Abstract
A rapid emergence of azole resistance has been observed in Aspergillus fumigatus in The Netherlands over the past decade. The dominant resistance mechanism appears to be of environmental origin and involves the TR34/L98H mutations in cyp51A. This resistance mechanism is now also increasingly being found in other countries. Therefore, genetic markers were used to gain more insights into the origin and spread of this genotype. Studies of 142 European isolates revealed that those with the TR34/L98H resistance mechanism showed less genetic variation than azole-susceptible isolates or those with a different genetic basis of resistance and were assigned to only four CSP (putative cell surface protein) types. Sexual crossing experiments demonstrated that TR34/L98H isolates could outcross with azole-susceptible isolates of different genetic backgrounds, suggesting that TR34/L98H isolates can undergo the sexual cycle in nature. Overall, our findings suggest a common ancestor of the TR34/L98H mechanism and subsequent migration of isolates harboring TR34/L98H across Europe.
INTRODUCTION
Aspergillus fumigatus is a saprophytic fungus that is capable of causing a wide range of diseases in various hosts. Invasive aspergillosis is the most severe manifestation of Aspergillus infection in humans, and this disease is associated with substantial mortality and morbidity. Medical triazoles, such as itraconazole, voriconazole, and posaconazole, play an important role in the management of Aspergillus diseases. However, azole resistance is an emerging problem in A. fumigatus and has been shown to be associated with increased probability of treatment failure (10, 11, 19, 20, 30, 35, 37, 39, 41).
Azole resistance is commonly due to mutations in the cyp51A gene, which encodes 14-α-demethylase in the ergosterol biosynthesis pathway. In azole-resistant clinical A. fumigatus isolates, a wide variety of cyp51A mutations, such as substitutions at codons G54, G138, P216, F219, M220, and G448, have been found (5, 11, 29). This is in contrast with a different pattern of resistance observed in isolates from The Netherlands. Here, a resistance mechanism consisting of the L98H substitution together with a 34-bp tandem repeat (TR34) in the promoter region of this gene (TR34/L98H) was found to be present in over 90% of itraconazole-resistant isolates, which also showed reduced susceptibility to voriconazole and posaconazole (30, 36). TR34/L98H isolates were recovered primarily from azole-naïve patients and were also recovered from the environment (28, 36). These observations suggest that azole-resistant Aspergillus is acquired by patients from an environmental source rather than arising through azole therapy. Recently, we provided evidence that exposure of A. fumigatus to 14-α-demethylase inhibitor (DMI) fungicides might provide a selective pressure leading to the emergence of TR34/L98H resistant isolates in the environment (27). On the basis of in vitro cross-resistance, molecule alignment studies, and docking simulations, five triazole fungicides that were highly similar to antifungal triazoles used in medicine were identified (27). The TR34/L98H resistance mechanism has been shown to be endemic in The Netherlands (36) and is also increasingly being reported in other European countries (11, 22, 24, 30, 34). Furthermore, the TR34/L98H genotype was recently reported outside Europe, in China and India (7, 18).
At present, it is unknown if the high frequency of the TR34/L98H genotype in azole-resistant isolates is due to migration from a common ancestral lineage or repeated independent development in genetically unrelated strains. Preliminary genotyping studies in TR34/L98H isolates using microsatellites showed shorter genetic distances between TR34/L98H isolates than those between wild-type controls (30), and another study showed that TR34/L98H isolates nest within a single population and have not spread across A. fumigatus populations (16). To gain more insights into the origin and spread of the TR34/L98H resistance mechanism, we studied the genetic relatedness of European A. fumigatus isolates containing the TR34/L98H mutations by analyzing several genetic markers. We also assessed the possible involvement of the recently described sexual cycle of A. fumigatus (25) in generating novel genetic diversity among isolates bearing the TR34/L98H genotype.
MATERIALS AND METHODS
Selection of TR34/L98H and control isolates.
A. fumigatus isolates containing the TR34/L98H resistance mechanism and originating between 1998 (the year in which the first TR34/L98H isolates were recovered in The Netherlands) (30) and 2007 were selected from the fungal culture collection of the Radboud University Medical Centre. Dutch TR34/L98H isolates, including isolates of clinical and environmental origin, were randomly selected. As controls, for each TR34/L98H isolate, an isolate with a susceptible phenotype, which was matched by year of isolation to the TR34/L98H isolate, was randomly selected. The controls also included both clinical and environmental isolates. To compare the TR34/L98H resistance mechanism with other resistance mechanisms, the culture collection was also searched for isolates with an azole-resistant phenotype that was not due to the TR34/L98H mechanism. Finally, TR34/L98H A. fumigatus isolates that originated from other European countries were included (see Table S1 in the supplemental material).
In vitro susceptibility testing.
In vitro activity of itraconazole, voriconazole, and posaconazole was tested according to the EUCAST broth microdilution reference method (31). The MIC was determined by the lowest antifungal concentration with a complete inhibition of growth after 48 h. MICs were interpreted based on recently proposed breakpoints (38).
DNA extraction and cyp51A sequencing.
DNA was isolated, and the full coding sequence of the cyp51A gene, as well as the promoter region, was determined by amplification and subsequent sequencing as previously described (5). Sequences were compared to a wild-type A. fumigatus cyp51A gene sequence (GenBank, National Center for Biotechnology Information [http://www.ncbi.nlm.nih.gov/], accession number AF338659) to detect mutations.
CSP typing, microsatellite analysis, and mating type determination.
CSP (putative cell surface protein) types were determined by PCR amplification and subsequent sequencing by following established protocols (13, 14). CSP types were assigned according to the CSP typing nomenclature described by Klaassen et al. (15).
Six microsatellite loci (STRAf 3A, 3B, 3C, 4A, 4B, and 4C) were analyzed as described previously (8, 28). The obtained genotypes were imported into BioNumerics v6.0 (Applied Maths, Sint-Martens-Latem, Belgium). A minimum spanning tree (MST) was constructed based on categorical treatment of the data, i.e., alleles were scored as either identical or nonidentical and the difference in numbers of repetitions at each locus between different genotypes was not taken into account.
Mating types were determined by a multiplex PCR-based mating type test, as described previously (26). The null hypothesis of a 1:1 ratio of the two mating types was tested using the χ2 test, with a P value of <0.05 considered significant (25).
Sexual reproduction.
To investigate the segregation of genetic markers and the TR34/L98H resistance mechanism following sexual reproduction, the wild-type isolate AfIR974 (25) was crossed with the clinical isolate v23-66, which harbored the TR34/L98H resistance mechanism. Mating experiments were performed on oatmeal agar plates (Difco oatmeal agar), and inoculations were performed as described previously (25). Plates were sealed with Parafilm and incubated at 30°C in the dark. Crosses were examined weekly, and when cleistothecia developed, ascospore suspensions were obtained as described previously (25). Ascospore suspensions were plated onto Sabouraud agar plates, and germinating ascospores were transferred to Sabouraud agar slants. Fifteen progeny were selected for further analysis, including in vitro susceptibility testing and mating type determination. In addition, the progeny were screened for the presence of the TR34/L98H resistance mechanism using the following PCR-based assays. The presence of the TR was investigated by amplifying part of the promoter region of the cyp51A gene using appropriate primers (5′-TGAGTTAGGGTGTATGGTATGCTGGA-3′ and 5′-AGCAAGGGAGAAGGAAAGAAGCACT-3′). For the L98H substitution, two PCRs were performed: an L98-specific PCR (primers 5′-CCTCTTCCGCATTGACATCCTGGA-3′ and 5′-TGACGGCAATCTTGCTCAATGTTGTTTA-3′) and an L98H-specific PCR (primers 5′-ACGAGTTTATTCTCAACGGCAAGGA-3′ and 5′-TTCGGTGAATCGCGCAGATAGTCC-3′). The cycling program consisted of a 2-min denaturation step at 94°C, followed by 35 cycles of 30 s at 94°C, 45 s at 60°C, and 45 s at 72°C and a final elongation step of 5 min at 72°C. Products of the TR detection PCR were separated on a 2% agarose gel together with a size marker. Amplicons of 188 bp in size do not contain a TR, while the presence of a TR will lead to amplicons of 222 bp (188 bp plus an extra 34-bp repeat). Amplification products of the L98- and L98H-specific PCRs were mixed and run on a 2% agarose gel with a size marker. The L98-specific product is 341 bp in size, while the L98H-specific product is 223 bp in size.
After the first sexual cross with a TR34/L98H isolate succeeded, additional TR34/L98H isolates were subjected to sexual crossing. For that, AfIR957 (MAT1-1) (25) and AfIR928 (MAT1-2) (25, 32) were crossed with various clinical isolates of the opposite mating type that contained the TR34/L98H resistance mechanism. The progeny of each cross were assessed both for the presence of the TR34/L98H mutations (by the PCR-based assays as described above) and for their CSP type.
RESULTS
TR34/L98H and control isolates.
The distribution of isolates over time is shown in Table 1. In total, 55 azole-resistant TR34/L98H isolates and 55 azole-susceptible wild-type controls that had been cultured between 1998 and 2007 in The Netherlands were selected. Of these, 86 were clinical isolates which originated from patients admitted to the Radboud University Nijmegen Medical Centre (71 isolates) or other Dutch hospitals (15 isolates). The remaining 24 isolates were of environmental origin and were recovered from soil, seeds, compost, leaves, water filter samples, and air samples (see Table S1 in the supplemental material).
Table 1.
Distribution of azole-resistant and azole-susceptible wild-type Dutch A. fumigatus isolates examined in this study according to the year of isolation
| Yr of isolation | No. of isolates with each phenotype and resistance mechanism |
||
|---|---|---|---|
| Wild type | Resistant TR34/L98H | Resistant non-TR34/L98H | |
| 1998 | 2 | 2 | |
| 2000 | 2 | 2 | |
| 2001 | 2 | 2 | |
| 2002 | 7 | 7 | 1 |
| 2003 | 4 | 4 | |
| 2004 | 7 | 7 | 1 |
| 2005 | 6 | 6 | |
| 2006 | 7 | 7 | |
| 2007 | 18 | 18 | 5 |
| Total | 55 | 55 | 7 |
The fungal culture collection comprising over 2,000 isolates of A. fumigatus contained seven resistant isolates without the TR34/L98H resistance mechanism that were cultured between 1998 and 2007 in The Netherlands (Table 1). Three isolates were of clinical origin and harbored a point mutation in the cyp51A gene, leading to the M220K, M220I, or M220V substitution. These mutations are known to be correlated with azole resistance (6, 9, 21). The other four azole-resistant isolates were obtained from the environment and did not have any mutation in the cyp51A gene.
In vitro susceptibility testing.
Results of in vitro susceptibility testing of the Dutch isolates are shown in Table 2. According to the proposed breakpoints (38), all of the 55 TR34/L98H isolates were resistant to itraconazole and all except two showed resistance or intermediate susceptibility to voriconazole. Ten isolates were susceptible to posaconazole, while the other 45 showed intermediate susceptibility or resistance to this drug. Of the seven non-TR34/L98H resistant isolates exhibiting resistance to azoles, five were itraconazole resistant, and of these, four isolates also showed intermediate or resistant phenotypes for voriconazole and/or posaconazole. The remaining two isolates were resistant to voriconazole, with a MIC of 4 mg/liter, while being susceptible to both itraconazole and posaconazole.
Table 2.
Results of in vitro susceptibility testing of Dutch A. fumigatus isolates
| Resistance mechanism (no. of isolates) | MIC geometric mean (range) (mg/liter)a |
||
|---|---|---|---|
| ITZ | VOR | POS | |
| Wild type (55) | 0.19 (0.031–1) | 0.5 (0.25–2) | 0.06 (0.016–0.5) |
| TR34/L98H (55) | 26.82 (4–32) | 4.42 (1–32) | 0.49 (0.25–1) |
| Non-TR34/L98H (7) | 8.83 (0.5–32) | 2.20 (0.5–4) | 0.67 (0.25–32) |
For the purpose of the analysis, all values >16 mg/liter were indicated as 32 mg/liter. ITZ, itraconazole; VOR, voriconazole; POS, posaconazole.
CSP genotyping and mating type.
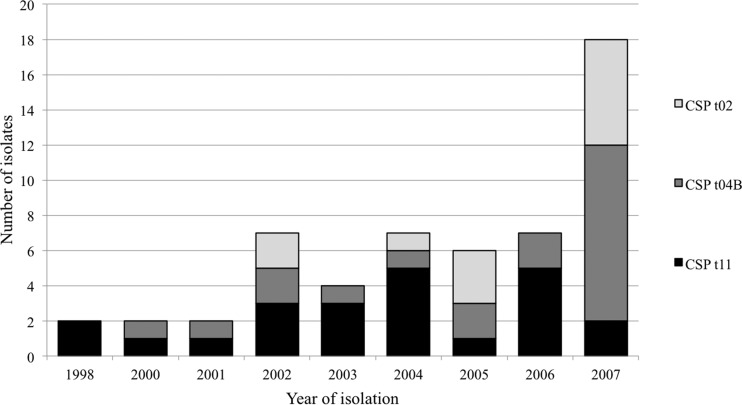
CSP typing showed that the azole-susceptible wild-type control isolates were spread across 11 CSP types (Table 3). The seven Dutch azole-resistant non-TR34/L98H isolates were distributed over CSP types t01, t02, t03, and t04A. In contrast, the 55 Dutch isolates with the TR34/L98H resistance mechanism grouped in only three CSP types: t02, t04B, and t11. Remarkably, CSP types t04B and t11 consisted exclusively of TR34/L98H isolates, while t02 types contained azole-susceptible and TR34/L98H and non-TR34/L98H resistant isolates. CSP types t02, t04B, and t11 all contained clinical as well as environmental TR34/L98H isolates. The distribution of CSP types over time within the group of TR34/L98H isolates is shown in Fig. 1. The first Dutch TR34/L98H isolates that were recovered from a patient in 1998 were of CSP type t11. TR34/L98H isolates of a second and third CSP type were then found in 2000 and 2002 (t04B and t02, respectively). From 2002 until 2007, no further new CSP types were observed among the Dutch TR34/L98H isolates.
Table 3.
Distribution of CSP types in the Dutch and European A. fumigatus isolates
| CSP type | No. (frequency [%]) of isolates from each group (n) |
|||
|---|---|---|---|---|
| Dutch wild type (55) | Dutch resistant non-TR34/L98H (7) | Dutch resistant TR34/L98H (55) | Other European TR34/L98H (25) | |
| t01 | 15 (27.3) | 4 (57.1) | ||
| t02 | 4 (7.3) | 1 (14.3) | 12 (21.8) | 12 (48) |
| t03 | 7 (12.7) | 1 (14.3) | ||
| t04A | 16 (29.1) | 1 (14.3) | ||
| t04B | 20 (36.4) | 8 (32) | ||
| t05 | 2 (3.6) | 1 (4) | ||
| t08 | 5 (9.1) | |||
| t09 | 1 (1.8) | |||
| t10 | 1 (1.8) | |||
| t11 | 23 (41.8) | 4(16) | ||
| t13 | 2 (3.6) | |||
| t14 | 1 (1.8) | |||
| t18 | 1 (1.8) | |||
Fig 1.
Distribution of CSP types of 55 TR34/L98H A. fumigatus isolates isolated between 1998 and 2007 in The Netherlands.
The multiplex PCR showed isolates of complementary MAT1-1 and MAT1-2 mating types to be present in all isolate groups; within the 55 wild-type isolates, a distribution ratio of 36% to 64% was found for MAT1-1/MAT1-2, while in the 55 TR34/L98H isolates, a ratio of 55% to 45% was detected, and in the final 7 non-TR34/L98H resistant isolates, a ratio of 57% to 43% for MAT1-1/MAT1-2 was found. There was no significant deviation from a 1:1 ratio in any of these groups according to a χ2 statistical analysis. With regard to CSP types, TR34/L98H isolates of both MAT1-1 and MAT1-2 mating types were present within all three CSP types (t02, t04B, and t11) (data not shown).
Other European TR34/L98H isolates.
The Radboud fungus culture collection contained 25 TR34/L98H isolates from seven other European countries (Austria, Belgium, Denmark, France, Italy, Norway, and United Kingdom) (see Table S1 in the supplemental material). Only the isolate from Norway was of environmental origin; the other 24 isolates were of clinical origin. CSP typing of these TR34/L98H isolates showed that they grouped to the same CSP types as the TR34/L98H isolates originating from The Netherlands (i.e., t02, t04B, and t11). There was only one exception: one of the five TR34/L98H isolates originating from Italy was of CSP type t05 (Table 3).
Microsatellite typing of Dutch and European isolates.
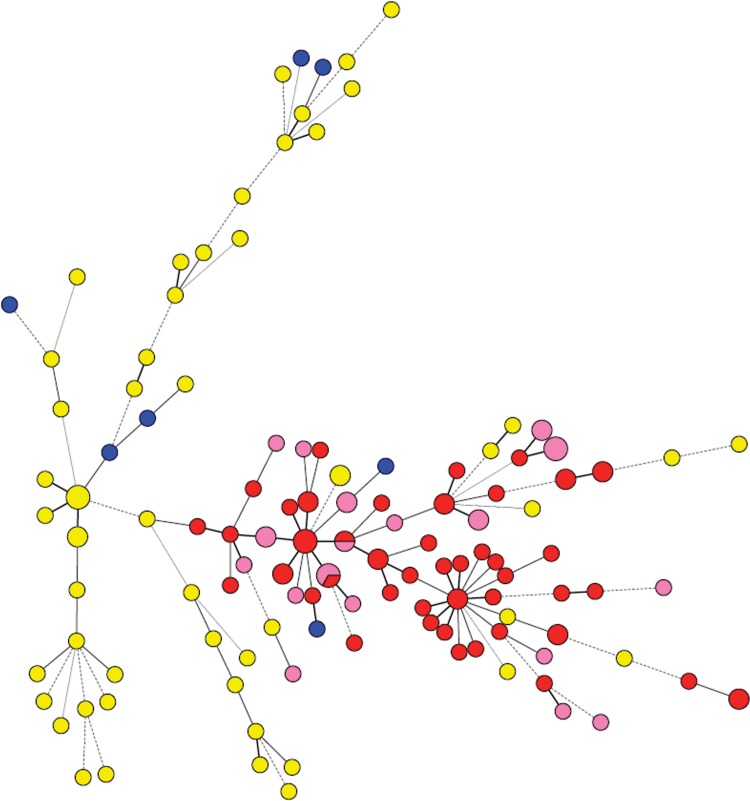
A minimum spanning tree (MST) was constructed based on the six STRAf microsatellite loci, with the majority of isolates having a unique genotype (Fig. 2). The Dutch TR34/L98H isolates formed a cluster that was almost entirely separate from the azole-susceptible wild-type isolates. In three cases, a clinical and an environmental TR34/L98H isolate had identical microsatellite genotypes.
Fig 2.
Minimum spanning tree showing the genotypic relationship between the azole-resistant and azole-susceptible A. fumigatus isolates. Each circle corresponds to a unique genotype, and the size of the circle corresponds to the number of isolates with that genotype (1, 2, or 3 isolates). Connecting lines correspond to the number of different microsatellite loci between the genotypes. Short bold line, 1 difference; black line, 2 differences; long gray line, 3 differences; dotted line, 4 or more differences. Red, azole-resistant TR34/L98H, The Netherlands (n = 55); yellow, azole-susceptible wild type, The Netherlands (n = 55); blue, azole-resistant non-TR34/L98H, The Netherlands (n = 7); pink, azole-resistant TR34/L98H, other European countries (n = 25).
The TR34/L98H isolates from other European countries also clustered with the Dutch TR34/L98H isolates. TR34/L98H isolates of two particular microsatellite genotypes were found in both The Netherlands and other European countries (see the dual-colored dots in Fig. 2). In addition, isolates from Belgium and Italy also shared the same genotype. The non-TR34/L98H resistant isolates did not cluster together and instead were more distributed throughout the tree.
Sexual crosses with TR34/L98H isolates.
In order to gain insight into the impact of sexual reproduction on the genetic markers, the wild-type isolate AfIR974 (MAT1-1, CSP type t02) was crossed with the clinical isolate v23-66, containing the TR34/L98H resistance mechanism (MAT1-2, CSP type t04B). After approximately 2 months of incubation, cleistothecia were formed and ascospores were isolated. As shown in Table 4, 15 progeny, of which eight (53%) harbored the TR34/L98H resistance mechanism and seven did not, were analyzed. The presence of TR34/L98H always corresponded with an azole-resistant phenotype. The presence of new combinations of both mating and CSP types in the progeny and the generation of new microsatellite genotypes provided evidence for recombination during the heterothallic sexual cycle (25). The combination of all genotypic markers (presence of TR34/L98H, six STRAf microsatellites, CSP and mating type) resulted in unique genotypes for 80% of the progeny. Surprisingly, in two of the progeny (AfIR974-v23-66-10 and AfIR974-v23-66-14), the number of repeats in the STRAf 3A marker increased from 80 to 82, a result which is suggestive of microvariation (1, 2).
Table 4.
Genotypes and in vitro azole susceptibility profiles of the parental isolates and 15 ascospore progeny from a cross between an azole-resistant TR34/L98H A. fumigatus isolate (v23-66) and an azole-susceptible wild-type isolate (AfIR974)
| Isolate | MIC (mg/liter)a |
TR34 presence | Codon 98 | Mating type | CSP type | No. of repeats of each STRAf microsatellite |
Genotypeb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITZ | VOR | POS | 3A | 3B | 3C | 4A | 4B | 4C | ||||||
| AfIR974 | 0.25 | 0.5 | 0.063 | − | L98 | MAT1-1 | t02 | 34 | 9 | 10 | 8 | 10 | 30 | A |
| v23-66 | >16 | 4 | 0.5 | + | L98H | MAT1-2 | t04B | 80 | 9 | 9 | 8 | 10 | 11 | B |
| AfIR974-v23-66-1 | >16 | 4 | 0.5 | + | L98H | MAT1-1 | t04B | 34 | 9 | 10 | 8 | 10 | 11 | C |
| AfIR974-v23-66-2 | 0.25 | 0.5 | 0.063 | − | L98 | MAT1-2 | t02 | 80 | 9 | 10 | 8 | 10 | 30 | D |
| AfIR974-v23-66-3 | >16 | 4 | 0.5 | + | L98H | MAT1-2 | t02 | 34 | 9 | 10 | 8 | 10 | 11 | E |
| AfIR974-v23-66-4 | >16 | 2 | 0.5 | + | L98H | MAT1-1 | t04B | 80 | 9 | 10 | 8 | 10 | 11 | F |
| AfIR974-v23-66-5 | >16 | 4 | 0.5 | + | L98H | MAT1-1 | t04B | 34 | 9 | 10 | 8 | 10 | 11 | C |
| AfIR974-v23-66-6 | >16 | 4 | 0.5 | + | L98H | MAT1-1 | t02 | 80 | 9 | 9 | 8 | 10 | 30 | G |
| AfIR974-v23-66-7 | 0.25 | 0.5 | 0.063 | − | L98 | MAT1-2 | t04B | 80 | 9 | 9 | 8 | 10 | 11 | H |
| AfIR974-v23-66-8 | 0.25 | 0.5 | 0.063 | − | L98 | MAT1-2 | t02 | 80 | 9 | 10 | 8 | 10 | 30 | D |
| AfIR974-v23-66-9 | 0.25 | 0.5 | 0.063 | − | L98 | MAT1-2 | t04B | 80 | 9 | 10 | 8 | 10 | 30 | I |
| AfIR974-v23-66-10 | >16 | 4 | 0.5 | + | L98H | MAT1-1 | t02 | 82 | 9 | 9 | 8 | 10 | 30 | J |
| AfIR974-v23-66-11 | 0.25 | 0.5 | 0.063 | − | L98 | MAT1-2 | t02 | 34 | 9 | 9 | 8 | 10 | 30 | K |
| AfIR974-v23-66-12 | >16 | 4 | 0.5 | + | L98H | MAT1-2 | t04B | 80 | 9 | 10 | 8 | 10 | 30 | L |
| AfIR974-v23-66-13 | >16 | 4 | 0.5 | + | L98H | MAT1-2 | t02 | 80 | 9 | 9 | 8 | 10 | 30 | M |
| AfIR974-v23-66-14 | 0.25 | 0.5 | 0.063 | − | L98 | MAT1-1 | t02 | 82 | 9 | 10 | 8 | 10 | 30 | N |
| AfIR974-v23-66-15 | 0.25 | 0.5 | 0.063 | − | L98 | MAT1-1 | t02 | 34 | 9 | 10 | 8 | 10 | 30 | A |
ITZ, itraconazole; VOR, voriconazole; POS, posaconazole.
The different genotypes of the progeny isolates, defined by unique combinations of the presence/absence of TR34/L98H, mating type, CSP type, and six microsatellite markers, are identified by different letters of the alphabet.
In addition, six TR34/L98H isolates were subjected to sexual crossing to determine whether they could outcross with isolates from a different CSP type that was not observed among the Dutch TR34/L98H resistant isolates. Clinical TR34/L98H isolates of CSP types t02, t04B, and t11 and of each mating type were crossed to the environmental isolates AfIR957 (MAT1-1, CSP type t05) and AfIR928 (MAT1-2, CSP type t03). Four out of six crosses were fertile (see Table S2 in the supplemental material). Analysis of the progeny from each successful cross revealed the presence of isolates that were of the TR34/L98H genotype and of CSP types t03 and t05 (see Table S2 in the supplemental material). This demonstrated that the presence of the TR34/L98H mechanism is not necessarily restricted to CSP types t02, t04B, and t11.
DISCUSSION
There are an increasing number of studies reporting the TR34/L98H mutations as a key underlying resistance mechanism in azole-resistant A. fumigatus isolates. The TR34/L98H mechanism was found to be widespread in The Netherlands and is also found in other European and Asian countries (4, 7, 11, 12, 18, 22, 24, 30, 34). TR34/L98H isolates have been recovered from the environment in The Netherlands, Denmark, and Norway (24, 28, 30), and there is increasing evidence that this resistance mechanism has developed through a fungicide-driven route of resistance development (27). The present study was undertaken to gain insights into the origin and spread of this resistance genotype.
The first key finding was that isolates with the TR34/L98H azole resistance mechanism originating from The Netherlands and seven other European countries are genetically less diverse than azole-susceptible wild-type isolates and isolates bearing other forms of azole resistance (for the latter two groups, only isolates originating from The Netherlands were tested). The two genotyping methods used, CSP and microsatellites, gave different levels of discrimination. CSP typing has a lower discriminatory power than microsatellite analysis and has been suggested to be more suitable for typing at the subpopulation level (15). This typing method revealed the significant result that Dutch isolates with the TR34/L98H genotype were found only in three CSP types, namely, t02, t04B, and t11. This contrasted with the distribution of CSP types in the azole-susceptible wild-type control group from The Netherlands, where isolates were found in 11 different CSP groups, similar to previously published results (14, 15). Importantly, two CSP types (t04B and t11) were observed to comprise exclusively TR34/L98H isolates, with no non-TR34/L98H resistant or azole-susceptible isolates found within these groups. This is consistent with a previous report in which isolates from Australia (where TR34/L98H-mediated resistance has not been described to date) were analyzed which also failed to detect isolates of these two CSP types (14). However, the absence of azole-susceptible isolates in these CSP types may simply be due to a very low frequency of occurrence. Meanwhile, 24 out of 25 TR34/L98H isolates (96%) of European origin other than The Netherlands grouped to the same three CSP types, indicating a close genetic relationship despite the geographic distances involved. Results of the microsatellite analysis were consistent with the CSP typing, demonstrating a clustering of all TR34/L98H isolates, distinct from most wild-type, azole-susceptible isolates. This was also shown in collections of Dutch isolates (28, 30). Another study concluded that resistant clinical isolates were more distributed among susceptible isolates, although most TR34/L98H isolates clustered within the same clade of the phylogenetic tree (23). From the data presented here, a close genetic relationship between all European TR34/L98H isolates can be inferred. However, it should be cautioned that as no azole-susceptible isolates from European countries other than The Netherlands were included in the analysis, we can conclude only that the TR34/L98H grouping is distinct specifically from the Dutch azole-susceptible isolates.
Considering the CSP typing and microsatellite results as a whole, it is possible to speculate about the evolutionary origin and spread of the TR34/L98H azole resistance genotype within Europe. The relatively close genetic relationships and limited CSP type diversity of the TR34/L98H isolates, compared to those of the azole-susceptible wild-type isolates, indicates that the independent and repeated emergence of the TR34/L98H mechanism seems unlikely. An alternative explanation is that the TR34/L98H isolates developed from a common ancestor or restricted set of genetically related isolates. A common origin of the TR34/L98H mechanism was previously suggested based on the microsatellite typing of 144 Dutch TR34/L98H isolates, from which it was calculated that the first TR34/L98H isolate would have emerged in the Netherlands in 1997 (27). The appearance and spread of the TR34/L98H mutation from an apparent common source is in marked contrast to the evolution of the non-TR34/L98H resistant isolates detected in the present study. These were distributed throughout the total set of isolates (Fig. 2), consistent with the hypothesis that mutations, such as at codon M220 in cyp51A, can be induced in isolates through prolonged patient exposure to medical triazoles (40). One TR34/L98H isolate from Italy was of CSP type t05, but it still grouped with the other TR34/L98H isolates in the microsatellite analysis. Further work is required to determine if CSP type t05 azole-resistant isolates are widespread in Italy and/or have spread to other countries.
A second key finding of the present study was that sexual reproduction, recently discovered in A. fumigatus (25), can occur between isolates of different CSP types and TR34/L98H genotypes to give rise to isolates with novel combinations of the TR34/L98H resistance mechanism in other CSP types. The finding that TR34/L98H isolates are sexually fertile provides a possible explanation for their genetic diversity. It is hypothesized that very few sexual cycles in the field would give rise to progeny exhibiting the degree of CSP variation observed in this study. Indeed, this is supported by the presence of both MAT1-1 and MAT1-2 isolates in the t02, t04B, and t11 CSP types. The accessory role of sexual reproduction was also suggested by a recent report which found that the TR34/L98H genotype was nested within a single, predominantly asexual population (16). Figure 1 suggests that sexual reproduction played a role in the early stages of development of azole resistance, as all three CSP types were already present in TR34/L98H isolates in The Netherlands by the year 2002. Moreover, the Norwegian isolate from 2000, not included in the analysis of Dutch isolates, grouped to CSP type t02, indicating that all three CSP types were already present in the initial years of TR34/L98H emergence. After sexual reproduction, subsequent asexual reproduction would most probably lead to persistence of these isolates and CSP types, given that after 100 (in vitro) asexual generations, no variations in CSP type were found (C. H. W. Klaassen, unpublished data). In the meantime, the number of repeats in microsatellite markers might undergo subtle changes during asexual reproduction (C. H. W. Klaassen, unpublished data), leading to the observed diversity in microsatellite markers.
Overall, our findings suggest a common origin of the TR34/L98H mechanism and the subsequent migration of TR34/L98H isolates across Europe. A similar spread of fungicide resistance has been shown before in plant pathogens: in the wheat pathogen Mycosphaerella graminicola, mutations causing resistance arose locally and were then spread across Europe through wind-dispersed ascospores (3, 33), and in the grape pathogen Botrytis cinerea, resistant isolates probably migrated from French to German wine-growing regions (17). More research into the genesis of the TR34/L98H mechanism is needed in order to understand the conditions that facilitate azole resistance development in the environment so that measures can be implemented to prevent the emergence of new resistance mechanisms.
Supplementary Material
ACKNOWLEDGMENTS
We thank all international and national collaborators for sending A. fumigatus isolates.
C. M. O'Gorman and P. S. Dyer are supported by the Wellcome Trust (United Kingdom). P. E. Verweij received research grants from Basilea, Bio-Rad, Gilead, Merck, Pfizer, and Schering-Plough. J. F. Meis has been a consultant to Astellas, Basilea, Merck, and Schering-Plough and received speaker's fees from Gilead, Janssen Pharmaceutica, Merck, Pfizer, and Schering-Plough.
Footnotes
Published ahead of print 6 June 2012
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Araujo R, Amorim A, Gusmao L. 2010. Genetic diversity of Aspergillus fumigatus in indoor hospital environments. Med. Mycol. 48:832–838 [DOI] [PubMed] [Google Scholar]
- 2. Balajee SA, de Valk HA, Lasker BA, Meis JF, Klaassen CH. 2008. Utility of a microsatellite assay for identifying clonally related outbreak isolates of Aspergillus fumigatus. J. Microbiol. Methods 73:252–256 [DOI] [PubMed] [Google Scholar]
- 3. Brunner PC, Stefanato FL, McDonald BA. 2008. Evolution of the CYP51 gene in Mycosphaerella graminicola: evidence for intragenic recombination and selective replacement. Mol. Plant Pathol. 9:305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burgel PR, et al. 2012. High prevalence of azole-resistant Aspergillus fumigatus in adults with cystic fibrosis exposed to itraconazole. Antimicrob. Agents Chemother. 56:869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Camps SM, et al. 2012. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature. Antimicrob. Agents Chemother. 56:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J, Li H, Li R, Bu D, Wan Z. 2005. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J. Antimicrob. Chemother. 55:31–37 [DOI] [PubMed] [Google Scholar]
- 7. Chowdhary AS, et al. 2012. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India. J. Antimicrob. Chemother. 67:362–366 [DOI] [PubMed] [Google Scholar]
- 8. de Valk HA, et al. 2005. Use of a novel panel of nine short tandem repeats for exact and high-resolution fingerprinting of Aspergillus fumigatus isolates. J. Clin. Microbiol. 43:4112–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia-Effron G, et al. 2005. Differences in interactions between azole drugs related to modifications in the 14-α sterol demethylase gene (cyp51A) of Aspergillus fumigatus. Antimicrob. Agents Chemother. 49:2119–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hodiamont CJ, et al. 2009. Multiple-azole-resistant Aspergillus fumigatus osteomyelitis in a patient with chronic granulomatous disease successfully treated with long-term oral posaconazole and surgery. Med. Mycol. 47:217–220 [DOI] [PubMed] [Google Scholar]
- 11. Howard SJ, et al. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15:1068–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howard SJ, Pasqualotto AC, Denning DW. 2010. Azole resistance in allergic bronchopulmonary aspergillosis and Aspergillus bronchitis. Clin. Microbiol. Infect. 16:683–688 [DOI] [PubMed] [Google Scholar]
- 13. Hurst SF, et al. 2009. Interlaboratory reproducibility of a single-locus sequence-based method for strain typing of Aspergillus fumigatus. J. Clin. Microbiol. 47:1562–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kidd SE, Zulkepeli NA, Slavin MA, Morrissey CO. 2009. Utility of a proposed CSP typing nomenclature for Australian Aspergillus fumigatus isolates: identification of additional CSP types and suggested modifications. J. Microbiol. Methods 78:223–226 [DOI] [PubMed] [Google Scholar]
- 15. Klaassen CH, de Valk HA, Balajee SA, Meis JF. 2009. Utility of CSP typing to sub-type clinical Aspergillus fumigatus isolates and proposal for a new CSP type nomenclature. J. Microbiol. Methods 77:292–296 [DOI] [PubMed] [Google Scholar]
- 16. Klaassen CH, Gibbons JG, Fedorova ND, Meis JF, Rokas A. 2012. Evidence for genetic differentiation and variable recombination rates among Dutch populations of the opportunistic human pathogen Aspergillus fumigatus. Mol. Ecol. 21:57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kretschmer M, et al. 2009. Fungicide-driven evolution and molecular basis of multidrug resistance in field populations of the grey mould fungus Botrytis cinerea. PLoS Pathog. 5:e1000696 doi:10.1371/journal.ppat.1000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lockhart SR, et al. 2011. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob. Agents Chemother. 55:4465–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mavridou E, Bruggemann RJ, Melchers WJ, Mouton JW, Verweij PE. 2010. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob. Agents Chemother. 54:860–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mavridou E, Bruggemann RJ, Melchers WJ, Verweij PE, Mouton JW. 2010. Impact of cyp51A mutations on the pharmacokinetic and pharmacodynamic properties of voriconazole in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 54:4758–4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mellado E, Garcia-Effron G, Alcazar-Fuoli L, Cuenca-Estrella M, Rodriguez-Tudela JL. 2004. Substitutions at methionine 220 in the 14α-sterol demethylase (Cyp51A) of Aspergillus fumigatus are responsible for resistance in vitro to azole antifungal drugs. Antimicrob. Agents Chemother. 48:2747–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mellado E, et al. 2007. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob. Agents Chemother. 51:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mortensen KL, et al. 2011. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory-based study with focus on Aspergillus fumigatus azole resistance. J. Clin. Microbiol. 49:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mortensen KL, et al. 2010. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob. Agents Chemother. 54:4545–4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Gorman CM, Fuller HT, Dyer PS. 2009. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457:471–474 [DOI] [PubMed] [Google Scholar]
- 26. Paoletti MC, et al. 2005. Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Curr. Biol. 15:1242–1248 [DOI] [PubMed] [Google Scholar]
- 27. Snelders E, et al. 2012. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One 7:e31801 doi:10.1371/journal.pone.0031801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Snelders E, et al. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microbiol. 75:4053–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snelders E, Melchers WJ, Verweij PE. 2011. Azole resistance in Aspergillus fumigatus: a new challenge in the management of invasive aspergillosis? Future Microbiol. 6:335–347 [DOI] [PubMed] [Google Scholar]
- 30. Snelders E, et al. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5:e219 doi:10.1371/journal.pmed.0050219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST) 2008. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14:982–984 [DOI] [PubMed] [Google Scholar]
- 32. Sugui JA, et al. 2011. Identification and characterization of an Aspergillus fumigatus “supermater” pair. mBio. 2(6):e00234–11 doi:10.1128/mBio.00234-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Torriani SF, Brunner PC, McDonald BA, Sierotzki H. 2009. QoI resistance emerged independently at least 4 times in European populations of Mycosphaerella graminicola. Pest Manag. Sci. 65:155–162 [DOI] [PubMed] [Google Scholar]
- 34. van der Linden JW, Arendrup MC, Verweij PE, SCARE-Network 2011. Prospective international surveillance of azole resistance in Aspergillus fumigatus (SCARE Network), abstr M-490. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Chicago, IL [Google Scholar]
- 35. van der Linden JW, et al. 2009. Azole-resistant central nervous system aspergillosis. Clin. Infect. Dis. 48:1111–1113 [DOI] [PubMed] [Google Scholar]
- 36. van der Linden JW, et al. 2011. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007-2009. Emerg. Infect. Dis. 17:1846–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Leer-Buter C, Takes RP, Hebeda KM, Melchers WJ, Verweij PE. 2007. Aspergillosis—and a misleading sensitivity result. Lancet 370:102. [DOI] [PubMed] [Google Scholar]
- 38. Verweij PE, Howard SJ, Melchers WJ, Denning DW. 2009. Azole-resistance in Aspergillus: proposed nomenclature and breakpoints. Drug Resist. Updat. 12:141–147 [DOI] [PubMed] [Google Scholar]
- 39. Verweij PE, Mellado E, Melchers WJ. 2007. Multiple-triazole-resistant aspergillosis. N. Engl. J. Med. 356:1481–1483 [DOI] [PubMed] [Google Scholar]
- 40. Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect. Dis. 9:789–795 [DOI] [PubMed] [Google Scholar]
- 41. Warris A, Weemaes CM, Verweij PE. 2002. Multidrug resistance in Aspergillus fumigatus. N. Engl. J. Med. 347:2173–2174 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.