Abstract
We describe 3 patients with left-sided staphylococcal endocarditis (1 with methicillin-susceptible Staphylococcus aureus [MSSA] prosthetic aortic valve endocarditis and 2 with methicillin-resistant S. aureus [MRSA] native-valve endocarditis) who were successfully treated with high-dose intravenous daptomycin (10 mg/kg/day) plus fosfomycin (2 g every 6 h) for 6 weeks. This combination was tested in vitro against 7 MSSA, 5 MRSA, and 2 intermediately glycopeptide-resistant S. aureus isolates and proved to be synergistic against 11 (79%) strains and bactericidal against 8 (57%) strains. This combination deserves further clinical study.
TEXT
Daptomycin is approved for the treatment of skin and soft tissue infections, Staphylococcus aureus bacteremia, and right-sided native-valve endocarditis (3). There are, however, few data on the efficacy of daptomycin in the treatment of left-sided native-valve endocarditis caused by S. aureus. In a randomized clinical trial, only 1 (11%) of 9 patients treated with intravenous (i.v.) daptomycin at 6 mg/kg daily was cured (9). In a registry of patients at 45 U.S. institutions who received daptomycin for any indication, a successful clinical outcome was achieved in 12 (63%) of 19 cases of left-sided endocarditis caused by S. aureus (14).
The efficacy of daptomycin in left-sided native- or prosthetic-valve S. aureus endocarditis may be improved either by increasing the dose to 10 to 12 mg/kg daily or by achieving synergy by the addition of a second agent. The addition of gentamicin or rifampin did not, however, increase the activity of daptomycin in a rabbit model of methicillin-resistant S. aureus (MRSA) endocarditis (18). While fosfomycin is FDA approved only for the treatment of uncomplicated urinary tract infections, it has demonstrated good antimicrobial activity against a broad spectrum of pathogens, including methicillin-susceptible S. aureus (MSSA) and MRSA (8). Fosfomycin, which acts by inhibition of an early step in cell wall synthesis, has been used successfully in combination with beta-lactams to treat severe staphylococcal infections (22, 23). It also shows in vitro synergy when combined with daptomycin (4).
We describe 3 patients with S. aureus endocarditis who were successfully treated with the combination of high-dose daptomycin and fosfomycin, and we present evidence of in vitro synergy between these 2 agents.
(These data were presented in part at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA, 12 to 15 September 2009 [abstr. E-1449].)
The 3 cases are summarized in Table 1. One had MSSA aortic prosthetic-valve endocarditis, and two had MRSA native-valve endocarditis. They were successfully treated with high-dose daptomycin (i.v. at 10 mg/kg/day) plus fosfomycin (i.v. at 2 g every 6 h) for 6 weeks. All 3 patients had multiple extracardiac septic emboli, and two had small perivalvular abscesses. In 2 cases, 7 to 10 days of i.v. high-dose daptomycin failed to produce sterile blood cultures. The third patient (MRSA mitral valve endocarditis) remained bacteremic after 5 days of vancomycin (trough level of 20 μg/ml at day 5); vancomycin was switched to high-dose daptomycin plus fosfomycin because of nephrotoxicity. All isolates were susceptible to daptomycin and fosfomycin. No clinical or biological side effects were observed. None of the patients needed cardiac surgery.
Table 1.
Clinical characteristics and outcomes of 3 patients with S. aureus endocarditis treated with a combination of high-dose daptomycin and fosfomycin
| Parameter(s) | Patient 1 | Patient 2a | Patient 3 |
|---|---|---|---|
| Age (yr) | 54 | 53 | 71 |
| Gender | Female | Male | Male |
| Chronic underlying diseases | Scleroderma, severe pulmonary artery hypertension, elective aortic and mitral double-valve replacement with mechanical prostheses 11 yr ago, MRSEc prosthetic-valve endocarditis 10 years ago needing an aortic valve homograft | Diabetes mellitus, HIV-HBVb coinfection | Diabetes mellitus, diabetic foot infection, Child-Pugh class B liver cirrhosis, mild mitral regurgitation due to severe valve calcification |
| Drug allergies | Penicillin, vancomycin | None | None |
| Source of infection | Unknown | Left inguinal infection by MRSA 2 weeks after lymph node biopsy | MRSA-infected foot ulcer |
| Clinical characteristics | High-grade fever, cerebral and splenic emboli, coma, additive EuroSCOREe of 18 | High-grade fever, malaise, pain in neck and index finger of left hand | High-grade fever, splenic emboli |
| Blood cultures before treatment | 6/6 positive for MSSA | 6/6 positive for MRSA | 6/6 positive for MRSA |
| Antibiotic susceptibilities (MIC, μg/ml) | Vancomycin, 1; fosfomycin, 8; daptomycin, 1 | Vancomycin, 1.5; fosfomycin, ≤ 32; daptomycin, 1 | Vancomycin, 1.5; fosfomycin, ≤ 32; daptomycin, 1 |
| TEEd findings | 1-cm aortic homograft valve vegetation, 10 by 8 mm perivalvular abscess, no prosthetic valve dysfunction | Calcification of aortic and mitral valves, no evidence of vegetations or perivalvular abscesses | Mitral valve rupture with severe regurgitation, 1-cm mitral valve vegetation, 6 by 8 mm perivalvular abscess |
| Initial i.v. antibiotic therapy (no. of days) | Daptomycin at 10 mg/kg/day (7) | Daptomycin at 4 mg/kg/day (2), daptomycin at 4 mg/kg/day plus gentamicin at 240 mg/48 h (5), daptomycin at 8 mg/kg/day plus gentamicin at 240 mg/48 h (3) | Vancomycin at 15 mg/kg every 12 h; vancomycin trough plasma concentrations, 11 and 20 μg/ml on days 2 and 5, respectively |
| Complications of first-line treatment | None | Progressive renal failure; need for hemodialysis; signs of septic embolization along left sternal margin and on first left metacarpal joint, second and third right toes, right forefoot, and left sternocleidomastoid muscle at day 2; pyomyositis with compression of oropharynx at day 10 | Serum creatinine increase from 1.4 to 3.0 mg/dl (normal, ≤1.3 mg/dl) during first 24 h but return to normal level after 2 days |
| Follow-up blood cultures after initial treatment | Positive at days 3, 7 | Positive at days 3, 7, 10 | Positive at day 5 |
| No. of days from initial treatment to switch to daptomycin plus fosfomycin | 7 | 10 | 5 |
| Doses given i.v. (daptomycin, fosfomycin) | 10 mg/kg/day, 2 g/6 h | 10 mg/kg/day, 2 g/6 h | 10 mg/kg/day, 2 g/6 h |
| Time of first negative blood cultures after switch to daptomycin plus fosfomycin (h) | 72 | 48 | 48 |
| Duration of treatment (wk) | 8 | 6 | 6 |
| Complications of daptomycin plus fosfomycin treatment | None | Transmetatarsal amputation of right foot, chronic hemodialysis | None |
| Follow-up blood cultures after daptomycin plus fosfomycin | Negative at days 14, 42 | Negative at days 14, 28, 42, 90 | Negative at days 30, 90 |
| Clinical follow-up | Alive at 1 yr | Alive at 1 yr | Alive at 6 mo |
We used seven MSSA strains and seven MRSA strains to perform in vitro studies (Table 2). MICs were determined by a microdilution method according to the procedures of the Clinical and Laboratory Standards Institute (2). Time-kill methodology was used to test the activity of daptomycin plus fosfomycin against MSSA and MRSA strains according to previously described criteria (20). Viability counts were performed at 0, 4, 8, and 24 h (12). Synergy, indifference, antagonism, and bactericidal activity were defined as previously described (18).
Table 2.
S. aureus strains tested and MICs
| Straina | MIC (μg/ml) |
||
|---|---|---|---|
| Daptomycin | Fosfomycin | Vancomycin | |
| MSSA 1112 | 0.5 | 8 | 1 |
| MSSA P3 | 0.5 | 4 | 1 |
| MSSA P4 | 0.5 | 8 | 1 |
| MSSA P7 | 0.5 | 8 | 1 |
| MSSA 4297 | 0.5 | 1 | 1 |
| MSSA RN4220 | 0.5 | 4 | 0.5 |
| MSSA 678 | 1 | 8 | 1 |
| MRSA 277 | 0.25 | 4 | 2 |
| MRSA P8 | 0.5 | 4 | 1 |
| MRSA 2167 | 0.5 | 16 | 2 |
| MRSA 4194 | 0.25 | 8 | 1 |
| MRSA 726 | 0.25 | 16 | 0.5 |
| GISA PC3 | 2 | 8 | 8 |
| GISA ATCC 700788 | 0.5 | 16 | 8 |
All isolates were stored at −80°C in skim milk prior to testing. Daptomycin susceptibility testing was performed in Mueller-Hinton broth (MHB) adjusted to 50 μg/ml calcium in accordance with the standard methodology. Fosfomycin susceptibility testing was performed in MHB supplemented with 25 μg/ml glucose-6-phosphate in accordance with the standard methodology. S. aureus ATCC 29213 was used as the control strain. MSSA RN4220, GISA PC3, and GISA ATCC 700788 were used as reference strains. The remaining 11 strains were isolated from the blood of patients with S. aureus endocarditis (1 isolate per patient). MSSA 678 was from case 1, and MRSA 726 was from case 3 (see Table 1).
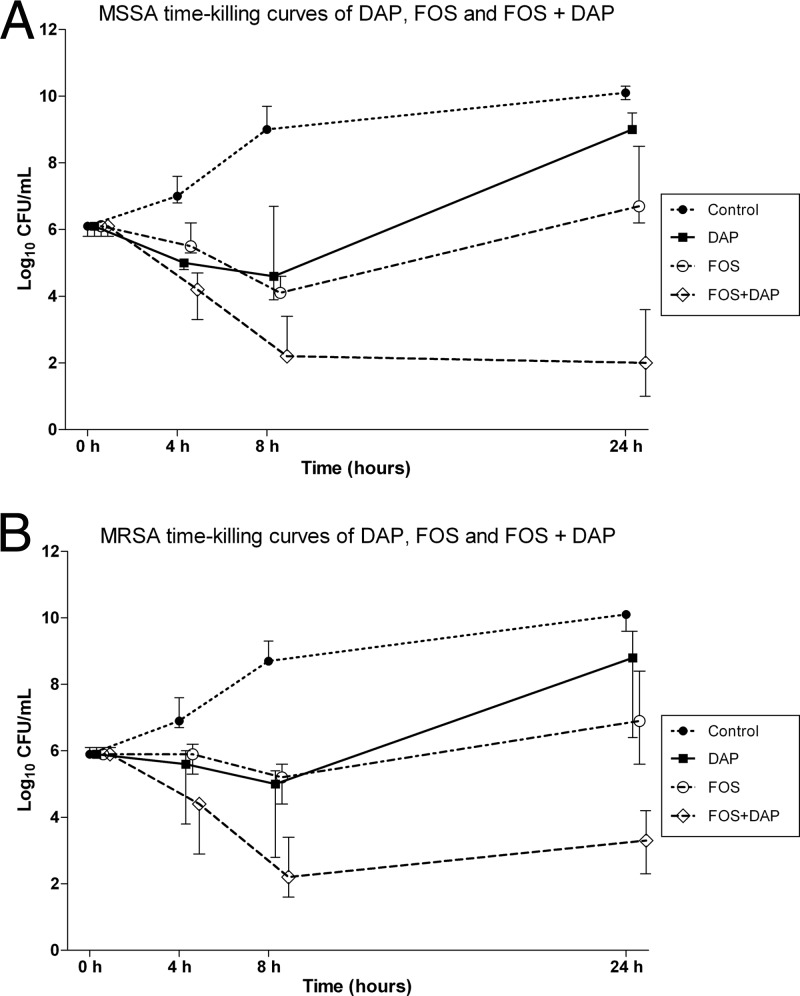
MIC data are presented in Table 2. Two of the MRSA strains, glycopeptide intermediately resistant S. aureus (GISA) PC3 and ATCC 700788, had only intermediate susceptibility to glycopeptides, with a vancomycin MIC of 8 mg/liter. Time-kill data are presented in Table 3 and Fig. 1. The combination of daptomycin and fosfomycin was synergistic against all 7 MSSA strains and was bactericidal against 5 of them at 8 and 24 h (Table 3). Daptomycin and fosfomycin demonstrated synergy against 3 of the 5 MRSA strains and bactericidal activity against 2 of them. Against 1 of the 2 GISA strains, ATCC 700788, daptomycin and fosfomycin demonstrated both synergy and bactericidal activity at 24 h; against strain PC3, the combination was both synergistic and bactericidal at 4 and 8 h but only synergistic at 24 h (Table 3).
Table 3.
MSSA and MRSA time-kill curves at antibiotic MICsa
| Strain (baseline [0 h] log10 no. of CFU/ml) | Change in log10 CFU/ml |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Fosfomycin |
Daptomycin |
Fosfomycin + daptomycin |
|||||||||
| 4 h | 8 h | 24 h | 4 h | 8 h | 24 h | 4 h | 8 h | 24 h | 4 h | 8 h | 24 h | |
| MSSA strains | ||||||||||||
| MSSA 1112 (6.1) | +0.9 | +3.6 | +4.1 | +0.1 | −2.1 | +0.4 | −1.2 | −1.1 | +2.9 | −1.7 | −3.9 | −2.5 |
| MSSA P4 (5.8) | +2 | +4.1 | +4.6 | −0.3 | −1.7 | +1.2 | −0.8 | −1.1 | +3.7 | −4.2 | −4.8 | −4.8 |
| MSSA P5 (6) | +0.8 | +3.6 | +4.3 | −0.5 | −2 | +3.5 | −2.2 | −2.4 | +3.4 | −2.7 | −3.9 | −5 |
| MSSA P7 (6.1) | +0.9 | +2.9 | +4 | −0.8 | −2.5 | +0.1 | −0.8 | +0.6 | +2.9 | −1.9 | −3.7 | −3.3 |
| MSSA 4297 (6.3) | +0.7 | +2.7 | +3.8 | −0.2 | −1.3 | +2.2 | −1.3 | −2.4 | +2.7 | −1.6 | −2.9 | −1.7 |
| MSSA RN4220 (5.7) | +0.8 | +3.1 | +4.2 | −5.4 | −1.1 | −0.7 | −1.2 | −2.4 | +3.8 | −1.8 | −3.6 | −4.7 |
| Patient 1 MSSA 678 (6.1) | +1.5 | +2.9 | +3 | +0.1 | −1.5 | +0.6 | −1 | −2 | +2.6 | −1.1 | −2.1 | −4.1 |
| MRSA strains | ||||||||||||
| MRSA 277 (5.6) | +1.1 | +3 | +4.5 | +0.2 | −0.3 | +3 | −0.8 | −1.1 | +4 | −1.2 | −3.4 | −2.3 |
| MRSA P8 (6.1) | +1.5 | +3.4 | +4.1 | −0.8 | −1.8 | −1.2 | −2.3 | −3.3 | −1.1 | −3.4 | −4.5 | −3.2 |
| MRSA 2167 (6) | +1 | +3.3 | +4.3 | −0.7 | −1.5 | +0.2 | −2.2 | −3.2 | +0.4 | −3 | −3.6 | −1.8 |
| MRSA 4194 (5.9) | +1 | +3.4 | +4.2 | +0.5 | +0.7 | +1 | +0.1 | −0.9 | +3.1 | 0 | −1.4 | −1.8 |
| Patient 3 MRSA 726 (6.1) | +1.5 | +2.6 | +2.9 | −0.2 | −1.7 | −0.5 | +0.6 | +1.9 | +2.7 | −1.6 | −2.7 | −4.1 |
| GISA PC3 (5.9) | +0.5 | +2.1 | +4.1 | +0.2 | −0.3 | +1.9 | −0.1 | −0.9 | +2.5 | −3 | −4.6 | −1.5 |
| GISA ATCC (5.9) | +1 | +2.7 | +4 | +0.3 | −0.7 | +2.5 | −0.3 | −0.5 | +3.8 | −1.4 | −4 | −3.6 |
Antibiotics were used alone and in combination at the MIC of each drug for the organism. If the MIC of a single drug eliminated viable organisms at 24 h, a concentration of 0.5 times the MIC was used so that the effect of a single drug would not obscure that of a drug combination. Daptomycin was tested at 0.5 times the MIC for two MSSA strains (1112 and P7) and one MRSA strain (726). Synergy was defined as a ≥2-log10 decrease in the number of CFU/ml between the combination and the most active agent tested alone and as a ≥2-log10 decrease in the colony count from the starting inoculum after 8 or 24 h. Indifference was defined as a <2-log10 change (increase or decrease) in the number of CFU/ml after 24 h between the combination and the most active agent tested alone. Antagonism was defined as a ≥2-log10 increase in the number of CFU/ml after 8 or 24 h between the combination and the most active agent tested alone. Bactericidal activity was defined as a decrease of ≥3 log10 CFU/ml after 24 h of incubation. The lower limit of detection for time-kill assays was 1 log10 CFU/ml.
Fig 1.
Time-kill curves of fosfomycin (FOS), daptomycin (DAP), and FOS plus DAP for 7 MSSA (A) and 7 MRSA (B) strains. Each result is expressed as a median and the interquartile range. Antibiotic concentrations were tested at the MIC, except for 2 MSSA strains (1112 and P7), where DAP was tested at 0.5 times the MIC.
Infective endocarditis caused by S. aureus causes considerable morbidity and mortality (10, 17). Treatment options, particularly for cases caused by MRSA, remain limited. The recently issued Infectious Diseases Society of America guidelines (15) recommend 6 weeks of either vancomycin or daptomycin at 6 mg/kg daily for the treatment of native-valve endocarditis caused by MRSA; the use of higher doses of daptomycin (8 to 10 mg/kg daily) were also considered. Along with increased doses, the addition of a second agent may provide synergy and thus enhance efficacy. Fosfomycin, a phosphonic acid derivative first identified in 1969 (11), has good tissue penetration and low protein binding and is available in an i.v. formulation (23). While it has demonstrated in vitro synergy in combination with a number of antibacterial agents, including daptomycin, against a variety of Gram-positive and -negative organisms (21–23), data on the use of the combination in patients are limited.
The initial failure of high-dose daptomycin (cases 1 and 2) and of vancomycin (case 3) may be explained by the high inoculum associated with disseminated staphylococcal infections and the difficulty of achieving adequate drug levels in sequestered foci of infection such as valve vegetations and abscesses. Therefore, these patients may have been ideal candidates for the synergistic action of two agents. Similar clinical conditions were identified by Fowler et al. (9) in those patients who had microbiological failure on daptomycin. While we did not see an increase in daptomycin MICs in our cases, the combination of daptomycin and fosfomycin has also proven effective, both in vitro and clinically, against daptomycin-nonsusceptible isolates of S. aureus (1, 13). These two agents interfere with different steps; fosfomycin inhibits peptidoglycan (cell wall) synthesis, while daptomycin acts via membrane depolarization (5, 19). Although the exact mechanism of synergy is unknown, it could involve a decrease in the positive surface charge of S. aureus, which in turn favors daptomycin membrane binding. This mechanism of action was demonstrated in vitro by Dhand et al. (6) in a daptomycin-nonsusceptible MRSA strain (MIC, 2 to 4 mg/liter) using the combination of daptomycin plus nafcillin, which is also an anti-staphylococcal cell wall agent.
In conclusion, the 3 cases discussed here and the in vitro results provide encouraging evidence that the combination of high doses of daptomycin plus fosfomycin can be effective in the treatment of both native- and prosthetic-valve endocarditis caused by MSSA or MRSA. This combination deserves further clinical study.
ACKNOWLEDGMENTS
The following (including some of the authors) are members of the Hospital Clínic Endocarditis Study Group, Hospital Clínic—IDIBAPS, University of Barcelona School of Medicine, Barcelona, Spain: José M. Miró, Asuncion Moreno, Ana del Río, Carlos Cervera, Juan M. Pericas, Ximena Castañeda, Jose M. Gatell (Infectious Diseases Service), Francesc Marco, Cristina Garcia de la Mària, Yolanda Armero, Manel Almela, Maria T. Jiménez-de-Anta (Microbiology Service), Carlos A. Mestres, Juan C. Paré, Carlos Falces, Ramón Cartañá, Salvador Ninot, Manel Azqueta, Marta Sitges, Magda Heras, José L. Pomar (Cardiovascular Institute), Jose Ramírez, Teresa Ribalta (Pathology Department), Merce Brunet (Toxicology Service), Dolors Soy (Pharmacy Service), and Iñaki Perez (statistician).
This work was supported in part by grants from the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (Madrid, Spain), the Spanish Network for Research in Infectious Diseases (REIPI RD06/0008), Fondo de Investigaciones Sanitarias (FIS; Madrid, Spain) grants FIS 080268 and FIS 1101131, and Fundación Máximo Soriano Jiménez (Barcelona, Spain). X.C. received an Academic Grant from Fundación Carolina-BBVA (Madrid, Spain) for an elective stay at our center during 2009 and 2010. In 2011, J.M.M. received an INT10/219 Intensification Research Grant (I3SNS and PRICS programs) from the Instituto de Salud Carlos III (Madrid, Spain) and the Departament de Salut de la Generalitat de Catalunya, Barcelona (Spain). M.V. has an Intensification Research Grant from the Instituto de Salud Carlos III (Madrid, Spain) and the Agencia Pedro Laín Entralgo (Madrid, Spain).
J.M.M. has received consulting honoraria and/or research grants from Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Cubist, Novartis, GlaxoSmithKline, Gilead Sciences, Pfizer, Roche, and Theravance. F.M has received consulting honoraria from Novartis and Janssen-Cilag. The rest of us have no conflicts of interest.
Footnotes
Published ahead of print 29 May 2012
REFERENCES
- 1. Chen LY, et al. 2011. High-dose daptomycin and fosfomycin treatment of a patient with endocarditis caused by daptomycin-nonsusceptible Staphylococcus aureus: case report. BMC Infect. Dis. 11:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clinical and Laboratory Standards Institute 2006. Performance standards for antimicrobial disk susceptibility tests; approved standard. Ninth edition Clinical and Laboratory Standards Institute, Wayne PA [Google Scholar]
- 3. Cubist Pharmaceuticals, Inc 2010. Cubicin (daptomycin for injection) prescribing information. Cubist Pharmaceuticals, Inc., Lexington, MA [Google Scholar]
- 4. Debbia E, Pesce A, Schito GC. 1988. In vitro activity of LY146032 alone and in combination with other antibiotics against gram-positive bacteria. Antimicrob. Agents Chemother. 32:279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dengler V, Meier PS, Heusser R, Berger-Bächi B, McCallum N. 2011. Induction kinetics of the Staphylococcus aureus cell wall stress stimulon in response to different cell wall active antibiotics. BMC Microbiol. 11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhand A, et al. 2011. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin. Infect. Dis. 53:158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. EuroSCORE Accessed 15 October 2011 http://www.euroscore.org/ EuroSCORE, Cambridge, United Kingdom [Google Scholar]
- 8. Falagas ME, Roussos N, Gkegkes ID, Rafailidis PI, Karageorgopoulos DE. 2009. Fosfomycin for the treatment of infections caused by Gram-positive cocci with advanced antimicrobial drug resistance: a review of microbiological, animal and clinical studies. Expert Opin. Investig. Drugs 18:921–944 [DOI] [PubMed] [Google Scholar]
- 9. Fowler VG, Jr, et al. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653–665 [DOI] [PubMed] [Google Scholar]
- 10. Fowler VG, Jr, et al. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021 [DOI] [PubMed] [Google Scholar]
- 11. Hendlin D, et al. 1969. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science 166:122–123 [DOI] [PubMed] [Google Scholar]
- 12. Isenberg HD. 2004. Time-kill assay, p 5.10.2.1–5.10.2.12 In Isenberg H. D. (ed), Clinical microbiology procedures handbook. ASM Press, Washington, DC [Google Scholar]
- 13. Joukhadar C, et al. 2010. Bactericidal effects of fosfomycin against daptomycin resistant strains of Staphylococcus aureus, abstr. E-1573b. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA [Google Scholar]
- 14. Levine DP, Lamp KC. 2007. Daptomycin in the treatment of patients with infective endocarditis: experience from a registry. Am. J. Med. 120(10 Suppl 1):S28–S33 [DOI] [PubMed] [Google Scholar]
- 15. Liu C, et al. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55 [DOI] [PubMed] [Google Scholar]
- 16. Mestres CA, et al. 2007. Preoperative risk stratification in infective endocarditis. Does the EuroSCORE model work? Preliminary results. Eur. J. Cardiothorac. Surg. 32:281–285 [DOI] [PubMed] [Google Scholar]
- 17. Miró JM, et al. 2005. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin. Infect. Dis. 41:507–514 [DOI] [PubMed] [Google Scholar]
- 18. Miró JM, et al. 2009. Addition of gentamicin or rifampin does not enhance the effectiveness of daptomycin in treatment of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:4172–4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muthaiyan A, Silverman JA, Jayaswal RK, Wilkinson BJ. 2008. Transcriptional profiling reveals that daptomycin induces the Staphylococcus aureus cell wall stress stimulon and genes responsive to membrane depolarization. Antimicrob. Agents Chemother. 52:980–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pillai SK, Moellering RCJ, Eliopoulos GM. 2005. Antimicrobial combinations, p 365–440 In Lorian V. (ed), Antibiotics in laboratory medicine, fifth edition Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 21. Popovic M, Steinort D, Pillai S, Joukhadar C. 2010. Fosfomycin: an old, new friend? Eur. J. Clin. Microbiol. Infect. Dis. 29:127–142 [DOI] [PubMed] [Google Scholar]
- 22. Portier H, et al. 1985. Cefotaxime in combination with other antibiotics for the treatment of severe methicillin-resistant staphylococcal infections. Infection 13(Suppl 1):S123–S128 [DOI] [PubMed] [Google Scholar]
- 23. Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. 2009. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int. J. Antimicrob. Agents 34:506–515 [DOI] [PubMed] [Google Scholar]