Abstract
An outbreak of egg drop disease occurred in many chicken and goose farms in China in 2011. By using an NS5-specific reverse transcriptase PCR (RT-PCR), we found that 56% of chicken and 38% of goose samples were positive for Tembusu-like virus (TMUV). Isolates showed high sequence homology to duck TMUVs, and chickens and geese showed signs of egg drop disease after experimental infection with duck TMUV. Our data suggest TMUV has adapted in domestic birds.
TEXT
The Tembusu-like virus, a member of the Flavivirus genus in the family Flaviviridae, consists of single-stranded positive-sense viral RNA encoding three structural proteins (C, prM/M, and E) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) in one open reading frame (5). The virion particle comprises a single copy of the viral RNA, encapsidated by the C protein and is enveloped by 180 copies of the prM/M and E glycoproteins. The E protein, which is glycosylated in most flaviviruses, is located on the virion surface and plays an important role in virus receptor binding, fusion, and cell entry. A novel duck flavivirus, closely related to Tembusu-like virus, has caused a substantial drop in egg production in infected ducks in China since April 2010 (2, 10). The infected flocks showed 3% to 5% death, 30% to 100% morbidity, and 30% to 60% egg production drop (10, 14). There were no reports of this infection in other domestic birds at that time.
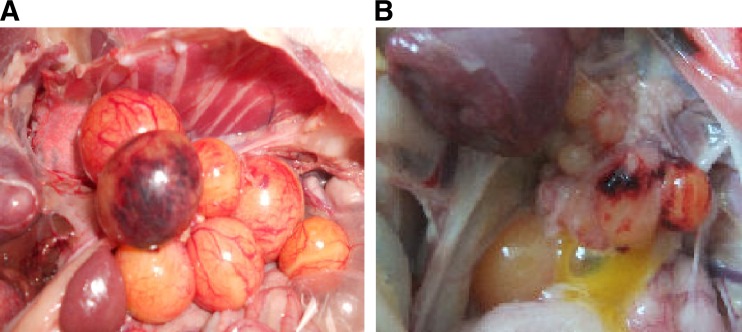
Since March 2011, a severe egg drop disease has occurred in chicken and goose farms in many provinces (data not shown). The affected chicken and geese presented with clinical symptoms that included weakness, anorexia, weight loss, and diarrhea, as well as heavy egg production drop. Postmortem examination of chickens and geese with egg production drop revealed notable ovary hemorrhage, ovaritis, and regression (Fig. 1). The disease was devastating on some chicken and goose farms, completely eliminating egg production and causing serious economic losses.
Fig 1.
Gross lesions of ovaries of infected birds. Shown are hemorrhage of ovarian follicles (A) and regression of ovarian follicles (B).
The egg production drop phenomenon raised suspicions that the novel duck Tembusu-like virus was responsible for this disease (2, 10). However, egg production drop is also observed in birds infected with eastern equine encephalitis virus (EEEV) (11) and birds infected with egg drop syndrome virus 76 (EDSV-76) (4). Therefore, to investigate the cause of egg drop disease in these chickens and geese, we developed a reverse transcriptase PCR (RT-PCR) using primers specific for the Tembusu-like virus that were based on the Tembusu-like NS5 gene and had the following sequences: NS5f, 5′TTTGGTACATGTGGCTCG3′; and NS5r, 5′ACTGTTTTCCCATCACGTCC3′. The presence of EEEV or EDSV-76 was determined by using a previously described PCR (1, 4). Liver, brain, kidney, spleen, and ovary samples from 245 chickens and 57 geese with egg drop disease were collected and analyzed.
The NS5-specific RT-PCR was conducted as follows: briefly, RNA was extracted as described previously (15). RT-PCRs were set up with 5 μl (5 ng) of viral RNA, 200 μM each primer, 1× PCR buffer (50 mM Tris-HCl [pH 8.3], 75 mM KCl), 50 mM MgCl2, 0.1 mM dithiothreitol (DTT), 1 U/μl of RNase inhibitor, 200 μM deoxynucleoside triphosphates (dNTPs), 1.125 U of Taq DNA polymerase, and 1 U of Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA); water was added to give a final volume of 50 μl. RNA samples, in a one-step reaction, were reverse transcribed for 1 h at 42°C followed by 30 PCR cycles of 94°C for 40 s, 55°C for 40 s, and 72°C for 1 min. Amplification of the products was confirmed by sequencing; the sequence data perfectly matched the expected nucleotide sequences. Neither EEEV nor EDSV-76 nucleic acid sequences were detected in the samples; however, the NS5-specific RT-PCR demonstrated the presence of Tembusu-like virus RNA.
The sensitivity of the NS5-specific RT-PCR was determined by testing serial dilutions (10−1 to 10−7) of allantoic fluids containing the duck Tembusu-like virus TA strain (initial titer, 7.9 × 106 PFU/ml) and by testing 115 duck samples (from liver, kidney, spleen, brain, and ovary). Virus isolations were performed by infecting six embryonated eggs with homogenates of corresponding clinical samples and confirmed the presence of Tembusu-like viral RNA in the allantoic fluids by the RT-PCR method and nucleotide sequencing as described previously (10). Using this approach, the sensitivity was determined to be 7.9 × 102 PFU/ml (10−4 dilution). The specificity of the NS5-specific RT-PCR method was determined by testing stock viruses, including avian influenza A virus, Newcastle disease virus, duck reovirus, duck hepatitis type 1 virus, and infectious bronchitis virus. None of these stock viruses was detected by the NS5-specific PCR. We also compared the NS5-specific RT-PCR method with the “gold standard” virus isolation method. By using the 115 duck samples, we calculated the sensitivity and specificity indexes of the NS5-specific RT-PCR method to be 95.7% (68/71) and 95.4% (42/44), respectively. The χ2 test found no significant differences between the NS5-specific RT-PCR and virus isolation methods (P > 0.05).
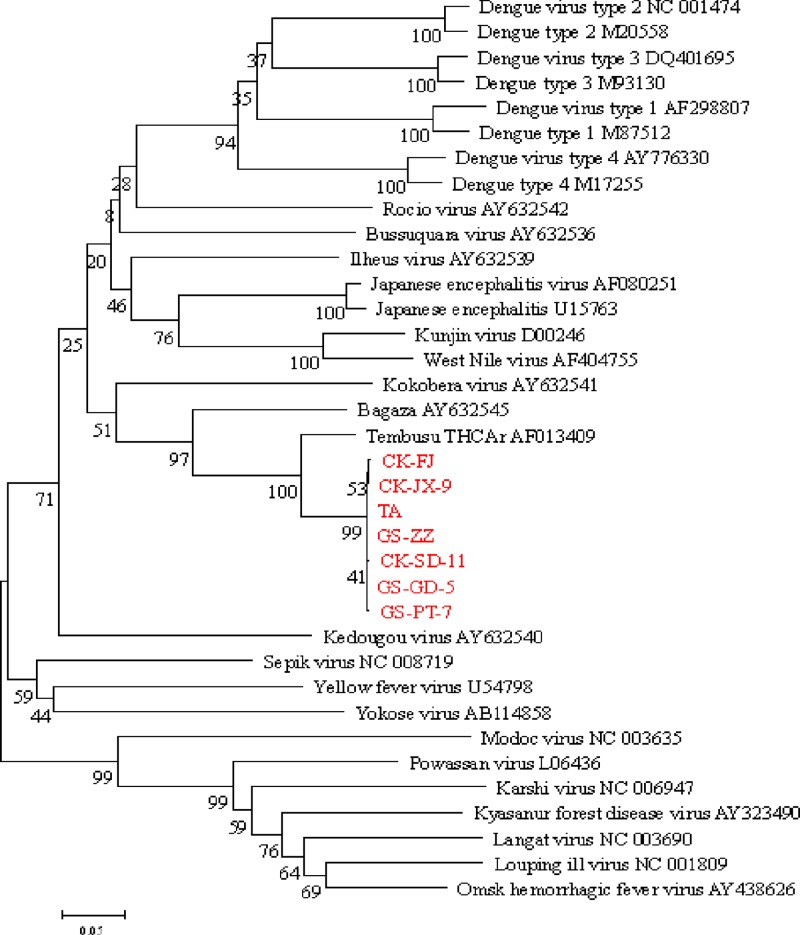
The NS5-specific RT-PCR method was then used to evaluate clinical specimens from diseased chickens and geese as well as specimens from healthy birds. Of the clinical samples tested, 138 of 246 (56.1%) chicken samples and 22 of 57 (38.6%) goose samples were positive for the Tembusu-like virus (Table 1), whereas the samples from 25 healthy chickens and 25 healthy geese were all negative (data not shown). Viral RNAs were most readily detected in the ovaries of chicken (82%) and goose (78%) samples. RT-PCR-amplified 350-bp products of the NS5 gene from the Tembusu-like virus-positive specimens were cloned into a pMD18-T vector as described previously (9, 16). Clones containing the insert were sequenced by using an ABI DNA sequencer (Applied Biosystems), and the sequences were submitted to GenBank. The nucleotide sequences were then compared with those from GenBank via BLAST analysis (www.ncbi.nlm.nih.gov). The NS5 sequences of isolates from chickens and geese were 99.7% to 100% homologous to the NS5 sequence of the duck Tembusu-like virus TA strain (GenBank no. JQ289550) (6) and showed 89.1% to 90.8% identity to that of the Tembusu virus THCAr strain (GenBank accession no. AF013409) (Fig. 2). Complete sequence analysis of isolates CK-SD-11 (GenBank no. JQ627862) and GS-PT-7 (GenBank no. JQ627864) revealed 99.3% to 99.6% homology to the sequences of duck Tembusu-like viruses.
Table 1.
Tembusu-like virus detection by using an NS5-specific RT-PCR method in chicken and goose clinical samples
| Sample type | % positive (no. positive/total) |
|---|---|
| Chickens | |
| Ovary | 82.9 (97/117) |
| Liver | 43.5 (20/46) |
| Kidney | 31.3 (10/32) |
| Spleen | 23.1 (9/39) |
| Brain | 16.7 (2/12) |
| Total | 56.1 (138/246) |
| Geese | |
| Ovary | 78.6 (11/14) |
| Liver | 25.0 (3/12) |
| Kidney | 36.4 (4/11) |
| Spleen | 20.0 (2/10) |
| Brain | 20.0 (2/10) |
| Total | 38.6 (22/57) |
Fig 2.
Phylogenetic relationships of the NS5 genes of viruses isolated in this study (shown in red) and those of other flaviviruses from GenBank.
To confirm that the egg drop disease was not caused by another virus, virus was isolated from the clinical samples by infecting six embryonated eggs, and allantoic fluids were collected and tested for other viruses. Hemagglutination tests confirmed the absence of avian influenza virus and Newcastle disease virus; an RT-PCR method (15) confirmed the absence of reovirus in the allantoic fluids. The egg drop disease was not, therefore, caused by these viruses.
Egg drop disease was reproduced by experimental infection of specific-pathogen-free (SPF) White Leghorn egg-laying chickens (20/group) and native healthy white geese (30/group) (housed in the Experimental Animal Center). The third-passage duck-origin Tembusu-like virus TA strain was selected for this experiment (6). Each bird was inoculated subcutaneously with 0.5 ml of 106 50% egg infective doses (EID50/ml) of the TA strain. Twenty chickens and 20 geese were inoculated with phosphate-buffered saline (PBS) for use as negative controls. Three randomly selected birds were sacrificed on days 3, 5, and 7 postinfection and tested for the presence of the flavivirus by using the NS5-specific RT-PCR method. Compared with the control birds, the infected birds experienced a 10% to 45% drop in egg production during the 7-day infection. The infected birds exhibited depression and anorexia, but no birds died during the experiment. The negative control birds showed no signs of either a drop in egg production or disease. Necropsies of infected chickens and geese showed obvious ovary hemorrhage on days 3, 5, and 7 postinfection. Twelve chickens and 10 geese in the infected groups experienced hemorrhage of enlarged livers and kidneys. No lesions obvious to the naked eye were present on the other organs examined. Viral RNAs were most readily detected in the ovaries of birds sacrificed on days 3 and 5 postinfection (Table 2), but no viral RNA was detected on day 7 postinfection.
Table 2.
Virus detection by an NS5-specific RT-PCR method in birds infected by the TA strain
| Day postinfection | Virus detection ina: |
||||
|---|---|---|---|---|---|
| Ovary | Liver | Kidney | Brain | Spleen | |
| 3 | 3/3 | 1/1 | 2/2 | 1/1 | 1/1 |
| 5 | 3/3 | 1/2 | 2/1 | 0/0 | 2/1 |
| 7 | 0/0 | 0/0 | 0/1 | 0/0 | 0/0 |
Shown is the number of positive organs from chickens/number of positive organs from geese out of a total of three tested.
This is the first report of Tembusu-like virus infection in chickens and geese. Genetic analysis of isolates from these birds revealed a high sequence similarity to those of duck Tembusu-like viruses in 2010, suggesting that this virus has adapted to domestic birds. This outbreak of the Tembusu-like virus in chickens and geese was remarkable given that ducks are not thought to be particularly susceptible to the Tembusu-like virus infection (2, 10), and its potential transmission to other wild birds should be considered.
Transmission of many flaviviruses is seasonal, reflecting mosquito activity. However, as with other flaviviruses (3), some Tembusu-like viruses were isolated in winter, which may suggest that mosquito is not the only route for virus transmission. Viral RNAs were most readily detected in the ovaries of birds in this study, and in an earlier report (2), suggesting the need to investigate whether transovarial transmission to eggs occurs. Bird migration and transport of infected birds could also be transmission mechanisms for this virus, similar to other flaviviruses (7, 8). Given its high homology to the Tembusu virus THCAr strain and earlier reports of the presence of antibodies against Tembusu virus in humans (12, 13), this virus should be investigated for its ability to infect humans. Moreover, effective prevention and elimination measures should be developed as quickly as possible.
This report highlights why the need for continued monitoring of the expansion and circulation of this virus is critically important for estimating its potential impact on avian farming communities in Asia.
Nucleotide sequence accession numbers.
The sequences of the viruses isolated from chicken and goose farms in China in this study have been submitted to GenBank under the following accession numbers (with the strain name given in parentheses): JQ627862 (CK-SD-11), JQ627860 (CK-FJ), JQ627861 (CK-JX-9), JQ627863 (GS-GD-5), JQ627864 (GS-PT-7), and JQ627865 (GS-ZZ-7).
ACKNOWLEDGMENTS
This study was supported by grant from the China Agriculture Research System (CARS-43-10) and the Chinese Special Fund for Agro-Scientific Research in the Public Interest (201003012).
Footnotes
Published ahead of print 12 June 2012
REFERENCES
- 1. Beckwith WH, Sirpenski S, French RA, Nelson R, Mayo D. 2002. Isolation of eastern equine encephalitis virus and West Nile virus from crows during increased arbovirus surveillance in Connecticut, 2000. Am. J. Trop. Med. Hyg. 66:422–426 [DOI] [PubMed] [Google Scholar]
- 2. Cao ZZ, et al. 2011. Tembusu virus in ducks, China. Emerg. Infect. Dis. 17:1873–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dawson JR, et al. 2007. Crow deaths caused by West Nile virus during winter. Emerg. Infect. Dis. 13:1912–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar NS, Kataria JM, Koti M, Dhama K, Toroghi R. 2003. Detection of egg drop syndrome 1976 virus by polymerase chain reaction and study of its persistence in experimentally infected layer birds. Acta Virol. 47:179–184 [PubMed] [Google Scholar]
- 5. Lindenbach BD, Thiel HJ, Rice CM. 2007. Flaviviridea: the viruses and their replication, p 1101–1152 In Knipe DM, Howley PM. (ed), Fields virology, 5th ed Lippincott, Williams, and Wilkins, Philadelphia, PA [Google Scholar]
- 6. Liu M, et al. 2012. Complete genomic sequence of duck flavivirus from China. J. Virol. 86:3398–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reed KD, Meece JK, Henkel JS, Shukla SK. 2003. Birds, migration and emerging zoonoses: west Nile virus, Lyme disease, influenza A, and enteropathogens. Clin. Med. Res. 1:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ritchie SA, Rochester W. 2001. Wind-blown mosquitoes and introduction of Japanese encephalitis into Australia. Emerg. Infect. Dis. 7:900–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sambrook R. 2002. Molecular cloning, p 1695–1697 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 10. Su JJ, et al. 2011. Duck egg-drop syndrome caused by BYD virus, a new Tembusu-related flavivirus. PLoS One 6:e18106 doi:10.1371/journal.pone.0018106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wages DP, Ficken MD, Guy JS, Cummings TS, Jennings SR. 1993. Egg production drop in turkeys associated with alphaviruses: eastern equine encephalitis virus and Highlands J virus. Avian Dis. 37:1163–1166 [PubMed] [Google Scholar]
- 12. Wallace RA, Rajagopal V. 1977. Activity of Tembusu and Umbre viruses in a Malaysian community—mosquito studies. Mosq. News 37:35–42 [Google Scholar]
- 13. Wolfe ND, Kilbourn AM, Karesh WB, Rahman HA, Bosi EJ. 2001. Sylvatic transmission of arboviruses among Bornean orangutans. Am. J. Trop. Med. Hyg. 64:310–316 [DOI] [PubMed] [Google Scholar]
- 14. Yan LP, Yan PX, Zhou JW, Teng QY, Li ZJ. 2011. An infectious disease of ducks caused by a newly emerged Tembusu virus strain in mainland China. Virology 417:1–8 [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y, et al. 2006. Detection and identification of avian, duck, and goose reoviruses by RT-PCR: goose and duck reoviruses are part of the same genogroup in the genus Orthoreovirus. Arch.Virol. 151:1525–1538 [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, et al. 2007. Characterization of M-class genome segments of Muscovy duck reovirus S14. Virus Res. 125:42–53 [DOI] [PubMed] [Google Scholar]