Abstract
A combination of drugs possessing different targets has been used as salvage therapy, although without scientific support. In vitro studies validating such combinations are scarce, and the methodology is very laborious and time-consuming. This study proposes a flow cytometric (FC) protocol as an alternative to evaluate the effect of the combination of anidulafungin (AND) with amphotericin B (AMB) and azoles (fluconazole and voriconazole), tested upon 39 and 36 Candida strains, respectively. The concentration assayed in the combination was 0.5× MIC of each drug. The membrane potential marker DiBAC4(3) [Bis-(1,3-dibutylbarbituric acid) trimethine oxonol] was used for AND-AMB, and the metabolic marker FUN-1 was used for AND-azoles. Drug interaction was determined by calculating a staining index (SI): the sum of the percentage of depolarized cells (DC) after treatment with drug combinations divided by the DC of the drug alone, and the sum of the mean intensity of fluorescence (MIF) displayed by cells treated with drug combinations divided by the MIF of the drug alone for FUN-1. An SI of <1 means antagonism, an SI between 1 and 4 means no interaction, and an SI of >4 means synergism. The combination of AND and AMB by FC and checkerboard was synergistic for 46 and 43% of isolates and antagonistic for 5 and 8%, respectively. For the combination of AND and azoles, it was synergistic for 36% and antagonistic for 3% by FC and synergistic for 44% and antagonistic for 3% by checkerboard. When the FC method was compared to the gold standard checkerboard method, the agreement was 0.91 (95% confidence interval [95% CI] of 0.88 to 0.94), sensitivity was 0.88 (95% CI of 0.73 to 0.95), and specificity was 0.95 (95% CI of 0.84 to 1). Thus, FC is a rapid and reliable method (<2 h) to assess the effect of antifungal combinations.
INTRODUCTION
Candida species represent an important cause of nosocomial infections with high morbidity and mortality rates (28, 33). Over the past few years, epidemiological changes have been registered: the incidence of C. albicans has been reduced followed by a growing incidence of non-albicans species (9, 21, 34). Late diagnosis and high mortality rates frequently foment the use of empirical antifungal combinations as salvage therapy without a sound scientific basis (1). The availability of new antifungal drugs with novel targets of action has enlivened the interest in combination therapy. Likewise, it is not possible to assume that the simultaneous administration of two or more drugs with distinct mechanisms of action would improve the clinical outcome compared to monotherapy (3). It is unknown whether a combination might reduce the effectiveness of each drug or increase the potential for drug interactions or even toxicity, keeping in mind that this carries a significantly increased cost to the health care system without previously proven clinical benefits (7). Thus, it is important and timely to critically evaluate the role of combination therapy.
The methods available for studying drug combinations are few and cumbersome and often provide contradictory results. The most commonly utilized are the checkerboard method (based on a mathematical model) and the time-kill assay, both impossible to implement in the routine of clinical laboratories because they are very laborious (15, 35). Therefore, the Etest was proposed as an alternative; however, it also has serious limitations, including the cost, despite its good correlation with the classical method. The Etest is difficult to interpret when dealing with the azoles, due to the inconsistent growth patterns, and it takes at least 24 h to provide results since it is based on microbial growth (15). Critical patients need a rapid response.
Flow cytometry (FC) is a valuable tool for studying antifungal susceptibility, since it can be used to detect different physiological cell stages by using the appropriate fluorescent markers (5, 8). FC susceptibility testing to azoles, amphotericin B (AMB), and echinocandins has already been described (23–25, 27, 30). The goal of this study was to develop an FC protocol to characterize the effects of the combinations of AMB or azoles with the echinocandin anidulafungin (AND) upon Candida spp.
MATERIALS AND METHODS
Fungal strains.
Thirty-nine Candida strains were tested regarding the association between AND and AMB: 14 Candida albicans, 8 Candida glabrata, 9 Candida parapsilosis, 4 Candida tropicalis, 1 Candida guilliermondii, 2 Candida krusei, and 1 Candida lusitaniae. For the association between AND and azoles (fluconazole [FLU] or voriconazole [VOR]), 36 strains were tested: 16 C. albicans, 9 C. glabrata, 7 C. parapsilosis, 7 C. tropicalis, and 1 C. krusei. Clinical isolates of Candida spp. with a previously characterized antifungal susceptibility phenotype were selected from the collection of the Department of Microbiology, Faculty of Medicine of Porto, Portugal, in order to study all possibilities: susceptible or nonsusceptible to both drugs or susceptible to one and nonsusceptible to the other. C. albicans ATCC 90028 was used as the reference strain. Before the initiation of each experiment, the yeasts were subcultured twice on Sabouraud agar (Liofilchem, Teramo, Italy) to ensure both the viability and that the culture was pure.
Drugs and chemicals.
Stock solutions of AMB (Sigma-Aldrich, Taufkirchen, Germany), FLU (Sigma), and VOR and AND (Pfizer, New York) were prepared as recommended by the CLSI protocol M27-A3 (6) and stored at −80°C. Fluorescent dyes 2-chloro-4-(2,3-dihydro-3-methyl-[benzo-1,3-thiazol-2-yl]-methylidene)-1 (FUN-1) and Bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3)] (both acquired from Molecular Probes, Leiden, Netherlands) were prepared in phosphate-buffered saline (PBS) (Sigma) and kept at −20°C.
Checkerboard microdilution studies.
Checkerboard assays were performed for AND plus AMB and for AND plus azoles (FLU or VOR). MIC values of each antifungal and for the associations were determined after 24 h of incubation. The concentrations tested for each antifungal ranged between 0.06 and 32 μg/ml for FLU, 0.015 and 8 μg/ml for VOR and AMB, and 0.06 and 2 μg/ml for AND. Endpoints were determined according to values defined by the M27-A3 protocol (6). The fractional inhibitory concentration index (FICI), which is defined as the sum of the MIC of each drug when used in combination divided by the MIC of the drug when used alone, was calculated to determine the interaction; a FICI of ≤0.5 represents synergy, >0.5 to 4 represents no interaction, and >4 represents antagonism (10, 18).
Flow cytometry studies.
For FC assays, the strains were subcultured in Sabouraud broth and incubated with agitation at 35°C until the exponential growth phase in order to obtain a homogenous population and thus to correlate the perturbations in cellular parameters observed by FC with the drug action and independently of growth phase. Then, 0.5 McFarland standard density yeast suspensions were prepared in PBS, corresponding to 106 yeast cells/ml. The cell suspensions were incubated at 35°C with subinhibitory concentrations (0.5× MIC value) of each antifungal alone and in combination as described above. In order to standardize the FC protocol for all strains, the 0.5× MIC of each drug was chosen since the breakpoints to AND and FLU are being reviewed and their values are species dependent (20, 22), because a new CLSI protocol is not already available, and because antagonistic and synergistic classifications usually rely on deviations from additivity (36). According to the Loewe additivity definition, 0.5× MIC of drug A combined with 0.5× MIC of drug B is equivalent to 1 MIC of drug A or 1 MIC of drug B in an additive drug pair. Even so, for strains inhibited at high MIC values and that do not present antagonism with the combination of 0.5× MIC, a new test was carried out using the breakpoint of the drug (e.g., 8 μg/ml for FLU and 4 μg/ml for AND) in order to evaluate its clinical significance.
Following 1 h of incubation, the cells were washed and incubated for 15 min in the dark at room temperature with 0.5 μg/ml of DiBAC4(3), a lipophilic anion able to diffuse across depolarized membranes, in the case of the association of AND plus AMB and with 0.5 μg/ml of FUN-1, a metabolic marker, in the case of association of AND plus azoles. The intensity of fluorescence of 30,000 cells was registered at FL1 (530 nm) for DiBAC4(3) and FL2 (575 nm) for FUN-1. The samples were analyzed in a FACSCalibur cytometer (BD Biosciences, Sydney, Australia) standard model equipped with 3 photomultiplayers (PMTs), standard filters, and a 15-mW 488-nm Argon laser and using CellQuest Pro software (version 4.0.2). Instrument controls followed the standard procedures described by the manufacturer. All trials were performed in triplicate. The evaluation of in vitro drug interactions by FC was determined according to the staining index (SI), which is similar to the FICI described above. The SI was calculated as the sum of the percentage of depolarized cells (DC) after treatment with drug combinations divided by the DC of the drug alone for DiBAC4(3) and the sum of mean intensity of fluorescence displayed by cells treated with drug combinations divided by the fluorescence of the drug alone for FUN-1. Hence, SI = (DC AND + AMB/DC AND) + (DC AND + AMB/DC AMB) for AND-AMB association and SI = (MIF AND + azole/MIF AND) + (MIF AND + azole/MIF azole) (MIF, ratio between mean intensity of fluorescence of treated cells and viable cells) for AND-azoles association. Taking into account the standard classification of the checkerboard results, an association provided by FC was defined as antagonism for SI of <1, no interaction for SI between 1 and 4, and synergy for an SI of >4.
Determination of viable cells.
The number of viable cells in each FC assay was determined by plating 100 μl of serial dilutions on Sabouraud agar medium and incubating at 35°C for 24 h. Afterwards, the number of CFU was determined. No carryover antifungal effect was detected. All assays were performed in triplicate.
Statistical analysis.
To evaluate the agreement between checkerboard and FC studies, the proportion of agreement (PA) and the value of Kappa (K) were calculated (31). In order to check the diagnostic validity of FC to detect the effect of AND with AMB and azoles, having the checkerboard method as the reference, sensitivity and specificity were calculated (with confidence intervals at 95%). For calculation of all measures, the SPSS program (version 19.0) was used.
RESULTS
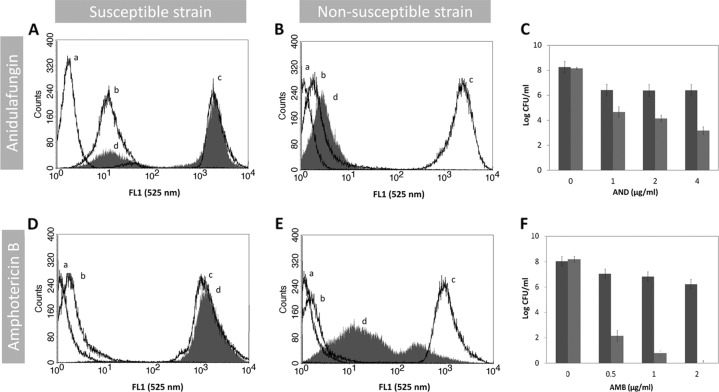
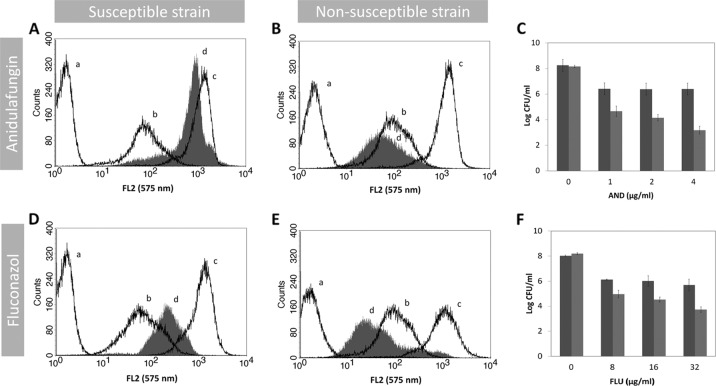
A protocol for evaluation of AND and AMB susceptibility by FC using DiBAC4(3) as a marker was optimized. Our method is indeed able to discriminate for both drugs the susceptible and nonsusceptible Candida strains. A typical example of FC analysis for antifungal susceptibility testing is represented in Fig. 1. Accordingly, for each strain, the autofluorescence of the cell population in analysis is measured. This value is always represented on the first decade of the log scale of intensity of fluorescence, while the ethanol-treated cells (dead cells, the positive control) showed a high increase in the green fluorescence intensity (FL1 [530 nm]) as expected. The viable nontreated cells stained with DiBAC4(3) had a slight increase in the fluorescence (2-fold) in comparison with the viable nontreated and nonstained cells (autofluorescence). Treatment of susceptible strains with AND produced a dose-dependent increase for the fourth decade of intensity of fluorescence of cells, which was not observed in the nonsusceptible strains (Fig. 1A, B). The results obtained after 2 h of incubation with the antifungal were similar to data after 1 h (data not shown). This increase in fluorescence intensity corresponds to a decrease in the number of CFU (Fig. 1A to C). Likewise, treatment of susceptible strains with 1 μg/ml of AMB also induced an increase in fluorescence intensity similar to that of the positive control after 1 h of incubation. In contrast, for strains having higher MIC values, only a slight increase in fluorescence intensity was observed after incubation with AND or AMB, highlighting the fact that plasma membrane depolarization is dependent on antifungal action and thus dependent on the antifungal susceptibility profile (Fig. 1D, E). The CFU values determined in similar conditions agree very well with the FC data (PA = 0.92) (Fig. 1C, F). Since azole treatment did not result in any increase in the intensity of fluorescence after DiBAC4(3) staining, even after 2 h of incubation, neither in susceptible nor in nonsusceptible strains (data not shown), a different fluorescent probe was chosen. FUN-1, a metabolic marker, has already been demonstrated by our group to be an excellent probe for azole susceptibility testing of Candida spp. (25). Thus, we developed an FC protocol to evaluate FLU, VOR, and AND susceptibility by FC using FUN-1 as a marker (Fig. 2). An increase in the intensity of fluorescence was registered only for susceptible strains after 1 h of incubation. Conversely, this increase was not found for nonsusceptible strains as expected (Fig. 2B, E). The CFU values were consistent with FC data (PA = 0.86) (Fig. 2C, F).
Fig 1.
In vitro antifungal activities of anidulafungin and amphotericin B. Distribution of fluorescence intensity of the C. albicans 0207 AND-susceptible strain (A), C. parapsilosis 0136 AND-nonsusceptible strain (B), C. albicans O207 AMB-susceptible strain (D), and C. lusitaniae D51 AMB-nonsusceptible strain (E). In each histogram, the autofluorescence is represented by line a, line b represents the fluorescence of untreated cells stained with DiBAC4(3), line c is the fluorescence of cells treated with 70% ethanol and stained with DiBAC4(3) (positive control), and line d is the fluorescence of cells treated with 1 μg/ml of antifungal drugs during 1 h and stained with DiBAC4(3). (C, F) Determination of the number of CFU (CFU/ml) of cell suspensions treated with different antifungal concentrations under conditions identical to those of the flow cytometric assay. The nonsusceptible strain is represented by the dark-gray bars and the susceptible strain by the light-gray bars.
Fig 2.
In vitro antifungal activities of anidulafungin and fluconazole. Distribution of fluorescence intensity of C. albicans 0207 AND-susceptible strain (A), C. parapsilosis 0136 AND-nonsusceptible strain (B), C. albicans O223 FLU-susceptible strain (D), and C. albicans O216 FLU-nonsusceptible strain (E). In each histogram, the autofluorescence is represented by line a, line b represents the fluorescence of untreated cells stained with FUN-1, line c is the fluorescence of cells treated with 70% ethanol and stained with FUN-1 (positive control), and line d is the fluorescence of cells treated with antifungal drugs (1 μg/ml of ANI and 16 μg/ml of FLU) during 1 h and stained with FUN-1. (C, F) Determination of the number of CFU (CFU/ml) of cell suspensions treated with different antifungal concentrations under conditions identical to those of the flow cytometric assay. The nonsusceptible strain is represented by the dark-gray bars and the susceptible strain by the light-gray bars.
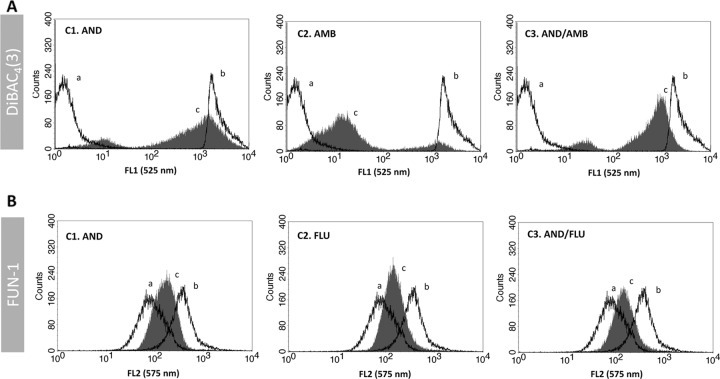
Regarding the antifungal association studies, the FC assays for antifungal associations were performed with subinhibitory concentrations (0.5× MIC values) of each drug either alone or in association using the previous optimized conditions (Fig. 1 and 2). These values were used to be able to standardize since the MIC varies for each strain. DiBAC4(3) was used for staining cells treated with AND-AMB and FUN-1 for staining cells treated with AND-FLU or AND-VOR. A typical synergistic interaction of AND-AMB evaluated by FC is represented in Fig. 3A. The lack of interaction between AND and FLU association is represented in Fig. 3B. Taking into account the FC data, the association between AND and AMB was synergistic in 46% of cases (18 of 39); there was no interaction in 49% of isolates (19 of 39), and the association was antagonistic in 5% (2 of 39) (Table 1). The association between AND and FLU was synergistic in 36% of cases (13 of 36), there was no interaction in 61% (22 of 36), and the association was antagonistic in 3% (1 of 36) (Table 2). The association between AND and AMB evaluated by the checkerboard microdilution method was synergistic in 43% of strains tested (17 of 39), there was no interaction in 49% (19 of 39), and the association was antagonistic in 8% (3 of 39). Antagonistic interaction was detected only for C. albicans. For C. glabrata isolates, this association was quite promising since there was no evidence of an antagonistic effect and “no interaction” was not a frequent event (Table 1). The results of AND and FLU association were similar to that for AND and VOR. Synergism was observed in 44% of strains (16 of 36), no interaction in 53% (19 of 36), and an antagonistic effect in 3% (1 of 36). These antagonistic events were detected for only one isolate of C. parapsilosis. Concerning C. glabrata, once again no antagonism was observed between AND and the azoles, and no interaction was also a rare event, it being detected only in two strains. For strains that are susceptible-dose dependent or resistant to FLU and those which are nonsusceptible to AND, not showing antagonism with the combination of 0.5× MIC, the results of the assays performed using the breakpoint concentration were similar to those obtained with 0.5× MIC (data not shown).
Fig 3.
Evaluation of antifungal combination effect using flow cytometry. (A) Flow cytometric analysis of the combination effect between anidulafungin and amphotericin B on the C. albicans 0215 strain, an example of synergistic association. Line a, fluorescence of untreated cells stained with DiBAC4(3); line b, fluorescence of cells treated with 70% ethanol and stained with DiBAC4(3); line c, fluorescence of cells treated with antifungal drugs and stained with DiBAC4(3); C1, cells treated with a subinhibitory concentration of AND (0.5× MIC); C2, cells treated with a subinhibitory concentration of AMB (0.5× MIC); and C3, cells treated with a subinhibitory concentrations of both antifungal drugs in association (AND 0.5× MIC + AMB 0.5× MIC). (B) Flow cytometric analysis of the combination effect between anidulafungin and fluconazole on the C. albicans OL196 strain, an example of indifferent association. Line a, fluorescence of untreated cells stained with FUN-1; line b, fluorescence of cells treated with 70% ethanol and stained with FUN-1; line c, fluorescence of cells treated with antifungal drugs and stained with FUN-1; C1, cells treated with a subinhibitory concentration of AND (0.5× MIC); C2, cells treated with a subinhibitory concentration of FLU (0.5× MIC); and C3, cells treated with subinhibitory concentrations of both antifungal drugs in association (AND 0.5× MIC + FLU 0.5× MIC).
Table 1.
In vitro interaction of anidulafungin and amphotericin B by the checkerboard and flow cytometry methods against 39 Candida speciesa
| Strain | Checkerboard MIC (μg/ml) |
FICI (interpretation) | Flow cytometry DC (%) |
SI (interpretation) | ||||
|---|---|---|---|---|---|---|---|---|
| AND | AMB | AND/AMB | AND | AMB | AND-AMB | |||
| C. albicans O236 | 0.25 | 0.25 | 0.125/0.03 | 0.62 (NI) | 45.04 | 13.53 | 22.42 | 2.15 (NI) |
| C. albicans O223 | 0.25 | 0.125 | 0.06/0.015 | 0.36 (S) | 71.23 | 14.58 | 69.08 | 5.71 (S) |
| C. albicans O216 | 0.125 | 0.25 | 0.06/0.015 | 0.54 (NI) | 49.68 | 8.24 | 25.17 | 3.56 (NI) |
| C. albicans O189 | 0.25 | 0.125 | 0.06/0.015 | 0.36 (S) | 26.33 | 1.78 | 24.77 | 14.86 (S) |
| C. albicans OL196 | 0.03 | 0.25 | 0.06/0.015 | 2.06 (NI) | 83.45 | 27.89 | 33.56 | 1.61 (NI) |
| C. albicans O207 | 0.015 | 0.125 | 0.06/0.015 | 4.12 (A) | 48.96 | 10.45 | 8.53 | 0.99 (A) |
| C. albicans O245 | 0.015 | 0.25 | 0.06/0.015 | 4.06 (A) | 48.13 | 37.23 | 20.21 | 0.99 (A) |
| C. albicans O237 | 0.25 | 0.25 | 0.06/0.015 | 0.30 (S) | 42.59 | 1.57 | 44.53 | 29.41 (S) |
| C. albicans O183 | 0.015 | 0.06 | 0.015/0.015 | 1.25 (NI) | 22.69 | 10.41 | 13.20 | 1.85 (NI) |
| C. albicans O222 | 1 | 0.125 | 0.06/0.015 | 0.18 (S) | 37.40 | 8.78 | 32.65 | 4.59 (S) |
| C. albicans O215 | 0.25 | 0.125 | 0.06/0.015 | 0.36 (S) | 68.42 | 13.05 | 65.04 | 5.93 (S) |
| C. albicans O190 | 0.25 | 2 | 0.125/0.015 | 0.51 (NI) | 19.34 | 11.08 | 17.57 | 2.49 (NI) |
| C. albicans O195 | 0.015 | 0.125 | 0.06/0.015 | 4.12 (A) | 53.12 | 12.98 | 17.89 | 1.72 (NI) |
| C. albicans ATCC | 0.03 | 0.06 | 0.015/0.015 | 0.75 (NI) | 61.34 | 37.12 | 53.11 | 2.30 (NI) |
| C. glabrata OL158 | 4 | 0.25 | 1/0.03 | 0.37 (S) | 79.44 | 19.36 | 75.85 | 4.87 (S) |
| C. glabrata O206 | 0.03 | 0.25 | 0.06/0.015 | 2.06 (NI) | 35.48 | 70.21 | 50.81 | 2.16 (NI) |
| C. glabrata O188 | 0.50 | 0.125 | 0.06/0.015 | 0.24 (S) | 82.83 | 3.95 | 58.39 | 15.49 (S) |
| C. glabrata OL163 | 0.25 | 0.25 | 0.06/0.015 | 0.30 (S) | 73.94 | 15.09 | 57.93 | 4.62 (S) |
| C. glabrata OL149 | 0.25 | 0.25 | 0.125/0.03 | 0.62 (NI) | 75.39 | 36.95 | 58.23 | 2.35 (NI) |
| C. glabrata O175 | 0.06 | 0.125 | 0.015/0.015 | 0.37 (S) | 66.85 | 10.44 | 63.11 | 6.99 (S) |
| C. glabrata O180 | 0.03 | 0.25 | 0.015/0.015 | 0.56 (NI) | 78.27 | 3.01 | 73.13 | 25.23 (S) |
| C. glabrata O181 | 4 | 0.06 | 0.015/0.03 | 0.50 (S) | 59.89 | 5.66 | 37.48 | 7.25 (S) |
| C. guilliermondii 33 | 1 | 0.125 | 0.25/0.03 | 0.49 (S) | 23.28 | 2.46 | 21.11 | 9.49 (S) |
| C. krusei OL16 | 0.25 | 0.25 | 0.06/0.015 | 0.30 (S) | 29.72 | 4.81 | 27.58 | 6.66 (S) |
| C. krusei O234 | 0.125 | 0.125 | 0.06/0.015 | 0.60 (NI) | 14.92 | 2.11 | 10.53 | 5.70 (S) |
| C. lusitaniae D51 | 0.5 | 2 | 0.25/0.25 | 0.63 (NI) | 79.93 | 49.79 | 69.93 | 2.28 (NI) |
| C. parapsilosis OL143 | 2 | 0.25 | 1/0.03 | 0.62 (NI) | 72.14 | 23.71 | 55.46 | 3.11 (NI) |
| C. parapsilosis O246 | 1 | 0.25 | 0.5/0.06 | 0.74 (NI) | 67.93 | 48.06 | 58.03 | 2.06 (NI) |
| C. parapsilosis ATO17 | 4 | 0.125 | 1/0.06 | 0.73 (NI) | 2.94 | 0.34 | 0.78 | 2.56 (NI) |
| C. parapsilosis OL144 | 2 | 0.25 | 0.06/0.03 | 0.15 (S) | 21.15 | 0.63 | 16.10 | 26.32 (S) |
| C. parapsilosis O204 | 4 | 0.125 | 2/0.06 | 0.98 (NI) | 82.33 | 31.18 | 80.53 | 3.56 (NI) |
| C. parapsilosis O136 | 2 | 0.125 | 1/0.03 | 0.74 (NI) | 15.58 | 14.50 | 16.98 | 2.26 (NI) |
| C. parapsilosis Cpo41 | 8 | 0.125 | 0.06/0.06 | 0.49 (S) | 8.87 | 5.98 | 15.06 | 4.22 (S) |
| C. parapsilosis O158 | 2 | 0.125 | 0.5/0.03 | 0.49 (S) | 45.08 | 4.92 | 21.61 | 4.87 (S) |
| C. parapsilosis O56 | 4 | 0.125 | 2/0.03 | 0.74 (NI) | 21.72 | 11.53 | 18.67 | 2.48 (NI) |
| C. tropicalis OL202 | 0.06 | 0.25 | 0.015/0.015 | 0.31 (S) | 90.22 | 3.45 | 89.79 | 27.02 (S) |
| C. tropicalis OL205 | 0.25 | 0.25 | 0.06/0.015 | 0.30 (S) | 70.98 | 23.04 | 53.85 | 3.10 (NI) |
| C. tropicalis OL193 | 0.03 | 0.5 | 0.06/0.015 | 2.03 (NI) | 42.01 | 11.21 | 11.01 | 1.24 (NI) |
| C. tropicalis 1304 | 1 | 0.06 | 0.5/0.03 | 1 (NI) | 11.54 | 1.98 | 3.96 | 2.34 (NI) |
S, synergism; A, antagonism; NI, no interaction; FICI, fractional inhibitory concentration index; DC, percentage of depolarized cells; SI, staining index. DC percentage is based on the use of 0.5× MIC of each drug. FICI = (MIC AND/AMB)/MIC AND + (MIC AMB/AND)/MIC AMB. SI = (DC AND + AMB)/DC AND + (DC AND + AMB)/DC AMB.
Table 2.
In vitro interaction of anidulafungin and fluconazole by the checkerboard and flow cytometry methods against 36 Candida speciesa
| Strain | Checkerboard MIC (μg/ml) |
FICI (interpretation) | Flow cytometry MIF |
SI (interpretation) | ||||
|---|---|---|---|---|---|---|---|---|
| AND | FLU | AND/FLU | AND | FLU | AND-FLU | |||
| C. albicans O189 | 0.25 | 32 | 0.06/0.06 | 0.24 (S) | 1.10 | 1.26 | 2.60 | 4.44 (S) |
| C. albicans O190 | 0.25 | 64 | 0.06/0.015 | 0.24 (S) | 0.48 | 0.48 | 1.24 | 5.13 (S) |
| C. albicans O195 | 0.015 | 4 | 0.015/0.06 | 1.02 (NI) | 2.70 | 1.62 | 2.34 | 2.31 (NI) |
| C. albicans O205 | 0.06 | 2 | 0.06/0.015 | 1.01 (NI) | 1.68 | 0.93 | 0.82 | 1.36 (NI) |
| C. albicans O207 | 0.015 | 4 | 0.015/0.06 | 1.02 (NI) | 2.30 | 1.74 | 1.55 | 1.57 (NI) |
| C. albicans O216 | 0.25 | 64 | 0.06/0.06 | 0.24 (S) | 4.77 | 3.16 | 7.72 | 4.06 (S) |
| C. albicans O223 | 0.25 | 1 | 0.06/0.06 | 0.30 (S) | 1.75 | 1.39 | 4.37 | 5.65 (S) |
| C. albicans O236 | 0.25 | 16 | 0.06/0.06 | 0.24 (S) | 1.10 | 1.03 | 2.17 | 4.08 (S) |
| C. albicans O237 | 0.25 | 64 | 0.06/0.06 | 0.24 (S) | 2.67 | 1.29 | 3.50 | 4.03 (S) |
| C. albicans O245 | 0.015 | 64 | 0.015/0.06 | 1 (NI) | 1.41 | 1.27 | 1.34 | 2.00 (NI) |
| C. albicans OL122 | 0.125 | 0.5 | 0.125/0.015 | 1.03 (NI) | 8.00 | 4.79 | 5.69 | 1.90 (NI) |
| C. albicans OL160 | 0.03 | 16 | 0.015/0.125 | 0.51 (NI) | 0.38 | 0.38 | 0.59 | 3.09 (NI) |
| C. albicans OL171 | 0.015 | 0.5 | 0.015/0.015 | 1.03 (NI) | 0.48 | 0.49 | 0.48 | 2.00 (NI) |
| C. albicans OL172 | 0.03 | 16 | 0.015/0.125 | 0.51 (NI) | 1.26 | 0.91 | 1.68 | 3.19 (NI) |
| C. albicans OL196 | 0.015 | 64 | 0.015/0.06 | 1 (NI) | 1.28 | 1.41 | 1.28 | 1.91 (NI) |
| C. albicans ATCC | 0.03 | 0.125 | 0.015/0.015 | 0.62 (NI) | 1.69 | 1.40 | 1.79 | 2.33 (NI) |
| C. glabrata O158 | 4 | 4 | 0.5/1 | 0.38 (S) | 7.93 | 1.01 | 8.90 | 9.93 (S) |
| C. glabrata O181 | 4 | 4 | 0.25/1 | 0.31 (S) | 2.48 | 0.51 | 2.83 | 6.72 (S) |
| C. glabrata O188 | 0.5 | 8 | 0.06/0.06 | 0.13 (S) | 3.77 | 2.81 | 7.15 | 4.44 (S) |
| C. glabrata O180 | 0.5 | 16 | 0.125/0.06 | 0.25 (S) | 0.46 | 1.02 | 1.30 | 4.09 (S) |
| C. glabrata O206 | 0.03 | 16 | 0.015/0.06 | 0.51 (NI) | 1.88 | 1.80 | 2.04 | 2.22 (NI) |
| C. glabrata OL149 | 0.25 | 16 | 0.06/0.06 | 0.24 (S) | 2.08 | 1.22 | 3.24 | 4.20 (S) |
| C. glabrata OL158 | 4 | 8 | 0.06/4 | 0.52 (NI) | 1.27 | 1.01 | 1.14 | 2.03 (NI) |
| C. glabrata OL163 | 0.25 | 16 | 0.06/0.06 | 0.24 (S) | 1.78 | 1.57 | 1.96 | 2.35 (NI) |
| C. glabrata OL164 | 0.25 | 8 | 0.06/0.015 | 0.24 (S) | 0.96 | 0.62 | 1.61 | 4.29 (S) |
| C. krusei OL16 | 0.25 | 64 | 0.125/0.06 | 0.51(NI) | 1.10 | 1.06 | 1.15 | 2.13 (NI) |
| C. parapsilosis Cpo41 | 8 | 0.125 | 0.06/0.5 | 4.01 (A) | 1.34 | 1.11 | 0.59 | 0.97 (A) |
| C. parapsilosis O136 | 4 | 2 | 0.25/2 | 1.06 (NI) | 1.64 | 0.91 | 0.92 | 1.57 (NI) |
| C. parapsilosis O246 | 2 | 0.5 | 0.25/0.25 | 0.625 (NI) | 1.53 | 1.72 | 1.66 | 2.05 (NI) |
| C. parapsilosis O56 | 4 | 0.5 | 0.5/0.25 | 0.625 (NI) | 2.33 | 4.06 | 3.07 | 2.07 (NI) |
| C. parapsilosis OL143 | 2 | 2 | 0.25/0.5 | 0.38 (S) | 3.37 | 4.37 | 4.96 | 2.61 (NI) |
| C. parapsilosis ATO16 | 4 | 0.25 | 0.5/0.06 | 0.37 (S) | 3.10 | 3.53 | 4.31 | 2.61 (NI) |
| C. parapsilosis OL144 | 2 | 2 | 0.5/1 | 0.75 (NI) | 1.95 | 1.18 | 1.94 | 2.65 (NI) |
| C. tropicalis OL193 | 0.015 | 2 | 0.015/0.06 | 1.03 (NI) | 1.12 | 1.68 | 1.53 | 2.27 (NI) |
| C. tropicalis OL202 | 0.015 | 2 | 0.015/0.06 | 1.03 (NI) | 0.47 | 0.75 | 0.46 | 1.60 (NI) |
| C. tropicalis OL295 | 0.25 | 2 | 0.06/0.06 | 0.27 (S) | 1.43 | 0.98 | 2.57 | 4.42 (S) |
S, synergism; A, antagonism; NI, no interaction; FICI, fractional inhibitory concentration index; MIF, mean intensity of fluorescence; SI, staining index. MIF was determined using 0.5× MIC of each drug. FICI = (MIC AND/FLU)/MIC AND + (MIC FLU/AND)/MIC FLU. SI = (MIF AND + FLU)/MIF AND + (MIF AND + FLU)/MIF FLU.
Our FC assay showed three more cases of “no interaction” than the checkerboard method, and there was one more case of antagonism in the checkerboard method related to one C. albicans strain (O195) in comparison with the FC method. The Kappa value obtained between both methods was 0.83 (95% CI of 0.79 to 0.87), it being 0.82 (95% CI of 0.76 to 0.88) for AND-AMB association and 0.84 (95% CI of 0.78 to 0.90) for the AND-azoles association. The proportion of agreement calculated was 0.91 (95% CI of 0.88 to 0.94), namely 0.90 (95% CI of 0.85 to 0.95) for the AND-AMB association and 0.92 (95% CI of 0.87 to 0.97) for the AND-azoles association. Regarding sensitivity and specificity of the FC method, considering the checkerboard assay as the reference methodology, sensitivity was 0.88 (95% CI of 0.73 to 0.95) for detection of synergic effects, and specificity was 0.95 (95% CI of 0.84 to 1). In order to detect an antagonistic interaction, FC sensitivity was 0.75 (95% CI of 0.3 to 0.95), and its specificity was 1.
DISCUSSION
The use of drugs with different mechanisms of action in association may play a key role in treatment of invasive fungal infections (3, 7). In the past few years, this combined antifungal therapy has received increased attention. Antifungal interaction involving Cryptococcus has been studied; however, reports on the effect of antifungal combinations involving the most frequent Candida spp. are less common. Most of these studies have demonstrated synergism, whereas others have reported no interaction and occasionally antagonism (3, 11, 13, 29, 32).
Echinocandins are a novel class of antifungals which have the cell wall as their target. The literature addressing the relationship between such drugs and membrane-active drugs such as polyenes or azoles against Candida is still somewhat limited (12, 13, 15). Importantly, drugs with different targets of action could reinforce each other, allowing a decrease in doses and thus reducing side effects for patients (12, 16). AND acts by inhibiting the 1,3-β-d-glucan synthesis, the major component of the fungal cell wall (26). AMB and azoles are membrane-active drugs; the former acts by making holes in the membrane and the latter by inhibiting its synthesis. Echinocandins probably enhance the effect of membrane-active drugs by increasing their access to the target (1, 12). Nevertheless, it cannot be assumed that the use of two or more effective drugs with distinct mechanisms of action would produce an improved outcome compared to the results seen with a single compound (3, 7).
Like in most previously published studies, the association between azoles or AMB with AND resulted, for the majority of the strains, in a synergy or no interaction (13, 17, 19). In fact, in our study, antagonism for AND-AMB association was observed only for three strains of C. albicans (O207, O245, O195) and one for the AND-FLU association in a strain of C. parapsilosis (Cpo41). Each drug alone had very low MIC values for these strains, but they increased following the association, although still remaining low. With regard to echinocandins, most authors found no antagonism between micafungin and azoles or AMB (4, 17, 32). Barchiesi et al. did not find advantages in associating caspofungin and the polyene, with the exception of C. parapsilosis, but the study included a much more limited number of strains (2). In some Candida strains, echinocandins are highly active, and so the fungicidal activity may be difficult to improve after combination (17). Moreover, we have shown that the drug interaction potential is species and strain dependent, which enhances the importance of the novel protocol described here for the first time.
The mathematic model used for the checkerboard method, which allows a quantification analysis after calculating the FICI, has been the most commonly used procedure to characterize the activity of antimicrobial combinations in clinical laboratories. Other methods, such as time-kill assays and Etest, have been used (13); however, despite their good correlation with the checkerboard assay, all of them take at least another 24 h to provide results. The terminology used to assign the results into interpretative categories is often a subject of debate and confusion, with difficult resolution (10). Synergism and antagonism have clear and intuitive meanings, although “no interaction” is a somewhat subjective category without a clear clinical relevance (10).
At the moment, few but relevant reports have helped to demonstrate the value of cytometric assays as excellent yet underexplored tools in clinical microbiology (8, 23, 30). Flow cytometry is a powerful high-throughput technology which allows the characterization of several thousands of cells per second, distinguishing between different physiological states. Differentiations between viable, intermediate, and nonviable cells are possible using fluorescent dyes. Using these tools, fast, reliable data could be obtained with great benefit for the patient. Echinocandin antifungal activity was studied using DiBAC4(3) or FUN-1, although for azoles only FUN-1 could be used after a short incubation time. Regarding AMB, previous studies reported the impossibility of studying its activity with FUN-1 (25). Thus, the study of the AND-AMB association was performed using DiBAC4(3) and that of the AND-azoles association was performed using FUN-1 (Fig. 1 to 3). DiBAC4(3) is a membrane potential marker that can enter depolarized cells, where it binds to intracellular proteins or membranes and exhibits enhanced fluorescence; and FUN-1 is a metabolic activity staining that passively diffuses through yeast cell walls. In metabolically inactive cells, FUN-1 remains in the cytoplasm, displaying a green fluorescence, while in active cells it is processed, which results in the formation of distinct vacuolar structures that exhibit a red fluorescence, accompanied by a reduction in the green cytoplasmic fluorescence (14, 24). For both markers, an increase in fluorescence intensity [FL1 (530 nm) for DiBAC4(3) and FL2 (575 nm) for FUN-1] agrees with the reduction of CFU counts, meaning a reduction of cell viability.
The FC protocols described show an excellent agreement with the checkerboard method and high sensitivity and specificity, thus allowing the study of different combinations of antifungal drugs in less than 2 h.
ACKNOWLEDGMENTS
This work was supported by Pfizer Laboratories grant WS718413 and by the startup SCREENPROFIND.
The work presented here is the subject of an international patent-pending application (no. PCT/IB2012/052807) on behalf of the authors.
Footnotes
Published ahead of print 12 June 2012
REFERENCES
- 1. Baddley JW, Pappas PG. 2005. Antifungal combination therapy: clinical potential. Drugs 65:1461–1480 [DOI] [PubMed] [Google Scholar]
- 2. Barchiesi F, Spreghini E, Tomassetti S, Giannini D, Scalise G. 2007. Caspofungin in combination with amphotericin B against Candida parapsilosis. Antimicrob. Agents Chemother. 51:941–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chamilos G, Kontoyiannis DP. 2006. The rationale of combination antifungal therapy in severely immunocompromised patients: empiricism versus evidence-based medicine. Curr. Opin. Infect. Dis. 19:380–385 [DOI] [PubMed] [Google Scholar]
- 4. Chaturvedi V, et al. 2011. Multilaboratory testing of two-drug combinations of antifungals against Candida albicans, Candida glabrata, and Candida parapsilosis. Antimicrob. Agents Chemother. 55:1543–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaturvedi V, Ramani R, Pfaller MA. 2004. Collaborative study of the NCCLS and flow cytometry methods for antifungal susceptibility testing of Candida albicans. J. Clin. Microbiol. 42:2249–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. CLSI 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. CLSI, Wayne, PA [Google Scholar]
- 7. Cuenca-Estrella M. 2004. Combinations of antifungal agents in therapy—what value are they? J. Antimicrob. Chemother. 54:854–869 [DOI] [PubMed] [Google Scholar]
- 8. Czechowska K, Johnson DR, van der Meer JR. 2008. Use of flow cytometric methods for single-cell analysis in environmental microbiology. Curr. Opin. Microbiol. 11:205–212 [DOI] [PubMed] [Google Scholar]
- 9. Erjavec Z, Kluin-Nelemans H, Verweij PE. 2009. Trends in invasive fungal infections, with emphasis on invasive aspergillosis. Clin. Microbiol. Infect. 15:625–633 [DOI] [PubMed] [Google Scholar]
- 10. Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones RN, Castanheira M, Pfaller MA. 2010. Fixed-ratio combination testing of an echinocandin, anidulafungin, and an azole, voriconazole, against 1,467 Candida species isolates. Antimicrob. Agents Chemother. 54:4041–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karlowsky JA, Zhanel HDGG, Goldstein BP. 2006. In vitro interactions of anidulafungin with azole antifungals, amphotericin B and 5-fluorocytosine against Candida species. Int. J. Antimicrob. Agents. 27(2):174–177 [DOI] [PubMed] [Google Scholar]
- 13. Kiraz N, et al. 2009. Antifungal activity of caspofungin in combination with amphotericin B against Candida glabrata: comparison of disk diffusion, Etest, and time-kill methods. Antimicrob. Agents Chemother. 53:788–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee W, Kwak Y. 1999. Antifungal susceptibility testing of Candida species by flow cytometry. J. Korean Med. Sci. 14:21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewis RE, Diekema DJ, Messer SA, Pfaller MA, Klepser ME. 2002. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemother. 49:345–351 [DOI] [PubMed] [Google Scholar]
- 16. Mukherjee PK, Sheehan DJ, Hitchcock CA, Ghannoum MA. 2005. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 18:163–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishi I, Sunada A, Toyokawa M, Asari S, Iwatani Y. 2009. In vitro antifungal combination effects of micafungin with fluconazole, voriconazole, amphotericin B, and flucytosine against clinical isolates of Candida species. J. Infect. Chemother. 15:1–5 [DOI] [PubMed] [Google Scholar]
- 18. Odds F. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 19. Pancham S, et al. 2005. Caspofungin for invasive fungal infections: combination treatment with liposomal amphotericin B in children undergoing hemopoietic stem cell transplantation. Pediatr. Transplant. 9:254–257 [DOI] [PubMed] [Google Scholar]
- 20. Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. 2011. Echinocandin and triazole antifungal susceptibility profiles for Candida spp., Cryptococcus neoformans, and Aspergillus fumigatus: application of new CLSI clinical breakpoints and epidemiologic cutoff values to characterize resistance in the SENTRY Antimicrobial Surveillance Program (2009). Diagn. Microbiol. Infect. Dis. 69:45–50 [DOI] [PubMed] [Google Scholar]
- 21. Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pfaller MA, et al. 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 14:164–176 [DOI] [PubMed] [Google Scholar]
- 23. Pina-Vaz C, Rodrigues AG, et al. 2010. Evaluation of antifungal susceptibility using flow cytometry methods. Mol. Biol. 638:281–289 [DOI] [PubMed] [Google Scholar]
- 24. Pina-Vaz C, et al. 2001. Susceptibility to fluconazole of Candida clinical isolates determined by FUN-1 staining with flow cytometry and epifluorescence microscopy. J. Med. Microbiol. 50:375–382 [DOI] [PubMed] [Google Scholar]
- 25. Pina-Vaz C, et al. 2001. Cytometric approach for a rapid evaluation of susceptibility of Candida strains to antifungals. Clin. Microbiol. Infect. 7:609–618 [DOI] [PubMed] [Google Scholar]
- 26. Pound MW, Townsend ML, Drew RH. 2010. Echinocandin pharmacodynamics: review and clinical implications. J. Antimicrob. Chemother. 65:1108–1118 [DOI] [PubMed] [Google Scholar]
- 27. Ramani R, Ramani A, Wong SJ. 1997. Rapid flow cytometric susceptibility testing of Candida albicans. J. Clin. Microbiol. 35:2320–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richardson MD. 2005. Changing patterns and trends in systemic fungal infections. J. Antimicrob. Chemother. 56(Suppl 1):i5–i11 [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez MM, et al. 2007. In vitro interaction of micafungin and fluconazole against Candida. J. Antimicrob. Chemother. 60:188–190 [DOI] [PubMed] [Google Scholar]
- 30. Rudensky B, et al. 2005. Rapid flow-cytometric susceptibility testing of Candida species. J. Antimicrob. Chemother. 55:106–109 [DOI] [PubMed] [Google Scholar]
- 31. Sabin C, Petrie A. 2000. Medical statistics at a glance. Blackwell Science, Malden, MA [Google Scholar]
- 32. Serena C, et al. 2008. In vitro interactions of micafungin with amphotericin B against clinical isolates of Candida spp. Antimicrob. Agents Chemother. 52:1529–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shao PL, Huang LM, Hsueh PR. 2007. Recent advances and challenges in the treatment of invasive fungal infections. Int. J. Antimicrob. Agents 30:487–495 [DOI] [PubMed] [Google Scholar]
- 34. Tortorano AM, et al. 2006. Candidaemia in Europe: epidemiology and resistance. Int. J. Antimicrob. Agents 27:359–366 [DOI] [PubMed] [Google Scholar]
- 35. White RL, Burgess DS, Manduru M, Bosso JA. 1996. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 40:1914–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yeh PJ, Hegreness MJ, Aiden AP, Kishony R. 2009. Drug interactions and the evolution of antibiotic resistance. Nat. Rev. Microbiol. 7:460–466 [DOI] [PMC free article] [PubMed] [Google Scholar]