Abstract
Apophysomyces variabilis is an emerging fungal pathogen that can cause significant infections in immunocompetent patients. We report a case of A. variabilis invasive wound infection in a 21-year-old male after a self-inflicted burn injury.
CASE REPORT
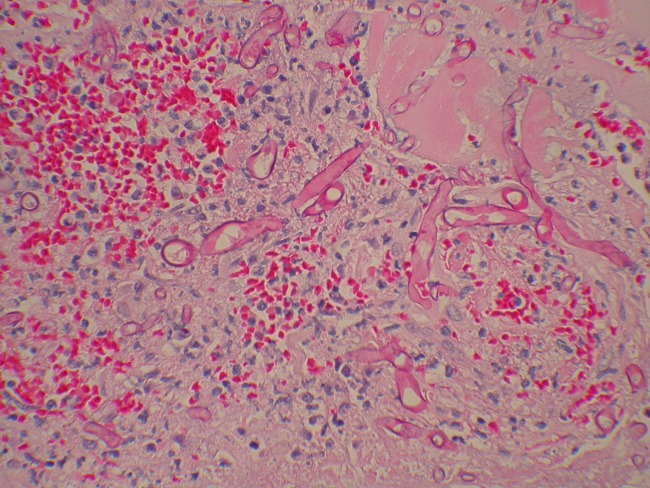
We report a case of a 21-year-old active-duty U.S. Marine with a known psychiatric history who suffered 90% total body surface area (TBSA) burns after self-immolation with gasoline while stationed in Okinawa, Japan. After the flames were doused by immersion in freshwater, he was taken immediately to a local emergency department, where he was intubated and underwent full-body escharotomies. He was transferred to a military hospital in Hawaii, where he underwent aggressive fluid resuscitation and received fresh frozen plasma for coagulopathy. Fiberoptic bronchoscopy was performed, which showed no inhalational injury. He was then transferred to the U.S. Army Institute of Surgical Research Burn Center in San Antonio, TX, approximately 4 days after the burning. Upon arrival, he underwent fascial bilateral excision of the lower and upper extremities with tangential excision of the anterior-posterior torso. A 4:1 mesh sandwich was applied to the anterior/posterior torso, and an allograft was applied to 80% of the TBSA. On postburn day 8, he was found to have suspected fungal growth on the allograft on his lower back. Fascial excision was performed, and histopathology of the excised tissue showed numerous large-diameter aseptate hyphae located mostly in the nonviable adipose tissue but with evidence of microinvasion of blood vessels (Fig. 1). Due to the clinical concern of fungal invasion beyond the burn wound, the patient was initially empirically treated with voriconazole and liposomal amphotericin B. Deep-wound cultures subsequently grew a fungus that was tentatively identified as a species within the order Mucorales. Blood cultures were negative for the fungus. Amphotericin B was continued to cover for the mucoralean species, while voriconazole was continued to empirically cover other possible fungal pathogens that might have been infecting this patient. Despite aggressive debridement of infected tissue and systemic antifungal administration, fungal infection persisted. The patient eventually died of extensive burn wounds and multiorgan failure syndrome. The fungal isolate from lateral back tissue was referred to the Fungus Testing Laboratory, University of Texas Health Science Center at San Antonio, where it was identified as Apophysomyces variabilis by phenotypic and genotypic testing.
Fig 1.
Histopathology (hematoxylin and eosin stained) of excised tissue from the left lower back showing numerous large-diameter aseptate hyphae located mostly in nonviable adipose tissue with evidence of microinvasion of blood vessels.
Fungal identification.
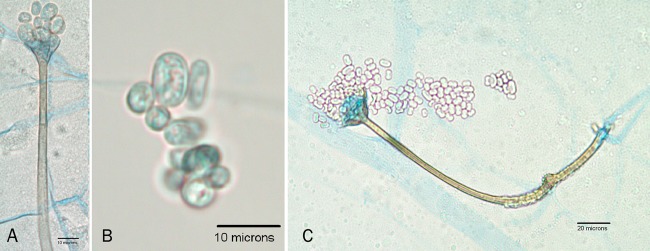
The isolate submitted on Mycobiotic agar (Remel, Lenexa, KS) was submitted to the Fungus Testing Laboratory collection as UTHSC 11-1354. Colonies were white and woolly, filling the agar slant tube, but failed to form any fruiting structures. Given the rapid growth and sterile nature of the isolate, the initial impression suggested either an Apophysomyces or a Saksenaea species. A carnation leaf agar (CLA) plate (16) and a water agar culture (18), both prepared in house, were inoculated and incubated at 25°C and 35°C, respectively, to induce sporangiospore formation. On CLA, the culture produced brown, unbranched sporangiophores, prominent apophyses, and pyriform sporangia similar to those observed in A. ossiformis and A. trapeziformis (2). However, a notable phenotypic difference in our isolate was the sporangiospore size and shape variability (Fig. 2A and B). A darkened area below the apophysis was also present—a feature described in other species within the genus (Fig. 2C). Temperature studies on potato flake agar slants (16) prepared in house and incubated for 7 days indicated 4+ growth at 37°C, 2+ growth at 40°C, and no growth at either 45 or 50°C. On the basis of the above features, the isolate was identified as Apophysomyces sp. and molecular characterization was initiated.
Fig 2.
Features of A. variabilis fruiting structures produced on CLA after 7 days of incubation at 25°C (bright-field microscopy, lactophenol cotton blue mount). Shown are a sporangiophore, the apophysis, and variable sporangiospores (A); sporangiospores of various sizes and shapes (B); and a fruiting structure showing a darkened area below the apophysis (C).
Molecular identification of this isolate was performed as described previously (19). Briefly, genomic DNA was prepared from a 24-h potato dextrose agar plate grown at 30°C using the Prepman Ultra reagent (Applied Biosystems, Foster City, CA). PCR assays were performed using the ITS1 and NL4 primers (19). The amplicon was purified with the Qiagen PCR purification kit (Qiagen, Valencia, CA) and then sequenced on both strands at the University of Texas Health Science Center at San Antonio Advanced Nucleic Acids Core Facility (19). The sequences were assembled using MacVector software (MacVector, Inc., Cary, NC), and the individual internal transcribed spacer (ITS) and D1/D2 regions were used to perform BLASTn searches of the NCBI database (http://www.ncbi.nlm.nih.gov/BLAST/). Individual search results were considered significant at identities of ≥97%. The three top hits in the ITS search were A. variabilis (accession number FN556442.1; identity, 708/708 [100%]), A. variabilis (accession number FN813492.1; identity, 701/701 [100%]), and A. variabilis (accession number FN813491.1; identity, 701/701 [100%]). The results of the D1/D2 search were similar, with the top three hits being A. variabilis (accession number FN554255.1; identity, 679/680 [99%]), A. variabilis (accession number FN554254.1; identity, 655/656 [99%]), and A. variabilis (accession number FN554253.1; identity, 651/652 [99%]). Thus, sequencing and phenotypic characterization confirmed the isolate as A. variabilis.
Antifungal susceptibility testing of the case isolate was performed, with MICs (μg/ml) as follows: amphotericin B, ≤0.03; posaconazole, 0.06; voriconazole, 8; itraconazole, 0.125; anidulafungin, >8; caspofungin, >8.
Discussion.
Apophysomyces was first isolated from soil samples in India in 1979 (14). Morphologically, this fungus typically produces pyriform sporangia; conspicuous funnel- and/or a bell-shaped apophyses; and clear, thin, and smooth-walled sporangiospores that are mostly oblong with rounded ends. It is a thermotolerant fungus that grows rapidly between 26 and 42°C (5, 14, 20). While most isolates have been reported from India, it also has been isolated in Australia, Southeast Asia, the United States, and South America, suggesting a broad distribution which covers tropical and subtropical climates. In the United States, Apophysomyces represents only 0.5% of the clinically significant Mucorales isolates (3).
Although Apophysomyces is typically an environmental mold, there have been an increasing number of human infections reported. Human infections by Apophysomyces involve a wide range of patients, the majority of which were immunocompetent (4). This is in contrast to infections caused by other members of the order Mucorales that tend to involve immunocompromised individuals and are most commonly seen in patients with poorly controlled diabetes. The most common mode of infection is traumatic implantation of contaminated soil or water leading to cutaneous or subcutaneous infections, rhino-orbital infection after facial trauma (9, 10, 12), or osteomyelitis after breakdown of the overlying skin (7, 13, 20). Renal infection by Apophysomyces has been reported, suggesting a possible hematogenous mode of infection following traumatic implantation through the skin (17). Disseminated infection with A. elegans has also been reported after kidney transplantation from a donor who died by drowning (15). Recently, a cluster of cutaneous mucormycosis cases has been reported in the aftermath of the Joplin, MO, tornado, in which 13 of the confirmed cases yielded A. trapeziformis (8). In our patient, the mode of infection is unclear but could have been traumatic implantation by his immersion in water after the burn.
Until recently, it was believed that Apophysomyces comprised a single species, A. elegans. However, sequence analysis of several genes, combined with physiological and morphological characteristics, has led to the recognition of four distinct Apophysomyces species (A. elegans, A. ossiformis, A. trapeziformis, and A. variabilis) (2). The incidence of human infection due to A. variabilis is unknown. Most reports of infections due to Apophysomyces have been attributed to A. elegans prior to the identification of the four distinct species of Apophysomyces. The organisms responsible for the first reported cases of human A. variabilis infection were identified as A. elegans morphologically and subsequently identified as A. variabilis by ITS sequencing (11). It is likely that some of the reported cases of A. elegans infection were due to A. variabilis, although the extent to which these infections are due to A. variabilis is uncertain. This also underscores the difficulty of identification by morphology and that definitive identification may require genetic analysis, although this approach may not be practical in routine laboratories. Interestingly, in the study where A. elegans was later identified as a complex of at least four species, the isolates that were identified as A. elegans were obtained from nonclinical samples (2), which lends support to the possibility that A. variabilis and possibly A. ossiformis and A. trapeziformis were the pathogenic species.
Most patients with Apophysomyces infections were treated with antifungal agents and aggressive debridement with variable results. The response was largely dependent on the patient's comorbid conditions and immune status. Amphotericin B is the recommended drug of choice for mucormycosis and has been used in most human infections with Apophysomyces species. Susceptibility studies have shown variable results. A study by Alvarez et al. (2) showed that the four species of Apophysomyces were susceptible to amphotericin B and posaconazole but not to voriconazole and caspofungin. A susceptibility study of Mucorales isolates by Dannaoui et al. (6) showed marginal activity of amphotericin B against Apophysomyces. Another in vitro susceptibility study of A. elegans also showed reasonable susceptibility of the fungus to posaconazole (1) and was used to successfully treat rhino-orbital Apophysomyces infection after the failure of treatment with amphotericin B (9). These reports suggest that amphotericin B may still be the drug of choice against Apophysomyces, as well as other genera of Mucorales as an adjunct to surgical debridement. Posaconazole may be used in patients who do not respond to amphotericin B or as an additional agent taking advantage of an alternative mechanism of antifungal activity. Retrospective susceptibility testing of the isolate showed sensitivity to amphotericin B and posaconazole similar to results obtained by Alvarez et al. (2).
A. variabilis was previously thought to be an environmental fungus but is emerging as a pathogen that can cause serious infections in humans and has some propensity to cause rare infections in immunocompetent patients. Infection is usually facilitated by traumatic implantation via trauma or burning. The true extent of A. variabilis infection is uncertain and requires genetic analysis of clinical isolates of Apophysomyces species. The mainstay of treatment continues to be amphotericin B, while posaconazole may be used as an alternative agent. To date, this is the first report to document infection by A. variabilis in a burn patient following the separation of this species within the A. elegans species complex.
Accession numbers.
The isolate described here has been deposited in the University of Alberta Microfungus Collection under accession number UAMH 11571. The sequences were deposited in GenBank under accession numbers JN980700 for the ITS sequence and JN980699 for the D1/D2 sequence.
ACKNOWLEDGMENTS
We acknowledge Dora McCarthy for antifungal susceptibility testing of the isolate.
The views expressed herein are ours and do not reflect the official policy or position of the Department of the Army, the Department of Defense, or the U.S. Government. W.P.D.C., T.P.C., M.E.G., C.E.W., S.H.K., and D.R.H. are employees of the U.S. Government.
Footnotes
Published ahead of print 23 May 2012
REFERENCES
- 1. Alastruey-Izquierdo A, et al. 2009. Activity of posaconazole and other antifungal agents against Mucorales strains identified by sequencing of internal transcribed spacers. Antimicrob. Agents Chemother. 53:1686–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez E, et al. 2010. Molecular phylogenetic diversity of the emerging mucoralean fungus Apophysomyces: proposal of three new species. Rev. Iberoam. Micol. 27:80–89 [DOI] [PubMed] [Google Scholar]
- 3. Alvarez E, et al. 2009. Spectrum of zygomycete species identified in clinically significant specimens in the United States. J. Clin. Microbiol. 47:1650–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chakrabarti A, et al. 2010. Apophysomyces elegans: epidemiology, amplified fragment length polymorphism typing, and in vitro antifungal susceptibility pattern. J. Clin. Microbiol. 48:4580–4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooter RD, Lim IS, Ellis DH, Leitch IO. 1990. Burn wound zygomycosis caused by Apophysomyces elegans. J. Clin. Microbiol. 28:2151–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dannaoui E, et al. 2003. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J. Antimicrob. Chemother. 51:45–52 [DOI] [PubMed] [Google Scholar]
- 7. Eaton ME, Padhye AA, Schwartz DA, Steinberg JP. 1994. Osteomyelitis of the sternum caused by Apophysomyces elegans. J. Clin. Microbiol. 32:2827–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fanfair RN, et al. 2011. Notes from the field: fatal fungal soft-tissue infections after a tornado—Joplin, Missouri, 2011. Morb. Mortal. Wkly. Rep. 60(29):992 [Google Scholar]
- 9. Ferguson TD, et al. 2007. Posaconazole treatment for Apophysomyces elegans rhino-orbital zygomycosis following trauma for a male with well-controlled diabetes. J. Clin. Microbiol. 45:1648–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia-Covarrubias L, Bartlett R, Barratt DM, Wassermann RJ. 2001. Rhino-orbitocerebral mucormycosis attributable to Apophysomyces elegans in an immunocompetent individual: case report and review of the literature. J. Trauma 50:353–357 [DOI] [PubMed] [Google Scholar]
- 11. Guarro J, et al. 2011. Apophysomyces variabilis infections in humans. Emerg. Infect. Dis. 17:134–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liang KP, Tleyjeh IM, Wilson WR, Roberts GD, Temesgen Z. 2006. Rhino-orbitocerebral mucormycosis caused by Apophysomyces elegans. J. Clin. Microbiol. 44:892–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meis JF, Kullberg BJ, Pruszczynski M, Veth RP. 1994. Severe osteomyelitis due to the zygomycete Apophysomyces elegans. J. Clin. Microbiol. 32:3078–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Misra PC, Srivastava KJ, Lata K. 1979. Apophysomyces, a new genus of the Mucorales. Mycotaxon 8:337–382 [Google Scholar]
- 15. Naguib MT, et al. 1995. Apophysomyces elegans infection in a renal-transplant recipient. Am. J. Kidney Dis. 26:381–384 [DOI] [PubMed] [Google Scholar]
- 16. Nelson PE, Toussoun TA, Marasas WFO. 1983. Fusarium species. An illustrated manual for identification. The Pennsylvania State University, University Park, PA [Google Scholar]
- 17. Okhuysen PC, Rex JH, Kapusta M, Fife C. 1994. Successful treatment of extensive posttraumatic soft-tissue and renal infections due to Apophysomyces elegans. Clin. Infect. Dis. 19:329–331 [DOI] [PubMed] [Google Scholar]
- 18. Padhye AA, Ajello L. 1988. Simple method of inducing sporulation by Apophysomyces elegans and Saksenaea vasiformis. J. Clin. Microbiol. 26:1861–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. 2010. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: a cautionary note. J. Clin. Microbiol. 48:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weinberg WG, Wade BH, Cierny G, Stacy D, Rinaldi MG. 1993. Invasive infection due to Apophysomyces elegans in immunocompetent hosts. Clin. Infect. Dis. 17:881–884 [DOI] [PubMed] [Google Scholar]