Abstract
Molecular typing of Mycobacterium tuberculosis can be used to elucidate the epidemiology of tuberculosis, including the rates of clustering, the frequency of polyclonal disease, and the distribution of genotypic families. We performed IS6110 typing and spoligotyping on M. tuberculosis strains isolated from HIV-infected subjects at baseline or during follow-up in the DarDar Trial in Tanzania and on selected community isolates. Clustering occurred in 203 (74%) of 275 subjects: 124 (80%) of 155 HIV-infected subjects with baseline isolates, 56 (69%) of 81 HIV-infected subjects with endpoint isolates, and 23 (59%) of 39 community controls. Overall, 113 (41%) subjects had an isolate representing the East Indian “GD” family. The rate of clustering was similar among vaccine and placebo recipients and among subjects with or without cellular immune responses to mycobacterial antigens. Polyclonal disease was detected in 6 (43%) of 14 patients with multiple specimens typed. Most cases of HIV-associated tuberculosis among subjects from this study in Dar es Salaam resulted from recently acquired infection. Polyclonal infection was detected and isolates representing the East Indian GD strain family were the most common.
INTRODUCTION
Molecular typing of Mycobacterium tuberculosis isolates has led to important insights about the epidemiology and pathophysiology of HIV-associated tuberculosis, which has implications for prevention strategies (17, 21). One of the most widely used typing methods is the determination of restriction fragment length polymorphisms based on the insertion sequence IS61110. Isolates that are unique on IS6110 typing are presumed to result from reactivation disease, while patterns that are part of a cluster (i.e., a group of identical or similar patterns collected in the same time frame) are more likely to represent recent transmission. Further, related IS6110 types can be grouped into families, which have been correlated with the geographic origin of the isolates (15).
In countries where HIV and tuberculosis are coendemic, tuberculosis due to clustered isolates is common (7, 16, 23). In Tanzania, a 1995 cross-sectional study found clustering in 37% of 68 HIV-infected patients (33), while another cross-sectional study found 83% of 88 isolates from patients with unreported HIV status in Northern Tanzania had similar IS6110 patterns (10). A recent meta-analysis of seven studies found a trend toward a higher proportion of clustering in HIV-infected individuals (16). These data show that new transmission M. tuberculosis is a greater driver of the expanding epidemic of HIV-associated tuberculosis than reactivation of latent infection. However, detailed clinical data are not typically available in cross-sectional typing studies. Further, only limited data are available on disease due to simultaneous infection with two or more strains of M. tuberculosis (polyclonal disease). The potential for polyclonal infection may be greater in HIV-infected patients who are at risk for concurrent reactivation and reinfection (5, 29).
M. tuberculosis genotypes, virulence, and drug resistance vary geographically. Some African countries have high rates of disease due to the W-Beijing family, which may be more pathogenic than other strains (23). The most common genotypic families among 147 tuberculosis isolates reported from Tanzania were the Central Asian (37%), Latin American Mediterranean (22%), and East-African Indian (17%) families (7).
During the DarDar Vaccine Trial in Tanzania, we collected isolates of M. tuberculosis from patients with HIV infection, as well as community controls, and performed IS6110 typing, spoligotyping, and drug susceptibility testing (30). These data were analyzed to determine the rates of clustering, the frequency of polyclonal disease, and the distribution of genotypic families.
MATERIALS AND METHODS
Isolates of M. tuberculosis.
The DarDar Vaccine Trial conducted in Dar es Salaam, Tanzania, from 2001 through 2008 documented the efficacy of a whole-cell mycobacterial vaccine, M. vaccae, in preventing tuberculosis in HIV-infected adults (30). We collected M. tuberculosis isolates from subjects screened for or enrolled in the trial which included (i) baseline isolates from 2,960 HIV-infected subjects screened and deemed ineligible for the trial and (ii) endpoint isolates at the time of a tuberculosis episode from 2,013 HIV-infected subjects enrolled in the trial and monitored prospectively for the development of tuberculosis. In addition, a nonrepresentative convenience sample of isolates from community cases of tuberculosis (e.g., treatment failure and retreatment cases) was obtained concurrently without identifiers from the National Tuberculosis and Leprosy Control Programme (NTLP) reference laboratory.
Three sputum samples were collected at the baseline screening and at the time of a suspected tuberculosis episode. The first specimen was collected at the time of the study visit and one each on the subsequent 2 days. Similarly, blood samples were collected at baseline and when a tuberculosis diagnosis was suspected; one specimen was collected concurrently with the first sputum specimen.
Subjects.
Eligible subjects for the DarDar Trial were HIV-infected adults recruited from HIV testing centers in Dar es Salaam with a baseline CD4 count of ≥200 cells/μl and a BCG scar. Subjects were screened for active tuberculosis at baseline with three expectorated or induced sputum samples and a mycobacterial blood culture. Screened subjects with culture-confirmed tuberculosis disease at baseline (167 subjects contributing 174 isolates) were ineligible to continue in the study. Eligible subjects were enrolled in the study, randomized 1:1 to receive vaccine or placebo, monitored for a median of 3.3 years, and evaluated with three expectorated sputum samples and a mycobacterial blood culture when a tuberculosis disease endpoint was suspected. At the time of enrollment, all subjects had a tuberculin skin test performed (TST), and those with reactions of ≥5 mm were treated with isoniazid at 300 mg daily for 6 months (24). Eligible DarDar subjects who subsequently developed tuberculosis or a CD4 count of <200 cells/μl were counseled and referred to a Tanzanian Ministry of Health Care and Treatment Center for antiretroviral therapy (ART).
Clinical data.
Detailed clinical and laboratory data were available for HIV-infected enrolled DarDar subjects who reached a tuberculosis endpoint: baseline CD4 count, HIV viral load, TST status, and history of tuberculosis (30). Screened subjects with tuberculosis at baseline were known to be HIV infected, but no other clinical data were available. Neither HIV status nor clinical data were available for community controls provided by the NTLP.
Microbiology.
Sputum samples were obtained by expectoration or induction for acid-fast bacilli stain and mycobacterial culture. Standard techniques were used and included culture on Löwenstein-Jensen medium and an automated mycobacterial blood culture (MB/BacT; bioMérieux, Durham, NC). Putative M. tuberculosis isolates were inoculated in duplicate into 7H9 liquid medium supplemented with oleic acid, albumin, dextrose, and catalase and 15% glycerol, frozen at −70°C and shipped to the United States, where they were tested for confirmation of M. tuberculosis complex using a DNA probe (Accuprobe; Gen-Probe, San Diego, CA) with a documented specificity of 100% (2, 25). For most patients, a single colony of M. tuberculosis from a single positive sputum or blood sample was selected for testing. In some instances, a single colony from a second positive sputum sample was also tested. Isolates that were not DNA probe positive for M. tuberculosis complex were not included in the analysis.
IS6110 typing and spoligotyping.
IS6110 typing was performed by the Public Health Research Institute (PHRI) Center in Newark, NJ, and the hybridization images were analyzed using Bio Image pattern matching software (Bio Image) (22). Spoligotyping was performed for 148 isolates by the New York State Department of Health in Albany, NY, and for 11 isolates at the Mycobacterial Reference Laboratory of the National Institute for Health and Welfare in Turku, Finland, following standard methods (21).
Cluster analysis.
A cluster was defined as two or more M. tuberculosis isolates that had IS6110 hybridization patterns that were either (i) indistinguishable or (ii) differed by one band and, when available, had a matching spoligotype result. Multiple isolates from the same patient were included in the determination of polyclonal disease but removed from all other analyses. If a patient had two isolates with distinct IS6110 patterns, they were considered to have polyclonal disease; if at least one IS6110 result met cluster criteria, the patient was classified as clustered. IS6110 results that did not meet cluster criteria were considered unique. Analyses were conducted using Excel and STATA 9 (StataCorp, College Station, TX).
Molecular analysis and family designation.
The PHRI database of IS6110 typing results comprises more than 27,000 M. tuberculosis isolates and more than 8,000 different fingerprint patterns (B. Kreisworth, unpublished data). Strain codes were assigned using methods previously described (3, 22). Strain patterns that are unique in the PHRI database did not qualify for a strain or family assignment and were given a default designation (i.e., “001”). The PHRI TB Center archived IS6110 fingerprint pattern library was mapped to one of nine M. tuberculosis major clusters as described by Gutacker et al. (15).
DST.
Isolates were sent to the Mycobacterial Reference Laboratory of the National Institute for Health and Welfare in Turku, Finland, where drug susceptibility testing (DST) was performed. Isolates were cultured on Löwenstein-Jensen medium, and DST for first-line drugs was performed on Middlebrook 7H10 agar by the disk-elutriation method using standardized protocols (32). Resistance to pyrazinamide was determined according to a method described by Brander (4). DST for the second-line drugs was performed with the narrow-range MIC method.
Human subject protection.
The study protocol of the DarDar Vaccine Trial was approved by the Dartmouth Committee for the Protection of Human Subjects, by the Muhimbili University of Health and Allied Sciences Research Ethics Committee, and by the Division of AIDS Clinical Science Review Committee, National Institutes of Health. Informed consent was obtained from all study participants. The study was conducted in compliance with good clinical practice guidelines.
Statistical analyses.
For two group comparisons, we used a Mann-Whitney U test, Student t test, and chi-squared test as appropriate. In all cases, we used a two-tailed threshold for a statistical significance of P < 0.05. Analyses were done with STATA 9 (StataCorp, College Station, TX).
RESULTS
Clustering.
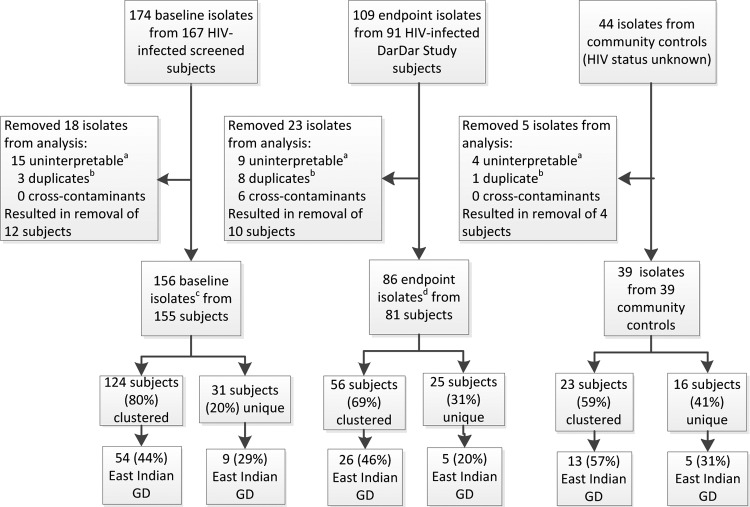
IS6110 typing was performed on 327 isolates (174 baseline, 109 endpoint, and 44 community isolates) and clustering determined for 281 isolates (156 baseline, 86 endpoint, and 39 community) from 275 subjects after removal of duplicate, false positive and uninterpretable results (Fig. 1). Clustering occurred in 203 (74%) of 275 subjects overall. Among 236 study subjects, clustering was observed in 180 (76%): in 124 (80%) of 155 subjects with baseline isolates and in 56 (69%) of 81 subjects with endpoint isolates (P = 0.063). A total of 45 clusters were identified involving study subjects; the median number of subjects in a cluster was 3, with a range from 2 to 27 (Table 1). Among the 39 community controls for whom no clinical data were available (Fig. 1), clustering was observed in 23 (59%) subjects.
Fig 1.
Diagram of M. tuberculosis isolate evaluation. This figure describes the breakdown of subjects and their isolates by category (screened subjects with baseline isolates, enrolled subjects with endpoint isolates, and community subjects with control isolates) included in the analysis and the degree of clustering and East Indian GD family representation in each. Baseline isolates were obtained from HIV-infected subjects when screened for the DarDar Vaccine Trial; endpoint isolates were obtained from enrolled HIV-infected subjects at the time of a tuberculosis diagnosis. Superscripts: a, uninterpretable results were mostly the result of mixed cultures caused by contamination or representative of a mixed infection; b, twelve duplicate results from nine subjects with multiple specimens were removed from the main analysis but included in the determination of polyclonal disease; c, includes one polyclonal disease subject with two isolates; d, includes five polyclonal disease subjects with two isolates each.
Table 1.
Cluster size frequency and distribution among 236 study subjectsa
| Cluster size | No. of clusters of that size |
|---|---|
| 2 | 21 |
| 3 | 9 |
| 4 | 3 |
| 5 | 5 |
| 6 | 2 |
| 7 | 1 |
| 8 | 1 |
| 10 | 1 |
| 11 | 1 |
| 27 | 1 |
Includes one study subject with polyclonal disease whose isolates belonged to two different clusters.
Among subjects with endpoint isolates, 25 (74%) of 34 vaccine-recipients belonged to one of 16 clusters and 31 (66%) of 47 placebo-recipients belonged to one of 20 clusters (P = 0.47). Among 20 subjects with disseminated tuberculosis (defined per protocol by positive blood culture), 14 (60%) were clustered.
There were no significant differences in characteristics of patients with clustered or unique isolates including the frequency of prior treatment for tuberculosis or positive TST at baseline (Table 2).
Table 2.
Characteristics of subjects with endpoint isolates who had clustered versus unique isolates
| Characteristica | Patients with: |
Pb | |
|---|---|---|---|
| Clustered isolates (n = 56) | Unique isolates (n = 25) | ||
| Median age (yrs) | 35 | 32 | 0.79 |
| No. of male subjects (%) | 11 (20) | 8 (32) | 0.23 |
| Baseline CD4 count (median cells/μl) | 348 | 342 | 0.79 |
| Baseline HIV viral load (mean log10)c | 4.4 | 4.3 | 0.63 |
| No. of subjects (%) with prior treatment for tuberculosis | 11 (20) | 4 (16) | 0.70 |
| Baseline ART (no.) | 0 | 0 | |
| TST ≥ 5 mm, no. (%)d | 33 (60) | 19 (76) | 0.16 |
| No. of subjects (%) that received isoniazid | 28 (50) | 13 (52) | 0.87 |
| No. of subjects (%) that received M. vaccae vaccine | 25 (45) | 9 (36) | 0.47 |
ART, antiretroviral therapy; TST, tuberculin skin test.
P values were determined by using the Mann-Whitney U test, Student t test, and chi-squared analyses as appropriate.
n = 53.
n = 55 clustered; n = 25 unique.
Polyclonal disease.
Polyclonal disease was detected in 6 (43%) of the 14 subjects with multiple isolates available for IS6110 typing. Of 6 subjects with at least two isolates from sputum, 3 (50%) had polyclonal disease. Five of these subjects (including two polyclonal cases) had both specimens collected over a 2-day period. The remaining subject was polyclonal based on isolates collected 5.5 months apart; the patient was successfully treated with 9 months of therapy. Among eight subjects with concurrent sputum and blood isolates, three (38%) had polyclonal disease and were previously reported in our study of disseminated tuberculosis (29).
All three subjects with polyclonal disease in sputum alone had one clustered isolate. Among the three patients with polyclonal disease in sputum and blood, one had neither isolate clustered, one had one isolate clustered and one had both isolates clustered (Table 3).
Table 3.
Polyclonal disease in 14 patients with HIV-associated tuberculosis
| Specimen source | No. polyclonal/no. tested (%) | No. of isolates clustered in polyclonal cases |
||
|---|---|---|---|---|
| None | One | Two | ||
| Sputum/sputum | 3/6 (50) | 0 | 3 | 0 |
| Sputum/blood | 3/8 (38) | 1a | 1 | 1 |
| Total | 6/14 (43) | 1/6 | 4/6 | 1/6 |
Clustered isolate was from blood.
Families.
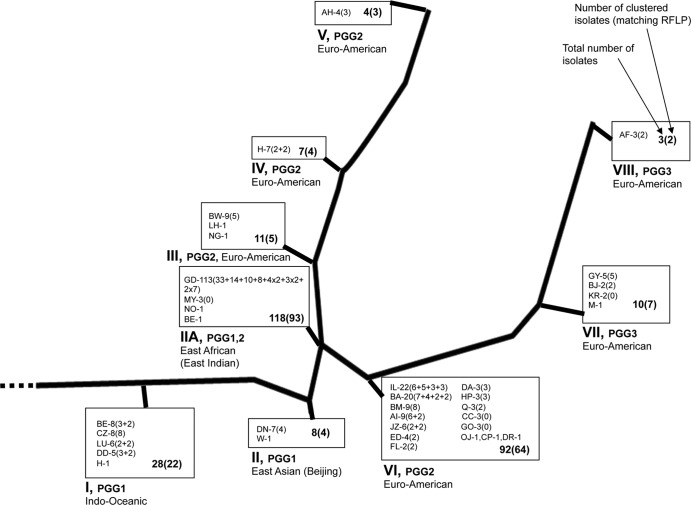
Thirty-one different IS6110 families were represented in the results. The East Indian GD family was the most common with 94 study subjects and 19 community control patients (41%) contributing (Fig. 2). Thirty-one (38%) enrolled subjects and 63 (41%) screened subjects had isolates from the GD family.
Fig 2.
M. tuberculosis strains from the study subjects in a phylogenetic framework. This figure depicts the evolutionary phylogenic tree of M. tuberculosis strain families and the number of isolates from each identified in our study population. RFLP, restriction fragment length polymorphism. A total of 327 isolates were examined, including 281 RFLP results from 275 subjects (one isolate per subject included in the analysis except for two isolates per subject for six subjects with polyclonal disease), 204 clustered isolates (from 203 subjects), and 77 nonclustered isolates (from 72 subjects).
DST.
DST for the first-line drugs was performed on 238 M. tuberculosis isolates from 233 study subjects. A total of 207 (89%) of the 233 subjects with DST results had isolates susceptible to all of the first-line drugs, while any resistance was detected in 26 (11%) isolates. Ten (13%) of the 78 endpoint isolates, and 16 (10%) of the 155 baseline isolates had any resistance detected. A similar rate of resistance was found among the community control isolates tested (5 of 37, 14%). Among clustered and unique isolates from study subjects with DST results, 16 of 178 (9%) and 10 of 56 (18%), respectively, had any resistance detected. Of the five polyclonal disease patients with more than one specimen tested, four had matching DST results (both pansensitive strains), and 1 had divergent results (no resistance detected in one strain and monoresistance to pyrazinamide found in the second strain tested). Seven isolates (five baseline and two endpoint, 3% overall) were multidrug-resistant tuberculosis (MDR-TB) No extensively drug-resistant isolates were detected.
DISCUSSION
This study of prospectively monitored HIV-infected patients in Tanzania shows that most cases of tuberculosis are clustered and are therefore likely to represent recent acquisition. Among those with known HIV infection, the 76% rate of clustering is higher than the average 68% found in a meta-analysis of seven studies, and among the highest reported rates (range, 37 to 79%) (16). Because our sample size was relatively small, with relatively few community isolates for comparison, the actual rate of clustering may even be higher, as has been shown with simulation modeling (13).
Recurrent tuberculosis is a major problem for individuals living in areas of tuberculosis endemicity (14). In our study, the prevalence of previous tuberculosis disease was similar among subjects whose isolates were and were not clustered. This suggests that in HIV-infected patients, previous tuberculosis disease does not enhance resistance to subsequent reinfection. In both HIV-infected and HIV-uninfected subjects with a first episode of tuberculosis disease, the risk of a second episode is higher than the risk of a first episode among persons living in the same community (12). In contrast, among patients without HIV infection and in animal models, latent tuberculosis infection has been shown to provide protection against tuberculosis disease upon re-exposure (1, 8), likely because an immunologic response that contains a tuberculosis infection also provides resistance to a new exposure.
The high rate of clustering is consistent with numerous studies that demonstrate most cases of tuberculosis in HIV are due to reinfection. This finding suggests that in addition to administration of isoniazid for 6 months to prevent reactivation, other strategies should focus on prevention of reinfection: for example, 36 months of isoniazid and prompt initiation of antiretroviral therapy (19, 26, 30). The DarDar Trial demonstrated vaccine administration reduced the incidence of definite tuberculosis for up to 4 years. The molecular analysis reported here indicates that the substantial majority of incident cases involved clustered isolates consistent with recent infection (30). We conclude that in the population studied the M. vaccae vaccine prevents new infections. The observation that the rates of clustering were not different between the vaccine and control groups may reflect the limitations of the molecular data set as discussed below.
Polyclonal disease, defined as simultaneous infection with two or more different M. tuberculosis strains, has been described as rare in some settings (9) but was found in 17% of new cases and 23% of retreatment cases in South Africa (31). We found polyclonal disease in 6 of 14 (43%) of tested subjects, and this rate may be an underestimate because most subjects had only one colony from a single specimen typed. In studies of disseminated M. avium in AIDS we have shown that detection of polyclonal disease increases when multiple colonies are tested from each positive clinical culture (27). Four of the six subjects with polyclonal disease had one clustered isolate and one unique isolate, raising the possibility that these subjects had simultaneous reactivation disease and new infection.
With disseminated M. avium infection in HIV we have observed differential drug susceptibility among patients with polyclonal disease and two different isolates (28). Only one of our subjects with polyclonal disease had different DST results for their two different strains. In regions where drug-resistant tuberculosis is more common than in Tanzania, polyclonal infection might involve one drug-sensitive and one drug-resistant isolate. Studies are needed of DST results from polyclonal isolates in these regions.
We found that the East Indian GD family of M. tuberculosis isolates was predominant among tuberculosis patients in Dar es Salaam, Tanzania. Isolates of the GD strain family are highly prevalent in India and Pakistan and among immigrants from this region. Presence of these genotypes in East Africa is not surprising given well-documented migration and trade-related travel patterns over several centuries, beginning as early as the 9th century. However, the predominance we observed contrasts with a previous study in which the Central Asian family was predominant among 147 M. tuberculosis isolates from patients in Dar es Salaam (7); no additional clinical information, including HIV status, was available regarding these patients. Further, other genotypic families predominate in neighboring countries: the European/American lineage in Malawi and the W-Beijing and the CC strain families in South Africa (7, 11, 24). In both the South Africa and Malawi studies, East Indian GD strain families were rare (2% in South Africa, 9% East African/Indian, and 2% East Asian in Malawi) (11, 23). Despite its prevalence in South Africa, a W-Beijing strain was present in only one subject in our study population. Consistent with other studies in Africa, most strains belong to the principal genetic groups I and II, the most ancestral strain types.
Most of the isolates evaluated here were drug susceptible, a finding consistent with the recent national report on tuberculosis drug resistance in Tanzania (6). In that report, isolates from 8.3% of new and 20.6% of retreatment patients had resistance to any of the first-line drugs. MDR-TB rates were very low at 1.1% in new and 3.9% in retreatment patients. In the present study, MDR-TB was identified in 3% of clustered isolates. Such results suggest tight control of tuberculosis drugs and compliance with the NTLP guidelines has been successful in limiting the emergence of drug-resistant strains.
The M. tuberculosis isolates studied here were identified through prospective, active case finding in the context of a phase III clinical trial (30). Further, baseline mycobacterial cultures were obtained regardless of baseline symptomatology, suggesting that our diagnostic work-up for tuberculosis was likely more sensitive than routine clinical practice in low-income countries.
There are several limitations to our study. Our study population was limited to patients with prior BCG vaccination and a CD4 count of ≥200 cells/μl and thus does not represent all patients with HIV infection. Our isolate sets did not represent all patients diagnosed with tuberculosis from Dar es Salaam in the given time frame, and we may have therefore missed identifying some cases as clustered. Our community isolates were not a representative sample since mycobacterial culture is not routinely performed on all patients with suspect tuberculosis but rather reserved for cases in which drug resistance is being considered (e.g., treatment failure and retreatment cases). Although enrolled subjects had both blood and sputum cultures obtained and tested by IS6110 per protocol, IS6110 testing of duplicated sputum samples from the same subject was not systematic, and rates of polyclonal infection in this subgroup may not be representative. Finally, although procedures were in place to prevent coughing subjects from exposure to other subjects, study subjects who contributed endpoint isolates for analysis were all seen in the same study outpatient facility, and we cannot exclude the possibility of nosocomial transmission of some cases and artificial elevation of apparent clustering rates.
In summary, we have shown that clustered and therefore newly acquired M. tuberculosis infection, particularly with the East African GD family, is the major cause of incident HIV-associated tuberculosis disease in Dar es Salaam, Tanzania. Polyclonal disease was documented, including simultaneous disease with both clustered and nonclustered isolates, suggesting that reactivation and new infection may occur together.
ACKNOWLEDGMENTS
This study was supported by grants AI 45407 and 5P20RR016437-08 from the National Institutes of Health, DAIDS, and grant D43-TW006807 from the Fogarty International Center.
We thank Outi Rautio and Betty Mchaki for their skillful conduct of the immunological assays in this study and all of the DarDar Vaccine Trial participants.
Footnotes
Published ahead of print 30 May 2012
REFERENCES
- 1. Andrews JR, et al. 2012. Risk of progression to active tuberculosis following reinfection with M. tuberculosis. Clin. Infect. Dis. 54:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Badak FZ, et al. 1999. Use of nucleic acid probes for identification of Mycobacterium tuberculosis directly from MB/BacT bottles. J. Clin. Microbiol. 37:1602–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bifani PJ, et al. 1999. 1999. Identification of a W variant outbreak of Mycobacterium tuberculosis via population-based molecular epidemiology. JAMA 282:2321–2327 [DOI] [PubMed] [Google Scholar]
- 4. Brander E. 1972. A simple way of detecting pyrazinamide resistance. Tubercle 53:128–131 [DOI] [PubMed] [Google Scholar]
- 5. Chaves F, Dronda F, Alonso-Sanz M, Noriega AR. 1999. Evidence of exogenous reinfection and mixed infection with more than one strain of Mycobacterium tuberculosis among Spanish HIV-infected inmates. AIDS 13:615–620 [DOI] [PubMed] [Google Scholar]
- 6. Chonde TM, et al. 2010. National anti-tuberculosis drug resistance study in Tanzania. Int. J. Tuberc. Lung Dis. 14:967–972 [PubMed] [Google Scholar]
- 7. Eldholm V, Matee M, Mfinanga SG, Heun M, Dahle UR. 2006. A first insight into the genetic diversity of Mycobacterium tuberculosis in Dar es Salaam, Tanzania, assessed by spoligotyping. BMC Microbiol. 6:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flahiff EW. 1939. The occurrence of tuberculosis in persons who failed to react to tuberculin, and in persons with positive tuberculin reactions. Am. J. Epidemiol. 30:69–74 [Google Scholar]
- 9. Garcia de Viedma D, Marin M, Serrano MJR, Alcala L, Bouza E. 2003. Polyclonal and compartmentalized infection by Mycobacterium tuberculosis in patients with both respiratory and extra-respiratory involvement. J. Infect. Dis. 187:695–699 [DOI] [PubMed] [Google Scholar]
- 10. Gillespie SH, et al. 1995. Restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolated from patients with pulmonary tuberculosis in northern Tanzania. Trans. R. Soc. Trop. Med. Hyg. 89:335–338 [DOI] [PubMed] [Google Scholar]
- 11. Glynn JR, et al. 2010. Changes in Mycobacterium tuberculosis genotype families over 20 years in a population-based study in Northern Malawi. PLoS One 5:e12259 doi:10.1371/journal.pone.0012259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glynn JR, et al. 2010. High rates of recurrence in HIV-infected and HIV-uninfected patients with tuberculosis. J. Infect. Dis. 201:704–711 [DOI] [PubMed] [Google Scholar]
- 13. Glynn JR, Vynnycky E, Fine PE. 1999. Influence of sampling on estimates of clustering and recent transmission of Mycobacterium tuberculosis derived from DNA fingerprinting techniques. Am. J. Epidemiol. 149:366–371 [DOI] [PubMed] [Google Scholar]
- 14. Glynn JR, et al. 2004. DNA fingerprint changes in tuberculosis: reinfection, evolution, or laboratory error? J. Infect. Dis. 190:1158–1166 [DOI] [PubMed] [Google Scholar]
- 15. Gutacker MM, et al. 2006. Single-nucleotide polymorphism-based population genetic analysis of Mycobacterium tuberculosis strains from 4 geographic sites. J. Infect. Dis. 193:121–128 [DOI] [PubMed] [Google Scholar]
- 16. Houben RM, et al. 2011. Human immunodeficiency virus associated tuberculosis more often due to recent infection than reactivation of latent infection. Int. J. Tuberc. Lung Dis. 15:24–31 [PubMed] [Google Scholar]
- 17. Kato-Maeda M, Metcalfe JZ, Flores L. 2011. Genotyping of Mycobacterium tuberculosis: application in epidemiologic studies. Future Microbiol. 6:203–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reference deleted.
- 19. Lawn SD, et al. 2010. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect. Dis. 10:489–498 [DOI] [PubMed] [Google Scholar]
- 20. Reference deleted.
- 21. Mathema B, et al. 2002. Identification and evolution of an IS6110 low-copy-number Mycobacterium tuberculosis cluster. J. Infect. Dis. 185:641–649 [DOI] [PubMed] [Google Scholar]
- 22. Mathema B, Kurepina NE, Bifani PJ, Kreiswirth BN. 2006. Molecular epidemiology of tuberculosis: current insights. Clin. Microbiol. Rev. 19:658–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Middlekoop K, et al. 2009. Molecular epidemiology of Mycobacterium tuberculosis in a South African community with high HIV prevalence. J. Infect. Dis. 200:1207–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Munseri PJ, Talbot EA, Mtei L, Fordham von Reyn C. 2008. Completion of isoniazid preventive therapy among HIV-infected patients in Tanzania. Int. J. Tuberc. Lung Dis. 12:1037–1041 [PubMed] [Google Scholar]
- 25. Peterson EM, et al. 1989. Direct identification of Mycobacterium tuberculosis, Mycobacterium avium, and Mycobacterium intracellulare from amplified primary cultures in BACTEC media using DNA probes. J. Clin. Microbiol. 27:1543–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Samandari T, et al. 2011. 6-Month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet 377:1588–1598 [DOI] [PubMed] [Google Scholar]
- 27. Slutsky AM, et al. 1994. Polyclonal infections due to Mycobacterium avium complex in patients with AIDS detected by pulsed-field gel electrophoresis of sequential clinical isolates. J. Clin. Microbiol. 32:1773–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. von Reyn CF, Jacobs NJ, Arbeit RD, Maslow JN, Niemczyk S. 1995. Polyclonal Mycobacterium avium infections in patients with AIDS: variations in antimicrobial susceptibilities of different strains of M. avium isolated from the same patient. J. Clin. Microbiol. 33:1008–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. von Reyn CF, et al. 2011. Disseminated tuberculosis in HIV infection: ineffective immunity, polyclonal disease and high mortality. Int. J. Tuberc. Lung Dis. 15:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Reyn CF, et al. 2010. Prevention of tuberculosis in bacille Calmette-Guérin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS 24:675–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Warren RM, et al. 2004. Patients with active tuberculosis often have different strains in the same sputum specimen. Am. J. Respir. Crit. Care Med. 169:610–614 [DOI] [PubMed] [Google Scholar]
- 32. Wayne LG, Krasnow I. 1966. Preparation of tuberculosis susceptibility testing mediums by means of impregnated disks. Am. J. Clin. Pathol. 45:769–771 [DOI] [PubMed] [Google Scholar]
- 33. Yang ZH, et al. 1995. DNA fingerprinting and phenotyping of Mycobacterium tuberculosis isolates from human immunodeficiency virus (HIV)-seropositive and HIV-seronegative patients in Tanzania. J. Clin. Microbiol. 33:1064–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]