Abstract
Klebsiella pneumoniae carbapenemases (KPCs) have recently been described in Chicago, IL, especially among residents of long-term acute care hospitals (LTACHs). These patients are frequently transferred to local Chicago hospitals for higher acuity of medical care, and rapid detection and isolation of KPC-colonized LTACH residents may interrupt the introduction of KPCs into acute care hospitals. We evaluated the performance of a real-time PCR for blaKPC from enrichment broth versus direct plating of rectal surveillance swabs on two selective culture media, CHROMagar extended-spectrum-β-lactamase (ESBL) and vancomycin, amphotericin B, ceftazidime, and clindamycin (VACC) plates. Rectal surveillance swabs were collected as part of a point prevalence study of KPC carriage rates among 95 residents of two Chicago area LTACHs. Discrepant results between PCR and culture were resolved by subculturing the enrichment broth. Overall, 66 of 95 patients (69.5%) were colonized with KPCs, using the cumulative results of culture as a reference standard. Real-time PCR from enrichment broth was positive in 64 of 66 (97%) colonized patients, including nine surveillance swabs that were missed by both selective culture media. PCR demonstrated higher sensitivity, 97.0%, than culture using either CHROMagar or VACC plates (both with sensitivity of 77.3%). In addition, turnaround time was significantly shorter for the PCR-based method than for culture, with a mean of 24 h versus 64 to 72 h for CHROMagar and VACC plates (P < 0.0001). Overall, PCR for blaKPC represents the best screening test for KPCs with significantly higher sensitivity and with less hands-on time, resulting in a shorter time to results.
INTRODUCTION
Klebsiella pneumoniae carbapenemases (KPCs) are currently the most common carbapenemase-producing enzymes among Enterobacteriaceae in the United States (19). The spread of KPC-producing organisms has been especially problematic in New York City, NY (3, 4), but they have also become widespread nationally, with KPC-producing isolates reported from 36 states, Washington, DC, and Puerto Rico (10). Hospital outbreaks have been reported in a number of U.S. cities and in countries around the world, including Israel and Greece (18, 22). Organisms that carry these enzymes are particularly difficult to treat as they are frequently resistant to other classes of antibiotics, including aminoglycosides, fluoroquinolones, and sulfonamides.
Recently, KPC-producing Enterobacteriaceae have been described in Chicago, IL, with long-term acute care hospitals (LTACHs) representing the sites of amplification and propagation of these organisms (27). Implementation of early interventions, including the rapid and accurate identification of patients asymptomatically colonized with KPCs, has been shown to be an important strategy for decreasing the spread of such organisms (2, 17). Culture-based surveillance methods using either Trypticase soy or MacConkey broth with imipenem disks followed by plating on MacConkey agar have been described (12). However, because of the long turnaround time (TAT) associated with the use of enrichment broths, simpler phenotypic methods using direct plating of surveillance swabs to chromogenic agar or MacConkey agar with ertapenem disks have recently been suggested (13, 24). In addition, PCR-based assays have also been developed for detection of carbapenem-resistant K. pneumoniae bacteria from rectal surveillance swabs (15). Importantly, the Centers for Disease Control and Prevention (CDC) have described the situations in which the need for surveillance is apparent (7), and our goal is to provide data on how best to do this testing.
We have described a sensitive and specific real-time PCR assay for the detection of KPC-producing Enterobacteriaceae in surveillance specimens (15). For the current report, we performed a prospective study using two different phenotypic culture methods and PCR for identification of rectal carriage of KPCs among hospitalized patients at two different Chicago area LTACHs.
MATERIALS AND METHODS
Sites, patient selection, and collection of surveillance specimens.
We conducted a point prevalence study of KPC rectal carriage among patients residing in two Chicago area LTACHs on separate days, 6 June and 22 June 2011. Rectal specimens were collected using premoistened double-headed rayon swabs (BBL Culture Swab; Becton, Dickinson, Sparks, MD) and placed in liquid Amies medium for transport to the microbiology laboratory for processing. Samples were deidentified before the double-headed swabs were separated to be tested individually at two laboratory sites: Rush University Medical Center (RUMC), Chicago, IL, and NorthShore University HealthSystem (NorthShore), Evanston, IL.
Culture-based method for KPC detection.
We used two different culture-based methods for detection of KPC-producing Enterobacteriaceae. Culture method 1 was performed at RUMC using a chromogenic agar, CHROMagar extended-spectrum-β-lactamase (ESBL) plate (CHROMagar Co., Paris, France). CHROMagar was freshly prepared on the day of the study according to the manufacturer's instructions and poured into 90-mm-diameter petri dishes. One of the double-headed rayon swabs was directly inoculated onto a CHROMagar plate and streaked to four quadrants for colony isolation. The plates were incubated overnight at 35°C in ambient air and then examined for growth (Klebsiella, Enterobacter, and Citrobacter spp., metallic blue colonies; Escherichia coli, dark pink or red colonies; and Proteus spp., brown halo). Each individual isolate was subcultured to MacConkey and 5% sheep blood agar plates (Remel, Lenexa, KS), and identification and susceptibility were determined using the Microscan Negative Urine Combo panel type 51 (MicroScan Walkaway 96 Plus; Siemens, Tarrytown, NY). Susceptibility was determined using the 2011 MIC criteria of the Clinical and Laboratory Standards Institute (CLSI, Wayne, PA) (8). K. pneumoniae isolates were classified as KPCs if MICs of both imipenem and meropenem were >8 μg/ml (we previously demonstrated that K. pneumoniae isolates for which the carbapenem MIC was >8 μg/ml were all blaKPC PCR positive [13, 27]). All other isolates for which the imipenem and/or meropenem MIC was ≥2 μg/ml were subjected to further testing using the modified Hodge test according to CLSI recommendations and blaKPC PCR testing (8, 15).
Culture method 2 was performed at NorthShore using selective agar containing vancomycin, amphotericin B, ceftazidime, and clindamycin (VACC plate; Remel). The second of the double-headed rayon swabs was directly inoculated onto a VACC plate and streaked to four quadrants for colony isolation. The swab was then placed into 5 ml of tryptic soy broth (Remel) containing one BD Sensi-Disc 30-μg ceftazidime disk (Becton, Dickinson), and the plates and broths were incubated overnight at 35°C in ambient air. An aliquot of the overnight enrichment broth culture was submitted for PCR testing for blaKPC (15). All individual isolates resembling Gram-negative bacilli on VACC plates were subcultured to MacConkey agar (Remel). Following overnight incubation, three to five isolated colonies were submitted for blaKPC PCR testing (15). KPC-positive isolates were further identified using a Vitek-2 automated system (bioMérieux, Durham, NC), and susceptibility testing was performed by the Kirby-Bauer disc diffusion method following CLSI guidelines (8).
PCR testing for blaKPC.
PCR detection of blaKPC was performed at NorthShore as previously described (15). Briefly, 250 μl of the overnight incubated enrichment broth with a ceftazidime disk was added to 200 μl of lysis buffer and heated for 10 min at 99°C; the sample was microcentrifuged, and 2 μl was used for real-time PCR on a LightCycler 2.0 instrument (Roche Diagnostics, Indianapolis, IN). The following primers and probes were used for blaKPC genes 1 to 11: KPC forward, (CAACCTCGTCGCGGAACCAT), KPC reverse (ACCACGGAACCAGCGCATTT), and a 6-carboxyfluorescein (FAM)-labeled hydrolysis probe (FAM-TTTCTTGCTGCCGCTGTGCTGG-Blackberry quencher [BBQ]). In addition, primers 16S792U22 (GTAGTCCACGCYGTAAACGATG) and 16S1062L22 (AACCCAACATYTCACRACACGA) and a Texas Red-labeled hydrolysis probe for 16S rRNA (16STAQ2, Texas Red-CGAWGCAACGCGAAGAACCTTACCT-BBQ) were used as an internal control of amplification. The limit of detection of the blaKPC assay is 1 to 100 bacterial genome copies per reaction (15).
Discrepant analysis.
For patient specimen swabs that were blaKPC PCR positive but negative for growth on CHROMagar or VACC plates, enrichment broth cultures were subcultured to new VACC agar plates and incubated at 35°C in ambient air. Any individual isolate resembling a Gram-negative bacillus was subcultured to MacConkey agar (Remel). Following overnight incubation, three to five colonies were submitted for blaKPC PCR testing (15) and further identified using a Vitek-2 automated system (bioMérieux, Durham, NC).
Statistical analysis.
The performance characteristics (sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]) were calculated for each method with VassarStats using the presence of eventual KPC growth (on CHROMagar [method 1] or VACC plates [method 2] or enrichment broth culture all combined) as the final reference standard. The results of the agar on the first attempt at recovery were used to determine the performance of each specific medium. We also calculated the TAT as the time elapsed from sample receipt in the laboratory to final confirmation of KPC growth or blaKPC detection by PCR. A paired t test was used for comparison between TATs using each method.
RESULTS
A total of 95 rectal surveillance swabs were collected from individual patients and underwent both culture and molecular testing; results are summarized in Fig. 1. Seventeen swabs (18%) were taken from patients with a previous positive clinical culture for KPCs. Overall, 66 patients (69.5%) were considered to have positive KPC surveillance rectal swabs (Fig. 1).
Fig 1.
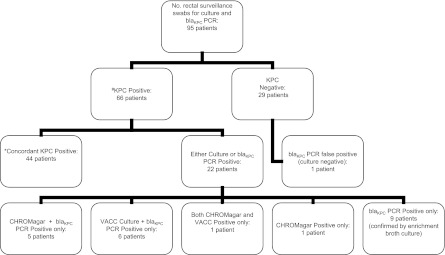
Results of KPC culture and blaKPC PCR for 95 rectal surveillance swabs. The reference standard for defining a sample as KPC positive (#) was isolation of a KPC-producing organism by any culture method using CHROMagar ESBL or VACC agar plates or tryptic soy enrichment broth. A result was considered to be concordant KPC positive (*) if it was KPC positive by both culture test methods, CHROMagar ESBL and VACC agar plates, and by blaKPC PCR.
Concordant results.
Concordant results between CHROMagar plates, VACC plates, and direct PCR from enrichment tryptic soy broth were documented for 72 surveillance swabs (76%); of these, 44 swabs tested positive by all methods, and 28 tested negative.
Performance of culture methods for recovery of KPCs.
Culture using CHROMagar (method 1) was positive for KPC growth in 51 surveillance swabs (54%). The majority of patient isolates (42 swabs) were K. pneumoniae; three surveillance swabs had E. coli, three swabs had K. pneumoniae and E. coli, one swab had K. pneumoniae and Citrobacter freundii, one swab had E. coli and Citrobacter koseri, and one swab had C. freundii. Culture using VACC plates (method 2) was also positive for KPCs in 51 surveillance swabs. The majority of swabs (45 patients) were positive for K. pneumoniae; three patient swabs were positive for E. coli, two had both K. pneumoniae and E. coli, and one had Pseudomonas aeruginosa.
Performance of PCR methods for recovery of KPCs.
A total of 65 surveillance swabs (68%) were positive for KPCs using blaKPC PCR detection from enrichment tryptic soy broth, including nine surveillance swabs that were missed by both CHROMagar and VACC cultures. Two KPC culture-positive surveillance swabs were blaKPC PCR negative and were considered false negatives. One blaKPC PCR-positive swab was negative for growth both on CHROMagar and VACC culture plates and in enrichment broth. The patient had no known history of clinical KPC infection, and the result was thus classified as a false-positive blaKPC PCR.
Discordant results.
Overall, 22 specimens showed discrepant results between the different test methods (Table 1). There were 10 PCR-positive swabs that were negative for growth on both CHROMagar and VACC plates; nine swabs yielded growth of KPC-producing K. pneumoniae bacteria using enrichment broth (including one KPC-producing isolate of P. aeruginosa) and hence were classified as true KPC-positive specimens. One swab was classified as a false-positive blaKPC PCR. There were two blaKPC PCR false-negative specimens; one swab had growth on both CHROMagar and VACC plates, and the second swab was positive on CHROMagar alone in a patient with a prior clinical history of KPC infection. The isolate was confirmed blaKPC PCR positive by testing of the colonies.
Table 1.
Laboratory analysis of 22 discrepant results and final interpretation
| CHROMagar plate |
VACC plate |
blaKPC PCR | Discrepant analysis using enrichment broth culture followed by blaKPC PCR | Final interpretation | ||
|---|---|---|---|---|---|---|
| Culture result | Colony blaKPC PCR | Culture result | Colony blaKPC PCR | |||
| Negative | NDa | Negative | ND | + | Positive | KPC positive |
| Positive | + | Negative | ND | + | Positive | KPC positive |
| Negative | ND | Negative | ND | + | Positive | KPC positive |
| Negative | ND | Negative | ND | + | Positive | KPC positive |
| Negative | ND | Positive | + | + | ND | KPC positive |
| Positive | ND | Negative | ND | + | Positive | KPC positive |
| Positive | ND | Negative | ND | + | Positive | KPC positive |
| Negative | ND | Negative | ND | + | Positive | KPC positive |
| Negative | ND | Positive | + | + | ND | KPC positive |
| Negative | ND | Negative | ND | + | Positive | KPC positive (P. aeruginosa) |
| Negative | ND | Negative | ND | + | Positive | KPC positive |
| Negative | ND | Positive | + | + | ND | KPC positive |
| Positive | ND | Negative | ND | + | Positive | KPC positive |
| Negative | ND | Positive | + | + | ND | KPC positive |
| Negative | ND | Negative | ND | + | Positive | KPC positive |
| Positive | ND | Negative | ND | + | Positive | KPC positive |
| Positive | + | Negative | ND | − | Negative | KPC positive (PCR false negative) |
| Negative | ND | Negative | ND | + | Negative | KPC negative (PCR false positive) |
| Negative | ND | Negative | ND | + | Positive | KPC positive |
| Negative | ND | Positive | + | + | ND | KPC positive |
| Negative | ND | Positive | + | + | ND | KPC positive |
| Positive | + | Positive | + | − | ND | KPC positive (PCR false negative) |
ND, not done.
Six culture-positive surveillance swabs showed growth on CHROMagar alone; i.e., they were VACC culture negative; five swabs were blaKPC PCR-positive from the enrichment broth, and the sixth was confirmed by PCR testing of the isolated colony. Six surveillance swabs were positive for growth of KPC-producing K. pneumoniae on VACC plates but not CHROMagar plates; all isolates were blaKPC PCR positive from the enrichment broth.
Analytical sensitivities of the three screening methods for identification of KPCs.
A summary of the performance characteristics of the different screening tests including sensitivity, specificity, PPV, and NPV is shown in Table 2. PCR from enrichment broth demonstrated higher sensitivity than culture using either CHROMagar or VACC plates. In addition, the TAT was significantly shorter for the PCR-based assay than for culture, with a mean of 24 h versus 64 h for CHROMagar (range, 18 to 90 h; P < 0.0001) and 72 h for VACC plates (range, 18 to 126 h; P < 0.0001).
Table 2.
Summary of analytic performance of three different screening methods
| Method | No. of false negatives | No. of false positives | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Mean TAT (h) |
|---|---|---|---|---|---|---|---|
| CHROMagar | 15 | 0 | 77.3 | 100 | 100 | 65.9 | 64 |
| VACC plates | 15 | 0 | 77.3 | 100 | 100 | 65.9 | 72 |
| blaKPC PCR | 2 | 1 | 97.0 | 96.6 | 98.5 | 93.3 | 24 |
DISCUSSION
Detection of multidrug-resistant organisms such as vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, and extended-spectrum beta-lactamase-producing Enterobacteriaceae is an important component of any infection control program (14, 20). Active screening for KPCs using rectal surveillance cultures has been shown to be highly effective, when part of a comprehensive infection control initiative, in halting the spread of KPCs in health care facilities (2, 17). LTACHs are well recognized as reservoirs for multidrug-resistant organisms, including more recently KPCs (6, 9). LTACH residents frequently are transferred to acute care hospitals for higher levels of medical care, allowing ample opportunity for introduction of KPCs into these facilities.
Since 2009, the Chicago, IL, area has witnessed the rapid emergence and spread of KPCs, primarily among patients transferred from LTACHs (16, 27). Because infections with KPCs are difficult to treat and are associated with increased morbidity and mortality, control of their spread is critically important (21). Reliance on clinical cultures taken from patients with suspected infection is likely to miss many patients harboring KPCs. There remains a potential window of opportunity within the metropolitan Chicago area to halt the spread of KPCs into acute care hospitals by screening for asymptomatic colonization, particularly in high-risk patients such as those admitted from LTACHs, so that proper infection control efforts can be instituted.
There is no consensus on the best microbiologic method(s) for identification of patients with rectal carriage of KPCs, and, additionally, there is no commercially prepared screening agar or molecular test available in the United States. We performed a point prevalence study of patients hospitalized in two different Chicago area LTACHs and found a very high colonization rate of 69% (66 of 95 patients tested). Using PCR for blaKPC performed directly on overnight enrichment broth allowed for accurate identification of 64 of 66 (97%) colonized patients. PCR was clearly more sensitive than culture using CHROMagar ESBL (method 1) or VACC plates (method 2), which were positive in only 51 of 66 colonized patients (77.3%; P = 0.0018). PCR detected KPC colonization in an additional 13 patients, including 1 patient with a blaKPC-positive isolate of P. aeruginosa. In addition, PCR was significantly more rapid than culture, with an average TAT of 24 h versus 64 to 72 h for CHROMagar (P < 0.0001). Our findings are similar to those of Schechner et al. comparing PCR to culture using MacConkey agar plates supplemented with 1 μg/ml imipenem for rectal screening of patients in a large tertiary medical center in Israel (25). The overall sensitivity of PCR in their study was 96.3% versus 77.8% for culture, with a significantly shorter TAT of 30 h for PCR versus 60 to 75 h for culture (25). Hindiyeh et al. compared blaKPC PCR on perianal/rectal swabs with culture using MacConkey agar with meropenem and ertapenem disks and reported that PCR detected an additional seven KPC-positive samples that were missed by culture (P = 0.016) while not missing any samples that were culture positive (11). In addition, the authors reported that their assay could be completed in 4 h, which was important for the rapid identification of cohorts of KPC carriers to reduce the chance of hospital spread of the organism (11).
The use of chromogenic agar has the advantage of allowing direct plating of surveillance swabs and easy interpretation after 24 h of incubation using color changes to identify various organisms (1). We used CHROMagar ESBL medium in preference to CHROMagar KPC medium because previously published data have demonstrated that CHROMagar KPC agar is less sensitive for detection of KPC isolates with a low level of resistance to carbapenems (5). However, that study was done using only serial dilutions of KPC-producing organisms for which the carbapenem MICs were variable instead of patient surveillance swabs (5). Our study is the first to demonstrate the utility of CHROMagar ESBL agar for patient surveillance swabs. CHROMagar ESBL agar has the advantage of allowing the identification of rectal carriage of ESBLs as well, which has been described in up to 33% of LTACH patients (26). Indeed, in our study CHROMagar ESBL agar identified another 38% of patients that were also colonized with ESBLs (data not shown): 22 patients with ESBL rectal colonization alone and 14 patients with ESBL and KPC cocolonization. The major disadvantages of the CHROMagar ESBL agar were its increased TAT (as bacterial growth requires further phenotypic testing to determine if the bacteria harbor ESBLs or KPCs) and significantly lower sensitivity for detecting KPC-producing bacteria. However, from a practical point of view, any patient with growth of pigmented organisms on CHROMagar ESBL agar would require contact isolation pending full identification of the organism, and therefore this method would not result in delayed implementation of infection control practices. In addition, culture allows for the identification of other mechanisms of carbapenem resistance. We identified one patient with a meropenem- and ertapenem-resistant isolate of K. pneumoniae on CHROMagar that was both blaKPC PCR and blaNDM-1 PCR negative. We also identified one KPC isolate that was positive only on CHROMagar in a patient with a prior clinical history of KPC infection.
VACC plates have been previously demonstrated to be a useful selective culture medium for ESBL isolation from rectal surveillance swabs (23). This medium has the advantage of being commercially available in the United States, and our study is the first to demonstrate its usefulness for detection of KPCs from surveillance swabs. We found that VACC plates had equal sensitivity to CHROMagar but, again, offered little advantage to PCR of enrichment broth culture and resulted in substantially increased use, in terms of time, of a microbiology technologist and a significant delay in TAT, with a mean of 72 h versus 24 h (P < 0.001).
The major advantages of blaKPC PCR was its ease of performance (thus minimizing required technologist time), high sensitivity, and a shorter time to results. The performance of KPC surveillance cultures is a very labor-intensive process due to the need to further isolate all species and colony morphologies growing on both selective and nonselective agar, which may also account for its lower sensitivity. We found that the use of enrichment broth culture increases the number of organism types and the amount of growth on plates and results in a further increase in TAT but allowed for the isolation of nine additional KPC-producing organisms that were missed by CHROMagar and VACC plates. The main disadvantage of blaKPC PCR is that no organism is available for further characterization, such as molecular typing, to determine clonality or strain typing unless one subcultures positive enrichment broth to recover the detected strain(s), as we did in this study. In addition, since PCR detects only blaKPC, its use is limited in settings where other carbapenemases are prevalent, such as blaNDM-1. Finally, PCR requires trained personnel and specialized equipment for molecular testing, which may not be readily available in smaller laboratories.
Our study had a number of limitations including a small patient sample size with a high prevalence of KPC carriage that will obviously impact the PPV and NPV of the test. In addition, since there is no reference standard for determining KPC carriage, we considered only culture-positive specimens by either the selective agars or enrichment broth as evidence of true carrier status. Consequently, one surveillance swab that was blaKPC PCR positive only was classified as a false-positive PCR result.
We conclude that blaKPC PCR has excellent sensitivity and specificity for screening patients for rectal carriage of KPCs. Its main additional advantage over culture-based methods is a significantly shorter time to result. The Chicago area is beginning to see an expanding number of patients colonized with KPCs, and if it is to avoid the epidemic dissemination of KPCs experienced by the New York City region, an early and aggressive approach to identify asymptomatically colonized patients using a rapid and highly sensitive PCR screening test may be necessary.
Footnotes
Published ahead of print 23 May 2012
REFERENCES
- 1. Adler A, et al. 2011. Laboratory and clinical evaluation of carbapenem-resistant Enterobacteriaceae from surveillance rectal swabs. J. Clin. Microbiol. 49:2239–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ben-David D, et al. 2010. Potential role of active surveillance in the control of a hospital-wide outbreak of carbapenem-resistant K. pneumoniae infection. Infect. Control Hosp. Epidemiol. 31:620–626 [DOI] [PubMed] [Google Scholar]
- 3. Bradford P, et al. 2004. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem hydrolyzing enzyme KPC-2 and inhibitor resistant TEM 30 β-lactamases in New York City. Clin. Infect. Dis. 39:55–60 [DOI] [PubMed] [Google Scholar]
- 4. Bratu S, et al. 2005. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City. Arch. Intern. Med. 165:1430–1435 [DOI] [PubMed] [Google Scholar]
- 5. Carrer A, Fortineau N, Nordmann P. 2010. Use of ChromID extended-spectrum β-lactamase medium for detecting carbapenemase-producing Enterobacteriaceae. J. Clin. Microbiol. 48:1913–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention 2011. Carbapenem-resistant Klebsiella pneumoniae associated with a long-term–care facility—West Virginia, 2009–2011. MMWR Morb. Mortal. Wkly. Rep. 60:1418–1420 [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention 2009. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb. Mortal. Wkly. Rep. 58:256–260 [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. Approved standard MS100-S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Endimiani A, et al. 2009. Emergence of blaKPC containing Klebsiella pneumoniae in a long-term acute care hospital: a new challenge to our healthcare system. J. Antimicrob. Chemother. 64:1102–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta N, Limbago BM, Patel JB, Alexander JK. 2011. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin. Infect. Dis. 53:60–67 [DOI] [PubMed] [Google Scholar]
- 11. Hindiyeh M, et al. 2008. Rapid detection of carbapenemase genes by real-time PCR. J. Clin. Microbiol. 46:2879–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Landman D, Salvani JK, Bratu S, Quale J. 2005. Evaluation of techniques for detection of carbapenem-resistant K. pneumoniae in stool surveillance cultures. J. Clin. Microbiol. 43:5639–5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lolans K, Calvert K, Won S, Clark J, Hayden MK. 2010. Direct ertapenem disk screening method for identification of KPC-producing Klebsiella pneumoniae and Escherichia coli in surveillance swab specimens. J. Clin. Microbiol. 48:836–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lucet JC, et al. 1999. Control of a prolonged outbreak of extended-spectrum beta-lactamase Producing Enterobacteriaceae in a university hospital. Clin. Infect. Dis. 29:1411–1418 [DOI] [PubMed] [Google Scholar]
- 15. Mangold K, et al. 2011. Real-time detection of blaKPC in clinical samples and surveillance specimens. J. Clin. Microbiol. 49:3338–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGuinn M, Hershow RC, Janda WM. 2009. Escherichia coli and Klebsiella pneumoniae carbapenemase in long-term care facility, Illinois, USA. Emerg. Infect. Dis. 15:988–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Munoz-Price S, et al. 2010. Successful control of an outbreak of Klebsiella pneumoniae carbapenamase-producing K. pneumoniae at a long-term acute care hospital. Infect. Control Hosp. Epidemiol. 31:341–347 [DOI] [PubMed] [Google Scholar]
- 18. Navon-Venezia S, et al. 2009. First report on a hyperepidemic clone of KPC-3 producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob. Agents Chemother. 53:818–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ostrowsky BE, et al. 2001. Control of vancomycin-resistant Enterococcus in health care facilities in a region. N. Engl. J. Med. 344:1427–1433 [DOI] [PubMed] [Google Scholar]
- 21. Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect. Control Hosp. Epidemiol. 29:1099–1106 [DOI] [PubMed] [Google Scholar]
- 22. Pournaras S, et al. 2009. Clonal spread of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains in Greece. J. Antimicrob. Chemother. 64:348–352 [DOI] [PubMed] [Google Scholar]
- 23. Reddy P, et al. 2007. Screening for extended-spectrum β-lactamase-producing Enterobacteriaceae among high-risk patients and rates of subsequent bacteremia. Clin. Infect. Dis. 45:846–852 [DOI] [PubMed] [Google Scholar]
- 24. Samra Z, et al. 2008. Evaluation of CHROMagar KPC for rapid detection of carbapenem-resistant Enterobacteriaceae. J. Clin. Microbiol. 46:3110–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schechner V, et al. 2009. Evaluation of PCR-based testing for surveillance of KPC-producing carbapenem-resistant members of the Enterobacteriaceae family. J. Clin. Microbiol. 47:3261–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trick W, et al. 2001. Colonization of skilled-care facility residents with antimicrobial resistant pathogens. J. Am. Geriatr. Soc. 49:270–276 [DOI] [PubMed] [Google Scholar]
- 27. Won SY, et al. 2011. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin. Infect. Dis. 53:532–540 [DOI] [PubMed] [Google Scholar]


