07 | 0.010 |
| Fluid or aspirate | 1.33 | 0.61–2.91 | 0.48 |
| Blooda | |||
| Hospital service | |||
| Medical | 1.07 | 0.85–1.34 | 0.57 |
| Oncology | 0.89 | 0.61–1.31 | 0.55 |
| Surgicala | |||
| ICU exposure | |||
| Current ICU admission | 0.81 | 0.63–1.03 | 0.08 |
| Prior ICU admission | 0.82 | 0.59–1.11 | 0.19 |
| No ICU admissiona | |||
| Antibiotic exposure | |||
| Previously receiving antipseudomonals | 1.88 | 1.43–2.48 | <0.0001 |
| Previously receiving only nonantipseudomonals | 1.80 | 1.38–2.35 | <0.0001 |
| No previous antibioticsa | |||
| Recent hospital admission | 1.80 | 1.42–2.29 | <0.0001 |
| Prior culture yielding Pseudomonas during current admission | 4.82 | 3.61–6.43 | <0.0001 |
| Neutropenia | 0.82 | 0.47–1.44 | 0.49 |
Referent category.
Implications of a 5-day cut-point to define antipseudomonal therapy.
Of the 7,668 positive cultures, 916 were obtained at least 2 days after admission but less than 5 days postadmission and so would be defined as early hospital-acquired infections by prevailing guidelines. In this time period, 52/916 (5.7%) positive cultures yielded P. aeruginosa, including 31/492 (6.3%) positive cultures obtained for patients with no ICU exposure and 19/215 (8.8%) positive respiratory cultures.
DISCUSSION
The probability that P. aeruginosa is the culprit cause of hospital-acquired infection continues to increase throughout the duration of hospital stay, such that one-quarter of all positive cultures are eventually attributable to this pathogen (Fig. 1). Although the absolute likelihood of isolating P. aeruginosa is higher in certain high-risk groups (those receiving prior antibiotics, exposed to intensive care units, or suffering from respiratory tract infection), the risk rises linearly over the duration of admission within all subgroups (Fig. 2). There is no threshold duration of admission at which P. aeruginosa prevalence changes abruptly, and therefore there is no obvious cut-point to distinguish early infection (for which antipseudomonal therapy can be spared) from later-onset infections (for which antipseudomonal therapy is necessary). Any such cut-point will be arbitrary and should explicitly consider the resulting likelihood of inadequate empirical coverage. For example, in the study institution, a traditional 5-day cut-point would lead to missed P. aeruginosa coverage in 5.7% of culture-positive episodes.
The linear increase in risk throughout the duration of a hospital stay is not surprising given that P. aeruginosa is an uncommon component of the microbial flora of healthy outpatients (so most individuals enter the hospital without P. aeruginosa colonization), but the organism flourishes in the hospital environment (16, 19). P. aeruginosa prefers moist environments and so can contaminate many of the aqueous solutions administered to hospitalized patients (ranging from disinfectants and soaps to irrigation and dialysis fluids), as well as the sinks and showers in patient rooms (5). Other admitted patients also serve as reservoirs, with colonization established most often on moist surfaces such as the perineum, axillae, oropharynx, nasal mucosa, and wounds (16). Therefore, each day in a hospital would be expected to result in large numbers of potential contacts with P. aeruginosa and a gradual increased risk of new colonization with this organism. Given the myriad virulence factors possessed by P. aeruginosa, and its resistance to most commonly used antibiotic agents, the risk of infection should likewise increase over the duration of hospital stay.
A number of prior studies have confirmed that P. aeruginosa contributes to a greater proportion of infections during late hospital admission, using diverse cut-points to distinguish early versus late infection (9, 22, 24). Using a threshold of ≥5 days, P. aeruginosa was significantly more common among patients with late-onset versus early-onset ICU pneumonia at Barnes-Jewish Hospital in St. Louis, MO (38% versus 25%, P = 0.003) (9). Thresholds as long as ≥16 days have yielded similar statistically significant associations with late acquisition of drug-resistant bacteria such as P. aeruginosa (22). We have generalized this finding by analyzing time of admission as a continuous variable and demonstrating consistent linear increases in the risk of P. aeruginosa infection which continue throughout the hospital stay.
Our model confirms other well-established risk factors for P. aeruginosa colonization and infection, including recent hospital admission, antibiotic exposure, and respiratory site of infection. Antibiotic exposure is a consistent risk factor for P. aeruginosa colonization and infection by suppressing other, more-susceptible Enterobacteriaceae and selecting out this notoriously resistant pathogen (13, 21). The increased likelihood of detecting P. aeruginosa in respiratory cultures is not surprising given that P. aeruginosa can colonize respiratory equipment, has a predilection for the respiratory tract, and is the number one cause of ventilator-associated pneumonia (17). Intensive care units are the site of the highest P. aeruginosa infection rates in most hospitals, likely related to the acuteness of patient illness, exposure to aqueous solutions and humidified air, and use of invasive devices (on which P. aeruginosa can form biofilm) (17). Although the crude prevalence of P. aeruginosa was higher in patients with current or prior ICU exposure, this association did not persist on multivariable adjustment. Importantly, our multivariable model confirms an independent impact of time from admission, even after accounting for all of these other important risk factors.
Although our study strengths include a hospital-wide analysis, with substantial sample size, and no loss to follow-up, there are some limitations inherent in the retrospective cohort design. We are unable to definitively distinguish between positive culture results that represent colonization versus invasive infection, and our study was not designed to analyze the impact of inadequate antipseudomonal coverage on clinical outcomes. However, we limited the studies to clinical specimens (for which the admitting team must at least suspect infection). Importantly, the relationship between time from admission and likelihood of isolating P. aeruginosa was persistent in the subgroup with positive sterile-site cultures (which more clearly represent clinically significant infection). Although we controlled for the most important recognized predictors of P. aeruginosa infection, we cannot rule out other unmeasured confounders. Our study was conducted within a single center, and so we cannot be sure that the results will be generalizable across all institutions, where perhaps the rate of acquisition over time may differ. Future research should reproduce our findings prospectively and across varied health care settings and evaluate potential clinical sequelae of inadequate empirical coverage of P. aeruginosa for early-onset hospital-acquired infections.
As the time from admission increases, we have documented a linear increase in the likelihood that P. aeruginosa is the causative organism for hospital-acquired infection. Any guidelines which distinguish early from late hospital-acquired infections must consider the implications of time point selection on the likelihood of inadequate empirical coverage for P. aeruginosa infections. Antipseudomonal coverage should be considered for patients with recent admission, for patients with prior antibiotic exposure or isolation of P. aeruginosa during the current admission, or for any hospitalized patient with respiratory infection.
ACKNOWLEDGMENTS
N. Daneman is supported by a clinician scientist award from the Canadian Institutes of Health Research.
We report no conflicts of interest relevant to this article.
Footnotes
Published ahead of print 6 June 2012
REFERENCES
- 1. American Thoracic Society, Infectious Diseases Society of America 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388–416 [DOI] [PubMed] [Google Scholar]
- 2. Brun-Buisson C, et al. 1989. Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli. Study of an outbreak in an intensive care unit. Ann. Intern. Med. 110:873–881 [DOI] [PubMed] [Google Scholar]
- 3. Chastre J, et al. 2003. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 290:2588–2598 [DOI] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2011. Analysis and presentation of cumulative antimicrobial susceptibility data. CLSI M39–A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Cross DF, Benchimol A, Dimond EG. 1966. The faucet aerator—a source of pseudomonas infection. N. Engl. J. Med. 274:1430–1431 [DOI] [PubMed] [Google Scholar]
- 6. Elligsen M, Walker S, Simor A, Daneman N. 2012. Prospective audit and feedback of antimicrobial stewardship in critical care: program implementation, experience, and challenges. Can. J. Hosp. Pharm. 65:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elligsen M, et al. 2012. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect. Control Hosp. Epidemiol. 33:354–361 [DOI] [PubMed] [Google Scholar]
- 8. Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146–155 [DOI] [PubMed] [Google Scholar]
- 9. Ibrahim EH, Ward S, Sherman G, Kollef MH. 2000. A comparative analysis of patients with early-onset vs late-onset nosocomial pneumonia in the ICU setting. Chest 117:1434–1442 [DOI] [PubMed] [Google Scholar]
- 10. Kollef KE, et al. 2008. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest 134:281–287 [DOI] [PubMed] [Google Scholar]
- 11. Kollef MH, Sherman G, Ward S, Fraser VJ. 1999. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462–474 [DOI] [PubMed] [Google Scholar]
- 12. Kumar A, et al. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 13. Lepelletier D, et al. 2006. Role of hospital stay and antibiotic use on Pseudomonas aeruginosa gastrointestinal colonization in hospitalized patients. Eur. J. Clin. Microbiol. Infect. Dis. 25:600–603 [DOI] [PubMed] [Google Scholar]
- 14. Lipsitch M, Bergstrom CT, Levin BR. 2000. The epidemiology of antibiotic resistance in hospitals: paradoxes and prescriptions. Proc. Natl. Acad. Sci. U. S. A. 97:1938–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lucet JC, et al. 1996. Outbreak of multiply resistant enterobacteriaceae in an intensive care unit: epidemiology and risk factors for acquisition. Clin. Infect. Dis. 22:430–436 [DOI] [PubMed] [Google Scholar]
- 16. Paterson DL. 2006. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin. Infect. Dis. 43(Suppl 2):S43–S48 [DOI] [PubMed] [Google Scholar]
- 17. Richards MJ, Edwards JR, Culver DH, Gaynes RP. 1999. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit. Care Med. 27:887–892 [DOI] [PubMed] [Google Scholar]
- 18. Rotstein C, et al. 2008. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can. J. Infect. Dis. Med. Microbiol. 19:19–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rutala WA, Weber DJ. 1997. Water as a reservoir of nosocomial pathogens. Infect. Control Hosp. Epidemiol. 18:609–616 [PubMed] [Google Scholar]
- 20. Schechner V, et al. 2009. Gram-negative bacteremia upon hospital admission: when should Pseudomonas aeruginosa be suspected? Clin. Infect. Dis. 48:580–586 [DOI] [PubMed] [Google Scholar]
- 21. Strateva T, Yordanov D. 2009. Pseudomonas aeruginosa—a phenomenon of bacterial resistance. J. Med. Microbiol. 58:1133–1148 [DOI] [PubMed] [Google Scholar]
- 22. Tacconelli E, et al. 2009. Antibiotic usage and risk of colonization and infection with antibiotic-resistant bacteria: a hospital population-based study. Antimicrob. Agents Chemother. 53:4264–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tan MW, Rahme LG, Sternberg JA, Tompkins RG, Ausubel FM. 1999. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc. Natl. Acad. Sci. U. S. A. 96:2408–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trouillet JL, et al. 1998. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am. J. Respir. Crit. Care Med. 157:531–539 [DOI] [PubMed] [Google Scholar]
- 25. Zeger SL, Liang KY. 1986. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121–130 [PubMed] [Google Scholar]