Abstract
All currently available vaccines against Streptococcus pneumoniae are based on selections of the over 90 different serotypes, which underlines the importance of serotyping for surveillance and vaccine efficacy monitoring. In this study, we modified and validated a PCR-based scheme for deducing the serotypes of the invasive pneumococci isolated in Finland. For validation, 170 isolates were serotyped using the new protocol with six sequential multiplex PCRs for the deduction of serotypes, supplemented with Quellung testing when needed. The results were compared with those obtained by traditional serotyping methods. We found that 98.8% (168/170) of the isolates were correctly serotyped by the new protocol. Subsequently, the scheme was taken into regular use for serotyping the invasive pneumococci isolated in Finland for serotype-specific surveillance purposes and has been applied in the serotyping of more than 1,500 invasive isolates so far. The sequential multiplex PCRs (mPCRs) have given a result for over 99% of the isolates and allowed us to both handle samples in bulk and noticeably reduce the cost of reagents. While serotyping primarily by PCR is precise and effective, Quellung testing remains the most reliable way to discover possible discrepancies between the DNA deduced and the phenotypic serotype of an isolate. Since implementing the protocol for regular use, two serotype 19F PCR-positive isolates were found to be serotype 19A by the Quellung reaction. While a rare occurrence, this is an important observation, which prompted a revision of our serotyping protocol to prevent possible underreporting of serotype 19A, a potential replacement serotype following large-scale vaccination.
INTRODUCTION
Streptococcus pneumoniae is a significant human pathogen expressing more than 90 different serotypes (4, 8, 14, 30). The polysaccharide capsule that determines the serotype is a major virulence factor contributing to the evasion of the host immune system (4). Currently, all clinically available vaccines are capsular polysaccharide based, which highlights the importance of serotyping for epidemiological surveillance and for monitoring vaccine efficacy. In Finland, notification of cases of invasive pneumococcal disease is mandatory, and all invasive pneumococci isolated from cerebrospinal fluid (CSF) or blood are sent to the National Institute for Health and Welfare (THL) for species confirmation and serotyping. Serotyping has been performed for several decades using both the traditional capsular swelling test, based on the Quellung reaction (2), and the more recent counterimmunoelectrophoresis (CIEP) method (22). While new personnel had to be trained due to retirements and reorganization, we decided to adapt and assess a recent PCR-based technique against the traditional serotyping protocol and analyze the cost-effectiveness of these methods. In practice, we modified the PCR-based serotyping scheme recommended by the Centers for Disease Control and Prevention (CDC), Atlanta, GA, to make it suitable for laboratory-based surveillance of invasive pneumococcal disease in Finland.
MATERIALS AND METHODS
Setting up the novel serotyping scheme.
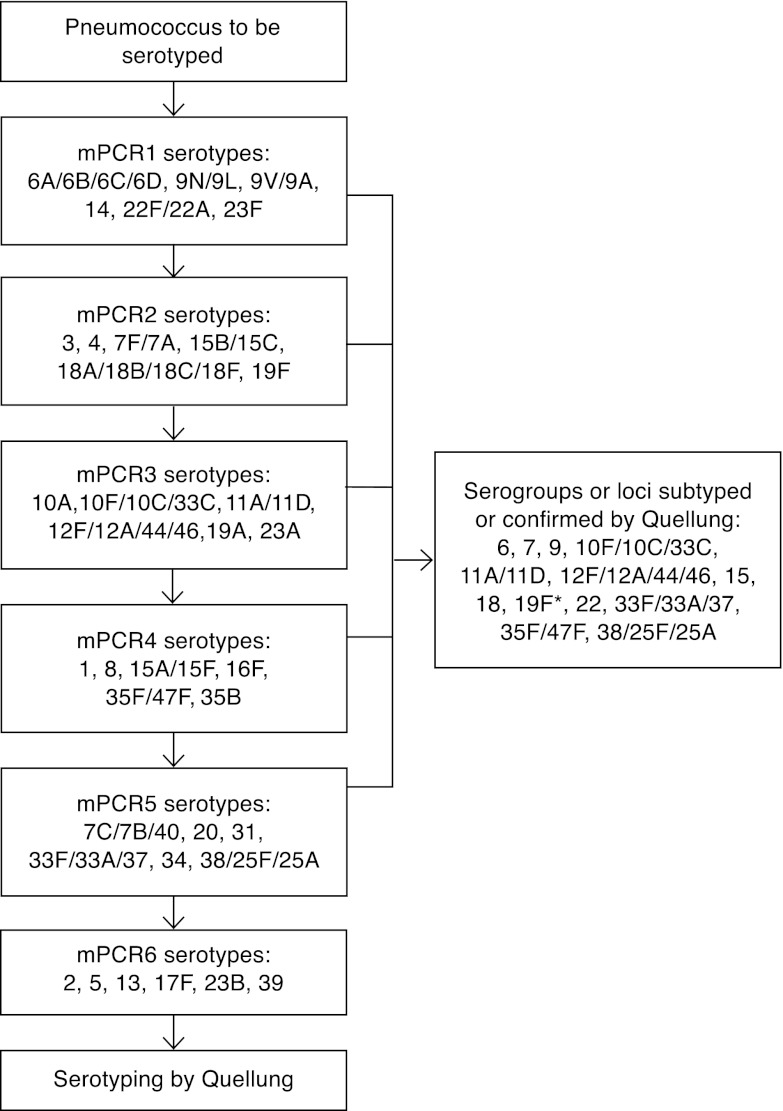
The serotype distribution of all 5,337 invasive isolates collected in the years 2002 to 2008 in Finland was analyzed when setting up the scheme for the PCR-based deduction of serotypes. Based on the serotype distribution, six sequential multiplex PCRs (mPCRs) were assembled, each detecting the capsular regulation gene wzg (cpsA) as an internal positive control along with six serotype or serogroup loci (Fig. 1). Relying on the surveillance data of the years 2002 to 2008, the most commonly occurring serotypes were included in the first two mPCRs. A separate mPCR (6C/6D mPCR) for detecting the presence of wzg (cpsA), the serogroup 6-specific wciP gene, and the 6C/6D wciNβ capsule gene was set up as described elsewhere (9).
Fig 1.
Flowchart of the mPCR-based protocol for deducing pneumococcal serotypes. *, 19F-positive mPCR results have been confirmed by Quellung testing since October 2011.
Bacterial isolates.
The designed protocol was validated by testing three sets of pneumococcal isolates (n = 170). Set 1 consisted of consecutive clinical invasive pneumococcal isolates (n = 70) collected in the year 2009 in Finland. Set 2 consisted of external quality assurance (EQA) isolates (n = 70) received previously from Statens Serum Institute (SSI), Denmark (19). Set 3 consisted of clinical invasive serogroup 6 isolates (n = 30) collected in the year 2009.
The isolates were blinded with respect to identification for serotyping. Aside from set 3, where the serogroup was known, the persons performing the serotyping had no prior knowledge of the serotype or serogroup distribution of the validation sets.
Positive controls were selected from among the invasive isolates from Finland that had been previously serotyped by CIEP, latex agglutination, and/or Quellung testing at a THL laboratory in Oulu, Finland. One positive-control isolate for each amplified locus in each mPCR was used for the PCR-based serotyping scheme.
Following validation, the novel serotyping protocol was taken into regular use and has been applied in the serotyping of all invasive pneumococci isolated in Finland since January 2010 (1,304 isolates over the initial 18 months).
Species verification.
Species verification was performed using optochin sensitivity testing and, when needed, bile solubility testing at the THL laboratories in Helsinki and Oulu and at SSI.
Serotyping by multiplex PCR supplemented with Quellung testing in Helsinki.
The new serotyping scheme (Fig. 1) was designed and tested at a THL laboratory in Helsinki. The genomic DNA was isolated by a lysis method as described previously (32). The amplification was performed in a 25-μl reaction volume with 35 thermal cycles and an annealing temperature of 54°C. All oligonucleotide primers used were previously published by the CDC (9, 10, 29, 31) or were available online (http://www.cdc.gov/ncidod/biotech/strep/pcr.htm). The serotypes of the isolates were deduced according to the serotyping flowchart (Fig. 1), with the exception of validation set 3, which focused on serogroup 6 and was tested first by 6C/6D mPCR and then subjected to Quellung subtyping. The PCR products were visualized by gel electrophoresis using 2% NuSieve 3:1 agarose gels (Lonza, Rockland, ME).
Because of primer cross-reactivity, the Quellung capsular reaction test was used to distinguish codetected serotypes, for subtyping when needed or for serotyping if no serotype- or group-specific product was obtained in any of the sequential mPCRs (Fig. 1). The antisera used were obtained from SSI Diagnostica, Denmark, and used according to the manufacturer's recommendation.
Serotyping by traditional methods in Oulu.
At a THL laboratory in Oulu, the three validation sets were serotyped with traditional methods using pneumococcal antisera (SSI Diagnostica, Denmark) and CIEP or, for neutral serogroup 7 and serotype 14, by latex agglutination; in addition, Quellung testing was used for confirmation where needed (16, 22).
Comparison of validation results and external serotyping.
The serotype results obtained by the different protocols in Helsinki and Oulu were compared. Confirmation of discrepant serotype results was performed at SSI, Denmark, by Quellung testing according to the instructions from antiserum manufacturer SSI Diagnostica (21) and/or at the University of Alabama at Birmingham (UAB), Birmingham, AL, by a strategy based on monoclonal antibodies (36).
Genotyping.
Because of discrepancies between the serotyping results determined on the basis of PCR and Quellung testing after the new protocol had been taken into continuous use, two isolates, IH163643-0 and IH166473-6, identified as 19F by mPCR but 19A by Quellung, were genotyped by multilocus sequence typing (MLST) in Helsinki. MLST was performed as described previously (32, 33); however, the gdh locus of isolate IH163643-0 was amplified using the alternative CS2 oligonucleotide primer pair (5), and Geneious Pro 5.5.6 software (Biomatters Ltd., Auckland, New Zealand) was used for sequence editing. The allelic profiles and isolate information were submitted to the MLST database located at Imperial College London (http://spneumoniae.mlst.net/). The wzy genes of these two isolates were sequenced using the following oligonucleotide primers: 19I cttgttttcaaayaaatgcaacttt, 19II agatttgaagaaatracataagaraaa, 19III gacgtatctgcttatatttttggatata, 19IV cctctatccgtgttcttatccgtgt, and 19V aagagtagtaaaacgctggaatacca. The sequences were compared with the sequences deposited in GenBank through the Basic Local Alignment Search Tool BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Cost analysis of the serotyping methods.
Based on material usage per isolate at the time of the validation of the protocol, the cost of reagents and materials was estimated for serotyping by Quellung testing and for the mPCR-based serotyping scheme, including Quellung testing when needed. The mean material cost of serotyping validation set 1 was considered for cost comparisons.
Nucleotide sequence accession numbers.
The nucleotide sequences for the wzy gene of isolate IH163643-0 and the wzy gene of isolate IH166473-6 were deposited in GenBank under accession numbers JX112900 and JX112901, respectively.
RESULTS
In all, the three sets consisted of 42 different serotypes (1, 2, 3, 4, 5, 6A, 6B, 6C, 6D, 7C, 7F, 8, 9N, 9V, 10A, 10, 11A, 12F, 14, 15A, 15B, 15C, 16F, 17F, 18C, 19A, 19F, 20, 21, 22F, 23F, 24F, 33, 34, 35F, 36, 38, 39, 40, 41, 42, and 48), three nonencapsulated isolates, and one nonpneumococcal isolate. In the first validation set, the results from the THL laboratories in Helsinki and Oulu were compatible for 97% (68/70) of the isolates, in the second set for 96% (67/70) of the isolates, and in the third set for 90% (27/30) of the isolates. Overall, the initial results differed for eight isolates (Table 1), and the combined compatibility of the results from the two THL laboratories was 95.3% for the total of 170 isolates. For certain serotypes, the protocols used differed in resolution power; accordingly, results such as serotype 10F/C versus serogroup 10 were not considered contradictory and required no further investigation.
Table 1.
Serotyping results for discrepant isolates included in the validation schemea
| Isolate | Validation set | Serotype(s) identified |
|||
|---|---|---|---|---|---|
| THL Helsinki | THL Oulu | SSI | UAB | ||
| IH 163205-2 | 1 | 6C | 6B | New type | 6D |
| IH 163216-4 | 1 | Not PNC | 19C | Not PNC | Nontypeable |
| SSI 22 | 2 | 14 | 21 | 21 | ND |
| SSI 44 | 2 | 19F | 19F and 19A | 19F | ND |
| SSI 69 | 2 | 42 | 35C | 42 | ND |
| IH 162439-1 | 3 | 6C | 6A | 6C | 6C |
| IH 162530-1 | 3 | 6C | 6A | 6C | 6C |
| IH 162476-5 | 3 | 6C | 6A | 6C | 6C |
PNC, pneumococcus; ND, not done.
Following the external verification of discrepant serotyping results performed at one or both of the reference laboratories at SSI and UAB (Table 1), the results obtained in Helsinki by the protocol under validation were compatible with the verified results for 98.6% (69/70) of the first validation set, 98.6% (69/70) of the second set, and 100% (30/30) of the third set. The combined compatibility of the results obtained in Helsinki by mPCR supplemented with Quellung testing and those obtained by the reference laboratory was 98.8% for the 170 isolates. For isolate IH 163205-2, the serotyping performed at UAB was considered decisive, as the UAB colleagues were the first to describe and name serotype 6D and detail its identification with current serotyping antisera (6, 7). The results for this isolate have been published separately (26). For isolate IH 163216-4, the result from SSI was considered decisive, as species verification tests had been performed there.
The first two mPCRs together were anticipated to give a result for over 80% of the invasive isolates when the new serotyping scheme was set up. This expectation has proved accurate, as 86% of the invasive pneumococcal isolates collected in the year 2010 and in the first 6 months of 2011 produced either a serotype- or serogroup-specific amplicon in one of the first two mPCRs. The six mPCRs combined were expected to produce a serotype- or serogroup-specific PCR product for 99.4% of the Finnish invasive isolates, and this number has been confirmed during the regular use of the protocol.
The two serotype 19A isolates that, following the continuous use of the novel protocol, had given discrepant results by mPCR and Quellung testing possessed novel genotypes according to MLST results. The allelic profile of isolate IH163643-0 was 1-5-41--5-9-1-18, sequence type (ST) 8025, and of isolate IH166473-6 was 13-8-4-5-9-1-18, ST8026. The wzy gene sequences (accession numbers JX112900 and JX112901) were identical in the two isolates and showed 93% to 92% identity with the serotype 19F wzy genes and 80% identity with the serotype 19A wzy genes deposited in GenBank.
The approximate mean material cost of serotyping by Quellung testing was €17 ($21.25) per isolate (range, €14 to €20 [$17.5 to $25.00]) and by mPCR-based serotyping was €6 ($7.50) per isolate (range, €3 to €13 [$3.75 to $16.25]) for validation set 1.
DISCUSSION
Although an indirect method, mPCR was proven precise and effective for serotyping the majority of invasive pneumococci for national surveillance. Since the validation of our protocol, the multiplex PCR serotyping scheme has been in use in Finland for all invasive pneumococci isolated since January 2010 and has been used thus far for over 1,500 pneumococcal isolates.
Since the implementation of the PCR-based serotyping scheme, the separate mPCR for detecting serotype 6C/6D isolates described by Carvalho et al. is no longer regularly used at our laboratory in Helsinki, first, because this particular mPCR alone is not able to distinguish between serotypes 6C and 6D, both of which carry the wciNβ gene, and second, because an antiserum and publications detailing its use are available to differentiate these serotypes (7). The newly identified serotype 6D was incorrectly identified with the methodology under validation (Table 1), but the growing number of publications about the typing of serotype 6D either with the help of antisera or by other PCR-based assays will make it possible to avoid this type of misidentification in the future (6, 20, 26, 27). In initial training, Quellung testing with factor antisera for serogroup 6 was considered challenging for certain isolates, which prompted the inclusion of a serogroup 6 set in the validation plan (set number 3), but we have found that, with the experience gained by the regular interpretation of Quellung reactions, this early uncertainty has been overcome.
During validation, the incorrect typing of a serotype 21 isolate as serotype 14 (Table 1) was most likely caused by a mixup of isolates or contamination at the DNA extraction level. In the new serotyping protocol, serotype 14 is specifically amplified in mPCR1 (Fig. 1) and does not require confirmation by Quellung testing; consequently, the error was not discovered until the results obtained by the different methods were compared. When DNA extraction and mPCR were repeated and Quellung testing was performed for this isolate, the serotype was determined to be 21. This incident emphasizes the importance of the careful handling of samples and pure cultures for DNA extraction as well as the use of appropriate negative controls at all stages of the process.
Serotypes 13, 23A, 23B, 31, and 35B were not included in the validation sets; thus, the amplification with these primers could not be confirmed with blinded samples. Since these serotypes are present in the invasive pneumococcal population in Finland, their detection by mPCR is included in our protocol applied to routine use, and isolates belonging to these serotypes have been properly identified by our serotyping protocol. Because of the ease of detection, the inclusion in particular of serotypes 23A and 35B in mPCR3 and mPCR4 has been helpful.
In recent years, a variety of DNA-based methods that rely on the capsular polysaccharide synthesis locus for the deduction of pneumococcal serotypes have been described, including approaches based on sequencing, restriction fragment length polymorphisms, hybridization assays, microarrays, and different PCR strategies (3, 11, 17, 18, 28, 35). The CDC serotyping schemes have been modified in different ways for use not only in Finland but in several other countries as well, which indicates that the oligonucleotide primers used for amplification can be combined in several ways (10, 15, 23, 24). This flexibility is important, as it allows the modification of a scheme according to the serotype distribution of the material to be serotyped, for example, that originating from different geographical regions of the world or representing colonizing or invasive isolates. Optimization should also take into account that the amplicons combined in any given mPCR should be of sufficiently different sizes to facilitate visual interpretation, an issue outlined by Miernyk et al. (23). For accurate serotyping by PCR, complementary phenotypic methods such as Quellung testing are needed for the subtyping and confirmation of the serotypes that are not distinguishable due to primer cross-reactivity.
Phenotypic serotyping also remains the most reliable way to discover possible false-positive results by PCR. One such case was discovered at the CDC in 2009, when a serotype 19F isolate produced a serotype 19A-specific PCR product; this led to the publication of an improved 19A-specific oligonucleotide primer pair that was also used in this study (31). Conversely, after the implementation of the new serotyping protocol in continuous use, one instance of cross-checking an mPCR serotyping result by Quellung testing at our laboratory revealed that an isolate that was serotype 19A by Quellung testing had given a false-positive serotype 19F PCR result. This isolate did not produce any amplicon with the 19A-specific oligonucleotide primers. A second, similar isolate was discovered soon after, and subsequently, all previous isolates (n = 68) that had yielded a 19F-positive PCR product were retrospectively examined by Quellung testing. No further discrepancies were revealed; we therefore conclude that this is a fairly rare occurrence. As serotype 19A is of particular interest as a potential replacement serotype following vaccination (12), the finding still prompted a change in our validated serotyping procedure, and as of October 2011, all isolates that are 19F positive in mPCR have been subtyped by Quellung testing to prevent possible underreporting of serotype 19A (Fig. 1). The two isolates producing discrepant serotyping results by mPCR and Quellung testing each had novel genotypes, ST8025 and ST8026, with four shared alleles. The closest match, sharing six alleles with ST8025, was genotype ST6970, represented by a serotype 19A isolate from the CSF of a child in Germany collected in the year 2005. The closest match for ST8026 is genotype ST7254, with six shared alleles. ST7254 is represented in the MLST database by a serotype 19F isolate from blood obtained in South Africa in the year 1985.
The capsular polysaccharide synthesis loci of serotype 19A and serotype 19F are known to be very similar; it has been proposed that differences in one gene, wzy (cpsI), give rise to the two different serotypes (25). This gene encodes the polymerase that links oligosaccharide repeat units to form lipid-linked capsular polysaccharides and determines the serotype by facilitating different types of bonds between the otherwise identical oligosaccharide repeat units in the capsules of serotype 19A and 19F (1, 4, 25). The nucleotide identity of wzy of 19A and wzy of 19F is 78.5%, and amino acid identity of the polymerases is 80.7% (25). The serotype 19A- and 19F-specific oligonucleotide primers in our use should target the respective wzy genes of these serotypes (28, 31). The sequences of the wzy genes in the two isolates that gave discrepant results by mPCR and Quellung testing showed 92% to 93% nucleotide identity with the serotype 19F wzy sequences and 80% identity with the serotype 19A wzy sequences in the database. In the light of this sequence data, it is less surprising that the gene is amplified with the 19F-specific primers, but the data offer little explanation of the contrasting phenotypic serotype result obtained by Quellung testing.
To discover possible discrepancies between serotyping results obtained by mPCR and Quellung as described above, a small sample of randomly selected isolates typed by mPCR alone should be examined also by Quellung testing for comparison. Participation in external quality assurance schemes is also necessary to detect possible discrepancies when the participating laboratories employ different serotyping methods.
The reagents used for PCR are commonly available in laboratories and relatively inexpensive; therefore, the material cost of serotyping based on deduction from mPCR results is affordable, compared with serotyping using antisera. The cost of serotyping isolates by the PCR-based protocol varies more than serotyping by traditional methods, as the materials for identification of the serotypes included in the first mPCR that do not require any confirmation by Quellung testing are very affordable, while the less common serotypes are costlier to determine, as they require several mPCRs due to the sequential nature of the protocol and the possible additional necessity of Quellung testing. This emphasizes the need for optimizing any PCR-based serotyping scheme according to the regional serotype distribution when known. When isolating DNA and performing mPCRs, the isolates can be handled in bulk, which reduces the hands-on time per sample, and with over 85% of the isolates being identified either fully or to the serogroup level in the first two mPCRs, the amount of work per isolate is very reasonable. The single drawback when handling samples in bulk is the delay in obtaining results for isolates with rare serotypes included in mPCR5 and mPCR6. In our laboratory, these mPCRs are performed only a couple of times a year, when a sufficient number of samples have been collected for a run.
As of September 2010, Finland introduced a 10-valent pneumococcal conjugate vaccine into the national childhood vaccination program. Some changes in the serotype distribution are to be expected, as has been reported elsewhere (13, 34). The laboratory-based surveillance of invasive pneumococcal disease requires the laboratory to be alert in order to identify the most frequent types quickly and efficiently and to adjust the protocol accordingly for serotype deduction even when the serotype distribution is changing.
ACKNOWLEDGMENTS
Gloria Carvalho and Leslie McGee at the CDC, Atlanta, GA, are thanked for encouragement and helpful discussions at the beginning of the process. Aila Soininen, Eeva-Liisa Korhonen, and Aili Hökkä are thanked for excellent technical assistance.
M.H.N. was supported by funding from the NIH (N01-AI-30021). The UAB has intellectual property rights in some of the reagents used, and M.H.N. is an employee of the UAB.
Footnotes
Published ahead of print 12 June 2012
REFERENCES
- 1. Aanensen DM, Mavroidi A, Bentley SD, Reeves PR, Spratt BG. 2007. Predicted functions and linkage specificities of the products of the Streptococcus pneumoniae capsular biosynthetic loci. J. Bacteriol. 189:7856–7876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Austrian R. 1976. The quellung reaction, a neglected microbiologic technique. Mt Sinai J. Med. 43:699–709 [PubMed] [Google Scholar]
- 3. Batt SL, Charalambous BM, McHugh TD, Martin S, Gillespie SH. 2005. Novel PCR-restriction fragment length polymorphism method for determining serotypes or serogroups of Streptococcus pneumoniae isolates. J. Clin. Microbiol. 43:2656–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bentley SD, et al. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2:e31 doi:10.1371/journal.pgen.0020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birtles A, et al. 2005. Multilocus sequence typing directly on DNA from clinical samples and a cultured isolate to investigate linked fatal pneumococcal disease in residents of a shelter for homeless men. J. Clin. Microbiol. 43:2004–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bratcher PE, Kim KH, Kang JH, Hong JY, Nahm MH. 2010. Identification of natural pneumococcal isolates expressing serotype 6D by genetic, biochemical and serological characterization. Microbiology 156:555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bratcher PE, Nahm MH. 2010. Cross-reactivity of current serogroup 6 factor sera from Statens Serum Institut with the recently described pneumococcal serotype 6d. J. Clin. Microbiol. 48:3044–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calix JJ, Moon NH. 2010. A new pneumococcal serotype, 11E, has a variably inactivated wcjE gene. J. Infect. Dis. 202:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carvalho MDG, et al. 2009. PCR-based quantitation and clonal diversity of the current prevalent invasive serogroup 6 pneumococcal serotype, 6C, in the United States in 1999 and 2006 to 2007. J. Clin. Microbiol. 47:554–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dias CA, Teixeira LM, Carvalho MDG, Beall B. 2007. Sequential multiplex PCR for determining capsular serotypes of pneumococci recovered from Brazilian children. J. Med. Microbiol. 56:1185–1188 [DOI] [PubMed] [Google Scholar]
- 11. Elberse KE, et al. 2011. Population structure of invasive Streptococcus pneumoniae in The Netherlands in the pre-vaccination era assessed by MLVA and capsular sequence typing. PLoS One 6:e20390 doi:10.1371/journal.pone.0020390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanage WP. 2008. Serotype-specific problems associated with pneumococcal conjugate vaccination. Future Microbiol. 3:23–30 [DOI] [PubMed] [Google Scholar]
- 13. Huang SS, et al. 2005. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics 116:e408–e413 [DOI] [PubMed] [Google Scholar]
- 14. Jin P, et al. 2009. First report of putative Streptococcus pneumoniae serotype 6D among nasopharyngeal isolates from Fijian children. J. Infect. Dis. 200:1375–1380 [DOI] [PubMed] [Google Scholar]
- 15. Jourdain S, et al. 2011. Sequential multiplex PCR assay for determining capsular serotypes of colonizing S. pneumoniae. BMC Infect. Dis. 11:100 doi:10.1186/1471-2334-11-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kilpi T, Herva E, Kaijalainen T, Syrjänen R, Takala AK. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20:654–662 [DOI] [PubMed] [Google Scholar]
- 17. Kong F, Brown M, Sabananthan A, Zeng X, Gilbert GL. 2006. Multiplex PCR-based reverse line blot hybridization assay to identify 23 Streptococcus pneumoniae polysaccharide vaccine serotypes. J. Clin. Microbiol. 44:1887–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kong F, et al. 2005. A molecular-capsular-type prediction system for 90 Streptococcus pneumoniae serotypes using partial cpsA-cpsB sequencing and wzy- or wzx-specific PCR. J. Med. Microbiol. 54:351–356 [DOI] [PubMed] [Google Scholar]
- 19. Konradsen HB. 2005. Validation of serotyping of Streptococcus pneumoniae in Europe. Vaccine 23:1368–1373 [DOI] [PubMed] [Google Scholar]
- 20. Kuch A, Sadowy E, Skoczynska A, Hryniewicz W. 2010. First report of Streptococcus pneumoniae serotype 6D isolates from invasive infections. Vaccine 28:6406–6407 [DOI] [PubMed] [Google Scholar]
- 21. Lambertsen L, Kerrn MB. 2010. Test of a novel Streptococcus pneumoniae serotype 6C type specific polyclonal antiserum (factor antiserum 6d) and characterisation of serotype 6C isolates in Denmark. BMC Infect. Dis. 10:282 doi:10.1186/1471-2334-10-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leinonen MK. 1980. Detection of pneumococcal capsular polysaccharide antigens by latex agglutination, counterimmunoelectrophoresis, and radioimmunoassay in middle ear exudates in acute otitis media. J. Clin. Microbiol. 11:135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miernyk K, et al. 2011. Serotyping of Streptococcus pneumoniae isolates from nasopharyngeal samples: use of an algorithm combining microbiologic, serologic, and sequential multiplex PCR techniques. J. Clin. Microbiol. 49:3209–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morais L, et al. 2007. Sequential multiplex PCR for identifying pneumococcal capsular serotypes from south-Saharan African clinical isolates. J. Med. Microbiol. 56:1181–1184 [DOI] [PubMed] [Google Scholar]
- 25. Morona JK, Morona R, Paton JC. 1999. Comparative genetics of capsular polysaccharide biosynthesis in Streptococcus pneumoniae types belonging to serogroup 19. J. Bacteriol. 181:5355–5364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nahm MH, et al. 2011. A report of Streptococcus pneumoniae serotype 6D in Europe. J. Med. Microbiol. 60:46–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oftadeh S, Satzke C, Gilbert GL. 2010. Identification of newly described Streptococcus pneumoniae serotype 6D by use of the Quellung reaction and PCR. J. Clin. Microbiol. 48:3378–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pai R, Gertz RE, Beall B. 2006. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J. Clin. Microbiol. 44:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pai R, et al. 2005. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J. Infect. Dis. 192:1988–1995 [DOI] [PubMed] [Google Scholar]
- 30. Park IH, et al. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45:1225–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pimenta FC, et al. 2009. Rarely occurring 19A-like cps locus from a serotype 19F pneumococcal isolate indicates continued need of serology-based quality control for PCR-based serotype determinations. J. Clin. Microbiol. 47:2353–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Siira L, et al. 2012. Clonality behind the increase of multidrug-resistance among non-invasive pneumococci in Southern Finland. Eur. J. Clin. Microbiol. Infect. Dis. 31:867–871 [DOI] [PubMed] [Google Scholar]
- 33. Siira L, et al. 2009. Temporal trends of antimicrobial resistance and clonality of invasive Streptococcus pneumoniae isolates in Finland, 2002 to 2006. Antimicrob. Agents Chemother. 53:2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singleton RJ, et al. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297:1784–1792 [DOI] [PubMed] [Google Scholar]
- 35. Tomita Y., et al. 2011. A new microarray system to detect Streptococcus pneumoniae serotypes. J. Biomed. Biotechnol. 2011:352736 doi:10.1155/2011/352736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu J, Lin J, Kim KH, Benjamin WH, Jr, Nahm MH. 2011. Development of an automated and multiplexed serotyping assay for Streptococcus pneumoniae. Clin. Vaccine Immunol. 18:1900–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]