Abstract
This study assessed the recovery rates of Gram-negative bacilli from stored endotracheal aspirates frozen with and without glycerol. Samples frozen with glycerol showed a significant difference in isolate recovery, 89.7% versus 69.2% (P = 0.02). This study demonstrates that it is possible to achieve high recovery rates of potentially pathogenic organisms from endotracheal aspirates when stored with glycerol, thus broadening the scope of active surveillance cultures for both clinical and research purposes.
TEXT
Active surveillance cultures identify a large group of colonized patients who would not be identified by clinically indicated cultures, potentially controlling the spread, colonization, and infection of additional patients (1, 7–9, 11). As active surveillance increases, it will be necessary for laboratories to find efficient and reliable methods of storing samples for later analysis and/or transport to outside facilities (2, 3).
Additionally, active surveillance cultures are being used to research the potential risk factors for acquisition, colonization, and epidemiology of targeted organisms, including Enterobacteriaceae and Acinetobacter baumannii (1, 7–9, 11). Commonly in this practice, cultures are stored and analyzed at a later date. We found high recovery rates (98% sensitivity) of organisms from frozen perirectal surveillance swabs stored in Trypticase soy broth (TSB) with 15% glycerol (6, 10). In this preliminary study, we assessed the recovery rate of Gram-negative bacilli (GNB) from frozen endotracheal aspirate samples.
Although the lower respiratory tract is believed to be a sterile environment, endotracheal aspirates have been shown to harbor potentially pathogenic organisms that are colonizing the patient, especially in the intensive care units (ICUs) and following intubation (4, 5). To our knowledge, this is the first study to assess the recovery of bacteria from patient endotracheal aspirates after freezing with a preservative.
Surveillance endotracheal aspirate samples were collected from patients in the medical and surgical ICUs. Upon receipt, all samples were split into two equal parts (A and B) (Fig. 1). All part A samples served as the control and were analyzed per the gold standard. A sterile cotton-tipped swab was dipped into the most purulent area of the sample and rolled onto one plate, and then the process was repeated. The samples were plated onto culture media in the following order: CHROMagar Acinetobacter (CHROMagar, Paris, France), Trypticase soy agar with 5% sheep's blood, chocolate, and MacConkey (BD BBL; BD, Sparks, MD). After plating, the part A sample was transferred to a 1.5-ml sterile freeze tube with an O-ring seal and stored without glycerol at −80°C (referred to as “frozen”).
Fig 1.
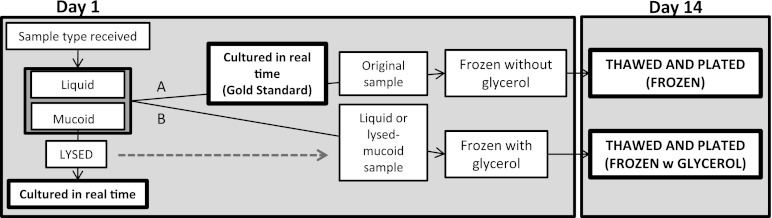
Flowchart of endotracheal aspirate sample workup. Day 14 results were plated and compared to the gold standard (bold). Samples were received in the lab and split into two equal parts. Part A was cultured in real time and frozen without the additive. The other half, part B, was frozen with glycerol. Part B mucoid samples were lysed before glycerol was added to allow for a homogeneous mixture with glycerol. Lysed samples were cultured in real time and compared to the gold standard to ensure no organism loss during lysis.
For comparison, the part B sample was frozen in an identical tube but was first mixed with TSB with 15% glycerol by volume and stored at −80°C (referred to as “frozen with glycerol”).
Due to the inability of mucoid samples to homogeneously mix with TSB with 15% glycerol, it was necessary to lyse mucoid part B samples prior to freezing, as shown in Fig. 1. Lysis was performed per the manufacturer's recommendations using a 1:10 dilution of Sputolysin reagent (Calbiochem; Merck KGaA, Darmstadt, Germany). The lysed part B samples were cultured and compared to the part A samples to ensure that lysis had no effect on the recovery of organisms. All part B samples (liquid upon receipt and lysed) were mixed with TSB with 15% glycerol by volume and stored at −80°C.
After 14 days, all samples were removed from the freezer, thawed, and plated. All organisms were identified using an established identification system (API 20E or Vitek 2; bioMérieux).
Statistical analysis was performed using McNemar's test. A statistically significant difference was considered to be a P value of <0.05.
Forty-eight (48) endotracheal aspirate samples were collected and analyzed. The gold standard culture method grew 39 GNB organisms from 17 samples (35% positivity rate). Lysed samples recovered 100% of organisms compared to the gold standard; therefore, lysis had no effect on the recovery of organisms. Frozen samples recovered 69.2% of GNB organisms (27/39; 95% confidence interval [CI], 54.7 to 83.7%). Frozen with glycerol samples recovered 89.7% of GNB organisms (35/39; 95% CI, 80.2 to 99.3%). See Table 1 for a list of the GNB organisms recovered.
Table 1.
Number of GNB isolates recovered from gold standard samples and from samples frozen with or without glycerol by organism
| Gram-negative isolate | No. of isolates from gold standard samples (% prevalence) | No. of isolates from frozen samples | No. of isolates from samples frozen with glycerol |
|---|---|---|---|
| Pseudomonas species | 10 (20.8) | 8 | 8 |
| Klebsiella pneumoniae | 6 (12.5) | 3 | 5 |
| Acinetobacter baumannii | 3 (6.3) | 3 | 3 |
| Proteus species | 3 (6.3) | 1 | 3 |
| Providencia stuartii | 3 (6.3) | 1 | 2 |
| Serratia marcescens | 3 (6.3) | 2 | 3 |
| Stenotrophomonas maltophilia | 3 (6.3) | 3 | 3 |
| Enterobacter aerogenes | 2 (4.2) | 1 | 2 |
| Enterobacter cloacae | 2 (4.2) | 2 | 2 |
| Escherichia coli | 2 (4.2) | 2 | 2 |
| Klebsiella oxytoca | 1 (2.1) | 0 | 1 |
| Providencia rettgeri | 1 (2.1) | 1 | 1 |
| Total | 39 | 27 | 35 |
Specimens frozen with glycerol yielded a statistically significant difference in the recovery rate of GNB organisms compared to freezing without glycerol (P = 0.02). No statistically significant difference was observed between mucoid and liquid samples in the recovery rates of either storage method. Gram-positive organisms were outside the scope of this study and were not included in the analysis.
To our knowledge, this is the first study to evaluate the recovery of viable bacteria following deep-freeze storage of endotracheal aspirate samples. Our results demonstrate that the recovery rate of GNB in endotracheal aspirates is significantly higher when the aspirates are frozen with glycerol than when they are frozen without glycerol. The state of the specimen (liquid or lysed mucoid) when frozen with glycerol appeared to have little impact on isolate recovery as long as glycerol was added.
A primary barrier to working with endotracheal aspirate samples in the clinical microbiology laboratory is a direct result of the nature of specimen. The samples vary greatly from patient to patient in color, blood content, volume of sample, as well as the viscosity of mucus. This sample inconsistency also contributes to the challenge of storing samples from large cohorts for the purpose of epidemiological investigations. Previous work by Zhao and colleagues demonstrated that storing a sputum sample from one cystic fibrosis patient at various temperatures (including −80°C) did not significantly alter the detection of bacteria using 16S rRNA detection methods (12).
The nature of the sample was a limitation of this study. Future studies could improve this method by performing a lysis step on all samples regardless of viscosity. This study was also limited by sample size.
We have previously shown that glycerol increases isolate recovery rates in stored nasal and perirectal swabs (5, 7). This study demonstrates that storage of endotracheal aspirate samples with glycerol significantly increases the recovery rate of GNB organisms versus storage without glycerol, thus broadening the scope of active surveillance cultures for both clinical and research purposes.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health, K12RR023250 (J.K.J.) and R01AI060859 (A.D.H.).
Footnotes
Published ahead of print 30 May 2012
REFERENCES
- 1. Calfee D, Jenkins S. 2008. Use of active surveillance cultures to detect asymptomatic colonization with carbapenem-resistant Klebsiella pneumoniae in intensive care unit patients. Infect. Control Hosp. Epidemiol. 29:966–968 [DOI] [PubMed] [Google Scholar]
- 2. Carter E, Stubbs JR, Bennett B. 2004. A model for consolidation of clinical microbiology laboratory services within a multihospital health-care system. Clin. Leadersh. Manag. Rev. 18:211–215 [PubMed] [Google Scholar]
- 3. Church D, Hall P. 1999. Centralization of a regional clinical microbiology service: the Calgary experience. Can. J. Infect. Dis. 10:393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Villota ED, Avello F, Granados MA, Arces M, Moles B. 1978. Early post-surgical bacterial contamination of the airways; a study on 28 open-heart surgery patients. Acta Anaesthesiol. Scand. 22:227–233 [DOI] [PubMed] [Google Scholar]
- 5. Drakulovic MB, et al. 2001. Initial bacterial colonization in patients admitted to a respiratory intensive care unit: bacteriological pattern and risk factors. Respiration 68:58–66 [DOI] [PubMed] [Google Scholar]
- 6. Green HP, et al. 2007. Impact of freezing on the future utility of archived surveillance culture specimens. Infect. Control Hosp. Epidemiol. 28:886–888 [DOI] [PubMed] [Google Scholar]
- 7. Harris A, et al. 2007. How important is patient-to-patient transmission in extended-spectrum β-lactamase Escherichia coli acquisition. Am. J. Infect. Control. 35:97–101 [DOI] [PubMed] [Google Scholar]
- 8. Harris A, et al. 2007. Patient-to-patient transmission is important in extended-spectrum β-lactamase-producing Klebsiella pneumoniae acquisition. Clin. Infect. Dis. 45:1347–1350 [DOI] [PubMed] [Google Scholar]
- 9. Karlowsky JA, Jones ME, Thornsberry C, Friedland R, Sahm DF. 2003. Trends in antimicrobial susceptibilities among Enterobacteriaceae isolated from hospitalized patients in the United States from 1998-2001. Antimicrob. Agents Chemother. 47:1672–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lautenbach E, et al. 2005. Test characteristics of perirectal and rectal swab compared to stool sample for detection of fluoroquinolone-resistant Escherichia coli in the gastrointestinal tract. Antimicrob. Agents Chemother. 49:798–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maragakis LL, Tucker MG, Miller RG, Carroll KC, Perl TM. 2008. Incidence and prevalence of multidrug-resistant Acinetobacter using targeted active surveillance cultures. JAMA 299:2513–2514 [DOI] [PubMed] [Google Scholar]
- 12. Zhao J, et al. 2011. Effect of sample storage conditions on culture-independent bacterial community measures in cystic fibrosis sputum specimens. J. Clin. Microbiol. 49:3717–3718 [DOI] [PMC free article] [PubMed] [Google Scholar]


