Abstract
In previous experiments, replacing the 10-mg/kg of body weight daily dose of rifampin with 7.5 to 10 mg/kg of rifapentine in combinations containing isoniazid and pyrazinamide reduced the duration of treatment needed to cure tuberculosis in BALB/c mice by approximately 50% due to rifapentine's more potent activity and greater drug exposures obtained. In the present study, we performed dose-ranging comparisons of the bactericidal and sterilizing activities of rifampin and rifapentine, alone and in combination with isoniazid and pyrazinamide with or without ethambutol, in BALB/c mice and in C3HeB/FeJ mice, which develop necrotic lung granulomas after infection with Mycobacterium tuberculosis. Each rifamycin demonstrated a significant increase in sterilizing activity with increasing dose. Rifapentine was roughly 4 times more potent in both mouse strains. These results reinforce the rationale for ongoing clinical trials to ascertain the highest well-tolerated doses of rifampin and rifapentine. This study also provides an important benchmark for the efficacy of the first-line regimen in C3HeB/FeJ mice, a strain in which the lung lesions observed after M. tuberculosis infection may better represent the pathology of human tuberculosis.
INTRODUCTION
The rifamycins are key sterilizing drugs in the treatment of tuberculosis (TB) (2). Addition of rifampin (RIF, or R) to regimens based on isoniazid (INH, or H) and streptomycin reduced the duration of treatment necessary for cure of human TB from 18 or more months to 9 months, before the subsequent addition of pyrazinamide (PZA, or Z) reduced it to 6 months (3, 4, 12, 13, 43). Data from an in vitro hollow fiber model (19), animal models (22, 49), and clinical studies (8, 23, 45) indicate RIF has dose-dependent bactericidal activity that is not optimized at the currently recommended 10-mg/kg of body weight (600 mg maximum) dose for adults (2). It follows that increasing rifamycin exposures may increase bactericidal and sterilizing activities and further shorten the duration of TB treatment (35). To this end, we have previously shown that replacing the 10-mg/kg daily dose of RIF with a 7.5- or 10-mg/kg daily dose of rifapentine (RPT, or P) in combination with INH and PZA reduces the duration of treatment needed to cure TB in mice by approximately 50%, owing to RPT's more potent antituberculosis activity and greater drug exposures obtained at a given dose (in mg/kg) (40, 41). A subsequent dose escalation trial in healthy volunteers showed daily RPT doses as high as 20 mg/kg were well tolerated (9). Daily regimens using RPT doses of 7.5 to 20 mg/kg in place of RIF are now under evaluation for efficacy, safety, and tolerability in at least 3 clinical trials (clinicaltrials.gov identifiers NCT00694629, NCT00728507, and NCT00814671).
Unlike RPT, RIF is already in use throughout the world for treatment of TB. Despite 40 years of clinical use, studies to define the highest well-tolerated daily dose are only now under way (clinicaltrials.gov identifiers NCT00760149, NCT01392911, and NCT01408914). To determine whether increases in the RIF dose result in improved sterilizing activity comparable to that observed with RPT and, if so, at which dose level, we conducted an experiment to determine the equipotent daily doses of RIF and RPT in combination with INH and PZA in the commonly used BALB/c mouse strain.
Although mice have served as a model for the evaluation of TB drug efficacy for more than 50 years, the lung pathology of Mycobacterium tuberculosis infection in BALB/c mice and other commonly used mouse strains does not resemble the typical lung pathology encountered in human TB (31). Whereas human TB is characterized by caseation necrosis and necrotizing granulomatous inflammation, wherein the majority of infecting tubercle bacilli reside extracellularly, such necrosis is not observed in mouse lungs, where virtually all tubercle bacilli reside inside phagocytic cells (15). Because RPT accumulates inside macrophages to a 5- to 6-fold-greater extent than RIF (30), it stands to reason that reliance on a model which disproportionately emphasizes intracellular infection in the absence of necrosis could lead to overestimation of the efficacy of RPT relative to RIF. Furthermore, the approximately 10-fold-higher protein binding of RPT over RIF may restrict the diffusion of free RPT into necrotic lesions to a greater extent than RIF (5, 39). Hypothesizing that pathological differences in experimental models influence the relative efficacies of RIF and RPT, we conducted a second experiment to compare equipotent daily doses of RIF and RPT, administered alone and in combination with INH, PZA, and ethambutol (EMB, or E), in BALB/c mice and in C3HeB/FeJ mice, a strain recently recognized to develop necrotic granulomas in response to M. tuberculosis infection (34).
(Some of the results of our studies have been previously reported in the form of oral presentations and a meeting abstract [17, 18, 47].)
MATERIALS AND METHODS
M. tuberculosis strain.
M. tuberculosis H37Rv was passaged in mice, subcultured in Middlebrook 7H9 (Fisher Scientific) supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC) complex (Becton Dickinson) and 0.05% Tween 80 (Sigma-Aldrich), and used for aerosol infection when the optical density at 600 nm was approximately 1.0.
Antimicrobials.
Drugs were obtained and stock solutions prepared as previously described (41). The MICs (in μg/ml) against M. tuberculosis H37Rv are 0.25 for RIF, 0.06 for RPT, and 0.1 for INH on 7H11 agar and 10 for PZA on Löwenstein-Jensen medium (pH 5.5) (16).
Pharmacokinetics of rifamycins and companion drugs in BALB/c and C3HeB/FeJ mice.
Steady-state rifamycin pharmacokinetics (PK) were assessed in uninfected 5- to 6-week-old female BALB/c mice (Charles River Laboratories). RIF doses of 10, 15, 20, and 40 mg/kg and RPT doses of 5, 10, and 20 mg/kg were administered by oral gavage, 5 days per week, for at least 2 weeks before mice were sacrificed at time points ranging from 0 to 48 h postdose for RIF and 0 to 168 h postdose for RPT. Drug concentrations in mouse serum were determined by validated high-performance liquid chromatography (HPLC) assays (41). Steady-state PK were assessed by using noncompartmental analysis, followed by modeling and simulations with a one-compartment open model with first-order elimination (WinNonlin; Pharsight, Cary, NC).
Single-dose serum PK profiles for RIF at 10 mg/kg, RPT at 10 mg/kg, INH at 10 mg/kg, and PZA at 150 mg/kg were determined in uninfected 5- to 6-week-old female C3HeB/FeJ mice (Jackson Laboratory) and compared to historical results for BALB/c mice (RIF, INH, and PZA) or contemporaneous BALB/c controls (RPT). Drug concentrations in mouse serum were determined by validated HPLC assays (32), and PK were evaluated as described above.
Aerosol infection.
Experiment 1 entailed a high-dose aerosol infection in which 496 6-week-old female BALB/c mice were aerosol infected with M. tuberculosis by using the Middlebrook inhalation exposure system (Glas-Col) and an undiluted log-phase broth culture. Mice were infected in five consecutive runs. Experiment 2 entailed a low-dose aerosol infection to enable sufficient time for the characteristic lung pathology to develop in each strain prior to treatment initiation. A total of 190 BALB/c and 190 C3HeB/FeJ mice, each 4 to 6 weeks old, were infected with a 1:50 dilution of a similar log-phase culture (2 runs per strain). All animal procedures were approved by the Johns Hopkins University Animal Care and Use Committee.
Chemotherapy.
Mice were block randomized by infection run into treatment groups. Treatment began 14 and 42 days after infection in experiments 1 and 2, respectively. Drugs were administered 5 days per week, by gavage.
The scheme of experiment 1 is presented in Table 1. Control mice in group 1 were left untreated in order to establish the baseline CFU counts and lethality of infection. To assess the impact of increasing RIF exposures in the first-line regimen, mice in groups 2, 3, 4, and 5 received RIF-INH-PZA (RHZ), where RIF was administered at 10, 15, 20, and 40 mg/kg, respectively. To assess the impact of increasing RPT exposures, mice in groups 6, 7, 8, and 9 received RPT-INH-PZA (PHZ), where RPT was administered at 5, 7.5, 10, and 20 mg/kg, respectively. Doses of INH and PZA were 25 and 150 mg/kg, respectively. Mice in groups 2 to 8 were treated for 12 weeks, whereas mice in group 9 were treated for 10 weeks. PZA was administered only during the first 8 weeks of treatment.
Table 1.
Scheme of experiment 1
| Treatment groupa | No. of mice sacrificed atb: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day −13 | Day 0 | Wk 2 | Wk 4 | Wk 8 | Wk 10 | Wk 12 | Wk 8 (+12) | Wk 10 (+12) | Wk 12 (+12) | |
| Untreated | 10 | 15 | 10c | |||||||
| RIF regimens | ||||||||||
| R10HZ | 5 | 5 | 5 | 5 | 5 | 15 | 15 | |||
| R15HZ | 5 | 5 | 5 | 5 | 5 | 15 | 15 | |||
| R20HZ | 5 | 5 | 5 | 5 | 5 | 15 | 15 | |||
| R40HZ | 5 | 5 | 5 | 5 | 5 | 15 | 15 | |||
| RPT regimens | ||||||||||
| P5HZ | 5 | 5 | 5 | 5 | 5 | 15 | 15 | |||
| P7.5HZ | 5 | 5 | 5 | 5 | 15 | 15 | 15 | |||
| P10HZ | 5 | 5 | 5 | 5 | 15 | 15 | 15 | |||
| P20HZ | 5 | 5 | 5 | 5 | 15 | 15 | ||||
Drugs were given orally at the following doses (in mg/kg): R at 10, 15, 20, and 40; P at 5, 7.5, 10, and 20; Z at 150; H at 25. All drugs were administered daily (5 of 7 days per week). Z was administered for the first 8 weeks of treatment.
The data for weeks 8 (+12), 10 (+12), and 12 (+12) are for mice that were sacrificed 12 weeks after completing 8, 10, or 12 weeks of treatment.
Ten untreated control mice were held for mortality observations beyond week 2.
The scheme of experiment 2 is presented in Table 2. Group 1 controls went untreated. To assess the dose-response relationship of RIF monotherapy, mice in groups 2, 3, and 4 received RIF at 10, 20, and 40 mg/kg, respectively. Group 5 received RHZ plus ethambutol (at 100 mg/kg), with RIF dosed at 10 mg/kg. Mice in groups 6, 7, and 8 received RPT monotherapy at 5, 10, and 20 mg/kg, respectively, and group 9 received PHZE, with RPT dosed at 10 mg/kg. E was added to the combinations evaluated in experiment 2 in order to mimic the drug combinations studied in 2 ongoing clinical trials (clinicaltrials.gov identifiers NCT00694629 and NCT00814671). Doses of INH and PZA were 10 and 150 mg/kg, respectively. This dose of INH produces a maximum concentration in serum (Cmax) and area under the concentration-time curve from 0 to 24 h (AUC0-24) values similar to those observed in humans with a rapid acetylator phenotype, whereas the higher dose employed in experiment 1 produces AUC0-24 values similar to those observed in slow acetylators (1). In order to provide a more conservative estimate of the contribution of INH to drug combinations under study, our laboratory has used the 10-mg/kg dose for combination experiments since 2009. Groups 2 to 4 and 6 to 8 were treated for 8 weeks. Groups 5 and 9 were treated for 16 and 12 weeks, respectively. PZA was administered only during the first 8 weeks of treatment.
Table 2.
Scheme of experiment 2 (performed in both BALB/c and C3HeB/FeJ mice)
| Treatment groupa | No. of mice sacrificed atb: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Wk −6 | Day 0 | Wk 4 | Wk 8 | Wk 4 (+12) | Wk 8 (+12) | Wk 12 (+12) | Wk 16 (+12) | |
| Untreated | 6 | 6 | 5 | 5 | ||||
| RIF regimens | ||||||||
| R10 | 5 | 5 | ||||||
| R20 | 5 | 5 | ||||||
| R40 | 5 | 5 | ||||||
| R10HZE | 5 | 5 | 15 | 15 | 15 | |||
| RPT regimen | ||||||||
| P5 | 5 | 5 | ||||||
| P10 | 5 | 5 | ||||||
| P20 | 5 | 5 | ||||||
| P10HZE | 5 | 5 | 15 | 15 | 15 | |||
Drugs were given orally at the following doses (in mg/kg): R, 10, 20, and 40; P at 5, 10, and 20; Z at 150; H at 10; E at 100. All drugs were administered daily (5 of 7 days per week). Z was administered for the first 8 weeks of treatment.
The data for weeks 4 (+12), 8 (+12), 12 (+12), and 16 (+12) are for mice that were sacrificed 12 weeks after completing 4, 8, 12, or 16 weeks of treatment.
Assessment of treatment efficacy.
Baseline lung log10 CFU counts were assessed the day after aerosol infection (day −13 [D−13] in experiment 1 and week −6 [W−6] in experiment 2) and at treatment initiation (day 0 [D0]). Treatment efficacy was assessed by comparing lung log10 CFU counts during treatment and by comparing the proportion of mice with culture-positive relapse 12 weeks after completion of treatment, as previously described (41). In experiment 1, CFU counts were compared after 2, 4, 8, 10, and 12 weeks of treatment, and relapse rates were compared after 8, 10, and 12 weeks of treatment. In experiment 2, CFU counts were compared after 4 and 8 weeks of treatment, and relapse rates were compared after 8, 12, and 16 weeks of treatment. Due to prior evidence of ready selection of drug-resistant mutants in C3HeB/FeJ mice, 20% of the undiluted lung homogenate from each mouse receiving rifamycin monotherapy was plated on 7H11 agar supplemented with RIF at 1 μg/ml.
Selection and analysis of rifamycin-resistant mutants.
For rifamycin-resistant mutants isolated from lung homogenates on RIF-containing plates, the extent of RIF resistance was determined. Colonies from each plate were scraped together, homogenized in a few drops of phosphate-buffered saline (PBS) by vortexing with glass beads, suspended in PBS, and inoculated onto 7H11 plates with RIF concentrations of 0, 0.25, 1, 4, 16, or 64 μg/ml.
Two representative colonies from each mouse were also subjected to mutation analysis following the method of Telenti et al. (48) with a few modifications. A 411-bp segment of rpoB, including the rifamycin resistance-determining region (RRDR), was amplified by PCR (GeneAmp PCR system 2700; Applied Bioystems), using 2 primer sets. The upstream primer was rpoB F (5′-TACGGTCGGCGAGCTGATCC). The downstream primer was rpoB R (5′-TACGGCGTTTCGATGAACC). The DNA was purified (using a QIAquick PCR purification kit [Qiagen]) and sequenced. The nucleotide sequences were analyzed by using the NCBI/BLAST/BLASTn program, with reference accession number NC_000962, Gene ID 888164.
Lung pathology.
To assess the pathological changes arising in each mouse strain in experiment 2, untreated mice were sacrificed 2 months into the treatment period (3.5 months after infection). One lung was homogenized for CFU counts, and the other was fixed in 10% neutral buffered formalin for histopathological assessment. After 1 week of fixation, lungs were sectioned and embedded in paraffin. Sections were cut to 5-μm thickness and placed on negatively charged glass slides. The sections were then stained with hematoxylin and eosin (H&E) and an acid-fast stain.
Statistical analysis.
Lung CFU counts were log10 transformed before analysis. Mean CFU counts were compared using one-way analysis of variance with Dunnett's posttest (Prism 4; GraphPad Software, San Diego, CA). The proportions of mice relapsing were compared using Fisher's exact test (STATA version 8.2; College Station, TX). Bonferroni's procedure was used to adjust the type I error rate for all multiple comparisons. All measures of statistical variation are expressed with standard deviations (SD).
RESULTS
Pharmacokinetics of rifamycins and companion drugs in BALB/c and C3HeB/FeJ mice.
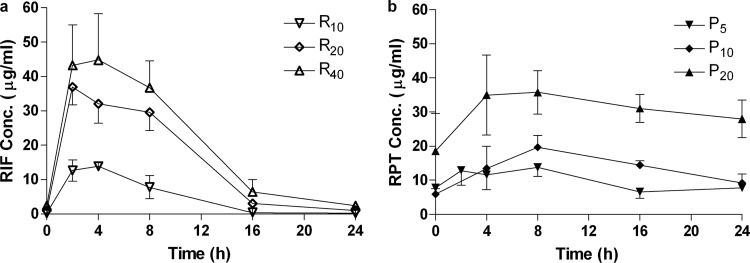
Rifamycin serum concentration-time curves determined after 2 weeks of dosing in BALB/c mice generally indicated dose-proportional PK (Fig. 1). Simulated PK parameters for R at 10 mg/kg (R10) and P at 7.5 or 10 mg/kg (P7.5 and P10, respectively) (Table 3) were comparable to those previously derived from single-dose data (41). Total drug AUC0–168 values for RPT were 2.53 times higher than for RIF at a given dose, leading to similar values for R20 and P7.5 and making R40 intermediate between P10 and P20 for this parameter. Cmax values were 1.37 times higher for RPT, with similar values for R10 and P7.5.
Fig 1.
Mean (± SD) serum drug concentrations of rifampin (a) and rifapentine (b) after 2 weeks of daily dosing (5 days per week). Sampling was performed after the 10th (R10), 11th (P10 and P20), or 13th (R20, R40, and P5) dose.
Table 3.
Simulated steady-state PK parameters for RIF and RPT in BALB/c mice
| Regimen | PK parameter for total drug |
|||
|---|---|---|---|---|
| Cmax (μg/ml) | t1/2 (h) | AUC0–24 (μg · h/ml) | AUC0–168 (μg · h/ml) | |
| R10 | 13.52 | 2.35 | 142.1 | 711 |
| R15 | 20.27 | 2.35 | 213.2 | 1,066 |
| R20 | 27.03 | 2.35 | 284.2 | 1,421 |
| R40 | 54.06 | 2.35 | 568.4 | 2,842 |
| P5 | 9.28 | 18.77 | 179.8 | 920 |
| P7.5 | 13.93 | 18.77 | 269.7 | 1,380 |
| P10 | 18.57 | 18.77 | 359.6 | 1,840 |
| P20 | 37.14 | 18.77 | 719.2 | 3,681 |
Single-dose PK for INH, PZA, and RIF in C3HeB/FeJ mice (see Fig. S1 in the supplemental material) were similar to data previously obtained for BALB/c mice (32, 39). Single-dose PK of RPT were similar in C3HeB/FeJ mice and in contemporaneous BALB/c controls (see Fig. S2 in the supplemental material).
Results of experiment 1.
On the day after infection (D−13), the mean CFU count (± SD) in the lungs was 3.53 ± 0.05 log10 CFU. By D0, the mean lung CFU count had increased to 7.52 ± 0.21, 7.48 ± 0.05, 7.57 ± 0.08, 7.38 ± 0.07, and 7.44 ± 0.08 log10 (P = 0.36) for mice infected during infection runs 1 through 5, respectively.
Lung CFU counts during treatment.
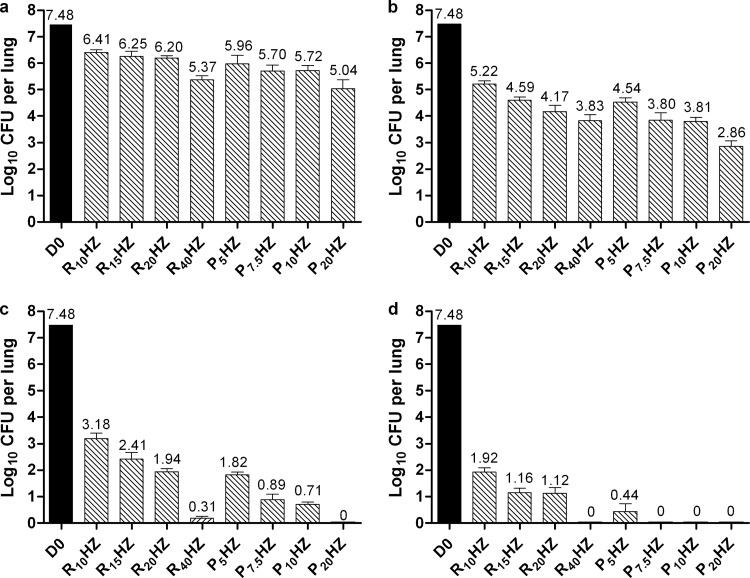
All PHZ regimens had significantly greater bactericidal activity than the control regimen of R10HZ at each time point (Fig. 2). Increasing the dose of R from 10 to 40 mg/kg in the RHZ regimen and the dose of P from 5 to 20 mg/kg in the PHZ regimen increased the bactericidal activity significantly. After 8 weeks of treatment, mice treated with P5HZ had lung CFU counts similar to mice treated with R20HZ, and mice treated with P7.5HZ and P10HZ had fewer than 10 CFU per lung, similar to mice treated with R40HZ (Fig. 2c). Only mice treated with P20HZ were culture negative.
Fig 2.
Mean (± SD) lung log10 CFU counts at treatment initiation (D0) and after 2 weeks (a), 4 weeks (b), 8 weeks (c), or 10 weeks (d) in experiment 1.
After 10 weeks of treatment, all mice treated with R40HZ, P7.5HZ, P10HZ, and P20HZ were culture negative, whereas all mice treated with R10HZ, R15HZ, and R20HZ had more than 1.0 log10 CFU per lung; mice treated with P5HZ had 0.44 log10 CFU per lung (Fig. 2d). Finally, after 12 weeks of treatment, the mean lung log10 CFU counts for mice receiving R10HZ and R15HZ were 1.08 and 0.69, respectively. Mice receiving R20HZ and P5HZ were culture negative with the exception of one mouse in each group, which harbored a single CFU in the entire lung homogenate.
Proportion of mice with culture-positive relapse.
After 8 weeks of treatment and 12 weeks of follow-up, all 15 mice receiving P7.5HZ or P10HZ had positive lung cultures (Table 4), consistent with the positive cultures observed from mice sacrificed at the completion of treatment (Fig. 2c). Only 6 (40%) of 15 mice treated with P20HZ relapsed.
Table 4.
Culture-positive relapse rates after treatment with escalating doses of RIF and RPT in combination with INH and PZA in experiment 1
| Treatment group | % (proportion) of mice relapsing after treatment for: |
||
|---|---|---|---|
| 8 wks | 10 wks | 12 wks | |
| RIF regimens | |||
| R10HZ | 100 (15/15) | 100 (15/15) | |
| R15HZ | 100 (15/15) | 87 (13/15) | |
| R20HZ | 100 (15/15) | 67 (10/15) | |
| R40HZ | 27 (4/15) | 0 (0/15) | |
| RPT regimens | |||
| P5HZ | 67 (10/15) | ||
| P7.5HZ | 100 (15/15) | 47 (7/15) | 13 (2/15) |
| P10HZ | 100 (15/15) | 33 (5/15) | 0 (0/15) |
| P20HZ | 40 (6/15) | 0 (0/15) | |
After 10 weeks of treatment, 7 (47%) and 5 (33%) of the 15 mice treated with P7.5HZ and P10HZ relapsed, respectively, whereas no mouse treated with P20HZ relapsed. On the other hand, mice treated with R10HZ, R15HZ, or R20HZ relapsed. Only 4 (27%) of the 15 mice treated with R40HZ relapsed, similar to the proportion observed with P10HZ.
After 12 weeks of treatment, no mouse receiving P10HZ or R40HZ relapsed, whereas 10 (67%) of the 15 mice treated with P5HZ or R20HZ relapsed. Nearly all mice treated with R10HZ (100%) or R15HZ (87%) relapsed.
Results of experiment 2.
On the day after infection (W−6), the mean log10 CFU counts were 2.00 ± 0.15 and 1.99 ± 0.11 in the lungs of BALB/c and C3HeB/FeJ mice, respectively. By D0, the mean lung counts had increased to 6.62 ± 0.16 and 7.29 ± 0.46 log10 CFU, respectively.
Lung CFU counts during treatment.
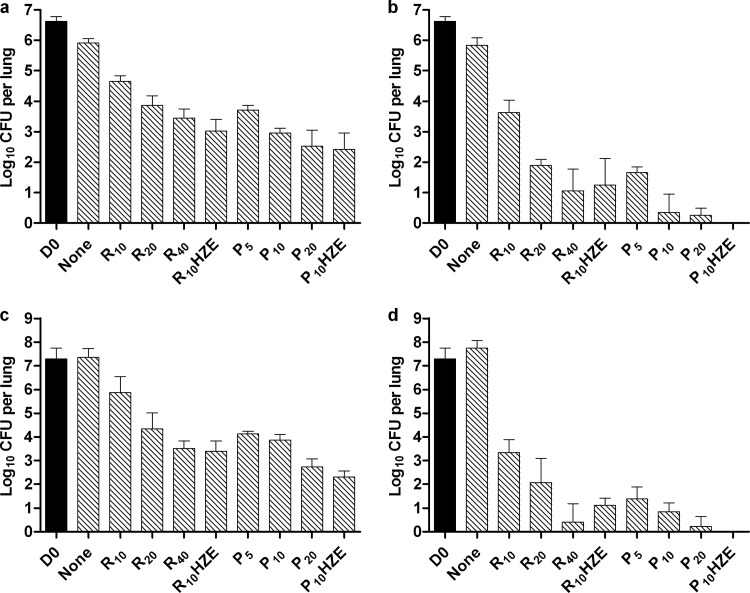
Dose-dependent activity was observed for each tested rifamycin in each mouse strain (Fig. 3). As previously described (20), lung CFU counts tended to increase in untreated C3HeB/FeJ mice (Fig. 3c and d), while decreasing in untreated BALB/c mice (Fig. 3a and b). Overall bactericidal activity, as measured by the reduction in CFU counts between day 0 and the end of treatment was, on average, 0.5 to 1.0 log10 greater in C3HeB/FeJ mice than in BALB/c mice. For each mouse strain at each time point, lung CFU counts were higher among mice receiving R10 than for all other monotherapy groups. In BALB/c mice, the activities of R20 and P5 were indistinguishable at 4 and 8 weeks, but P10 and P20 were more active than R20 at 4 weeks (P < 0.01 and <0.001, respectively) and at 8 weeks (P < 0.001). The activity of R40 was indistinguishable from that of P5 and P10, but inferior to that of P20 (P < 0.001), at 4 weeks and indistinguishable from all P doses at 8 weeks, at which time the mean CFU count approached 1 log10. These results were similar to results observed with combination therapy in experiment 1, confirming that RPT is roughly 4 times more potent than RIF in BALB/c mice.
Fig 3.
Mean (± SD) lung log10 CFU counts in BALB/c (a and b) or C3HeB/FeJ (c and d) mice at treatment initiation (D0) and after 4 weeks (a and c) or 8 weeks (b and d) in experiment 2.
In C3HeB/FeJ mice, the activity of R20 was indistinguishable from that of P5 or P10 at 4 weeks, but inferior to that of P10 (P < 0.05) at 8 weeks. P20 was superior to R20 at both time points (P < 0.001). The activity of R40 was superior to that of P5 (P < 0.05), not different from that of P10, and inferior to that of P20 (P < 0.01) at 4 weeks; it was indistinguishable from all P doses at 8 weeks, when the mean CFU count approached zero. Therefore, the superior potency of RPT over RIF is of a similar magnitude in both BALB/c and C3HeB/FeJ mice.
After 4 weeks of treatment, rifamycin resistance was detected in 2 of 5 C3HeB/FeJ mice receiving R10 monotherapy. The resistant subpopulation represented less than 1% of the total population in one mouse and less than 0.01% in the other. After 8 weeks of treatment, rifamycin resistance was detected in 3 of 5 C3HeB/FeJ mice receiving R10 monotherapy, 2 of 5 mice receiving R20, and 2 of 5 mice receiving P10. With the exception of one R10-treated mouse in which the resistant subpopulation represented approximately 0.1% of the total bacillary population, the resistant mutants had overgrown the susceptible population in these mice. Therefore, the CFU data for these mice were not included in Fig. 3d or in the analysis of bactericidal activity described in the preceding paragraph.
After 4 and 8 weeks of treatment, the 4-drug combination of PHZE produced lower CFU counts than RHZE in both mouse strains (P ≤ 0.05) (Fig. 3a to d). All 9 mice receiving PHZE for 8 weeks were culture negative, whereas only 1 BALB/c mouse receiving RHZE for 8 weeks was culture negative (P = 0.0004).
Proportion of mice with culture-positive relapse.
Treatment of BALB/c mice with RHZE for 8, 12, and 16 weeks resulted in relapse rates of 100%, 47%, and 13%, respectively (Table 5). The better outcomes with 12 weeks of RHZE in experiment 2 relative to the same duration of treatment with RHZ in experiment 1 were likely due to the lower bacterial burden at the start of treatment in experiment 2 rather than an additive sterilizing effect of E (55). A similar overall sterilizing activity of RHZE was observed in C3HeB/FeJ mice, in which 100%, 86%, and 7% of mice relapsed after 8, 12, and 16 weeks, respectively. The higher relapse rate among C3HeB/FeJ mice after 12 weeks of treatment also likely reflects a higher initial bacterial burden.
Table 5.
Culture-positive relapse rates after treatment with RIF- or RPT-containing combinations in BALB/c and C3HeB/FeJ mice in experiment 2
| Strain and treatment group | % (proportion) of mice relapsing after treatment for: |
|||
|---|---|---|---|---|
| 4 wks | 8 wks | 12 wks | 16 wks | |
| BALB/c mice | ||||
| R10HZE | 100 (15/15) | 47 (7/15) | 13 (2/15) | |
| P10HZE | 100 (14/14) | 7 (1/15) | 0 (0/15) | |
| C3HeB/FeJ mice | ||||
| R10HZE | 100 (15/15) | 86 (12/14) | 7 (1/15) | |
| P10HZE | 100 (13/13) | 21 (3/14) | 33 (5/15) | |
Four weeks of treatment with PHZE were not sufficient to prevent relapse in either mouse strain. On the other hand, 8 weeks of PHZE resulted in relapse in only 1 (7%) of 15 BALB/c mice and 3 (21%) of 14 C3HeB/FeJ mice (P < 0.0001 versus RHZE for both mouse strains). Moreover, 8 weeks of PHZE was more effective than 12 weeks of RHZE in both strains (P = 0.0352 and 0.0018 in BALB/c and C3HeB/FeJ mice, respectively).
After 12 weeks of treatment, relapse rates were roughly 50% lower in mice receiving PHZE compared to RHZE (P = 0.0063 and 0.0078 in BALB/c and C3HeB/FeJ mice, respectively). The proportion of C3HeB/FeJ mice relapsing after 12 weeks of PHZE was similar to the proportion observed after 8 weeks of treatment for unclear reasons. Only 1 of the 5 mice relapsing after 12 weeks of PHZE had more than 7 CFU in the entire lung homogenate, whereas the majority of those mice relapsing after 12 weeks of RHZE had more than 200 CFU per lung.
Mutational analysis of rifamycin-resistant mutants isolated from C3HeB/FeJ mice.
The RIF MIC was ≥64 μg/ml for each resistant strain isolated from mice receiving rifamycin monotherapy. RRDR mutational analysis revealed an S531→W531 (S531W) mutation in one strain and an S522→L522 (S522L) mutation in the other strain isolated from mice in the R10 group at 4 weeks. The same analysis revealed an H526→L526 (H526D) mutation in one strain isolated from one mouse in the R10 group at 8 weeks and an S531→L531 (S531L) mutation in both strains isolated from mice in the R20 group. The resistant strain isolated from one mouse that received 8 weeks of P10 monotherapy harbored an H526→R526 (H526R) mutation. The resistant isolates from two mice in the R10 group and one mouse in the P10 group were lost to contamination before they could be further analyzed.
Lung pathology.
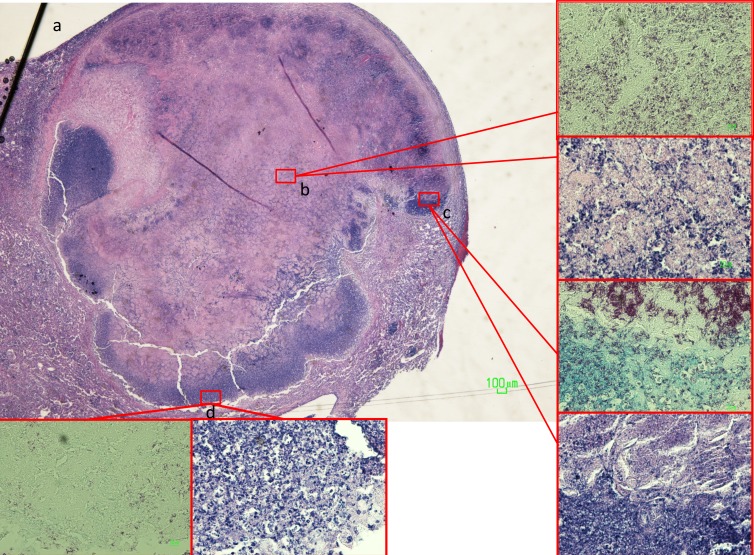
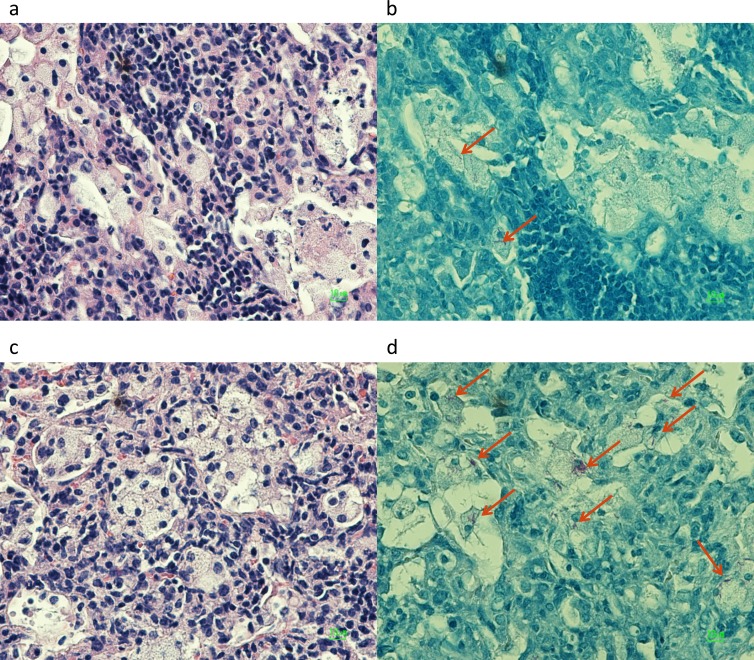
Conspicuous tubercles in C3HeB/FeJ mice demonstrated characteristic granulomas with central caseation and abundant extracellular acid-fast bacilli within the necrotic regions (7) (Fig. 4). Intracellular bacilli were found on the outer rim of the cellular cuff encompassing the granuloma. However, the lungs of both C3HeB/FeJ and BALB/c mice harbored similar zones of chronic inflammation with nests of foamy macrophages containing acid-fast bacilli (Fig. 5), as previously described for chronically infected mice (21, 56).
Fig 4.
(a and b) Histopathology of a necrotic granuloma (a) in untreated mouse lung 3.5 months after low-dose challenge with M. tuberculosis, demonstrating central caseation with abundant extracellular acid-fast bacilli (b). Magnification, ×20 (a) or ×500 (b, inset). (c, inset) Large numbers of extracellular bacilli were also found at the transition zone between the central necrosis and the cellular cuff, where evidence of more recent cellular necrosis was observed. Magnification, ×500. (d, inset) Intracellular bacilli were found in smaller concentrations at the outer margin of the cellular cuff. Magnification, ×200.
Fig 5.
Histopathology of nonnecrotic lung foci in infected BALB/c (a and b) and C3HeB/FeJ (c and d) mice, demonstrating chronic inflammation with foamy macrophages containing acid-fast bacilli (arrows). Magnification, ×500.
DISCUSSION
In this study, we first used a well-established BALB/c mouse model of TB to compare the bactericidal and sterilizing properties of RIF and RPT over a range of daily doses when administered together with INH and PZA. Although the treatment phase lasted only 12 weeks, the R10HZ control group performed similarly to prior experiments, in which 24 weeks of treatment were required to achieve a durable cure for all mice (32, 33, 39, 41, 46, 54). Escalation of the RIF dose to 40 mg/kg produced a stable cure in all mice after just 12 weeks of treatment. Remarkably similar results were observed with the 10-mg/kg dose of RPT, confirming prior results showing that 10 to 12 weeks of treatment with P10HZ were sufficient to achieve stable cure in all mice (40, 41). Likewise, the R20HZ and P5HZ regimens had similar activities, curing 33% of mice after 12 weeks of treatment and indicating they may well have produced a stable cure after 16 or 20 weeks, although this was not assessed. These results indicate that RPT is 4 times more potent that RIF in this murine model. In other words, daily administration of RPT at 5 and 10 mg/kg is equivalent to daily administration of RIF at 20 and 40 mg/kg, respectively, in the first-line combination regimen. These results are consistent with those of Lenaerts et al. (29), who previously found RPT at 5 mg/kg to be equipotent to RIF at 20 mg/kg in Swiss mice receiving monotherapy 1 week after intravenous infection with the Erdman strain of M. tuberculosis.
One virtue of studying these rifamycins in mice is that the serum PK profiles of both drugs are generally similar in mice and humans. After 2 weeks of daily dosing (5 days per week) for BALB/c mice, P5 and P10 produced total drug Cmax values of 9.28 and 18.57 μg/ml and AUC0–24 values of 179.8 and 358.6 μg · h/ml, respectively. In healthy volunteers taking RPT with food for 1 to 2 weeks (7 days per week), the 5- and 10-mg/kg doses produced mean or median total drug Cmax values of 10.9 to 15.7 and 21.7 to 23.8 μg/ml, respectively, and mean or median AUC0–24 values of 166 to 218 and 330 to 358 μg · h/ml, respectively (9, 26). In the present study, RIF at 10 mg/kg in BALB/c mice produced total drug Cmax and AUC0–24 values of 13.52 μg/ml and 142.1 μg · h/ml, respectively, after 2 weeks of dosing. Whereas single doses of 8 to 12 mg/kg in humans have resulted in average Cmax and AUC values of 8.5 to 13.6 μg/ml and 58 to 131 μg · h/ml, respectively, steady-state AUC values are generally lower (e.g., 40 to 80 μg · h/ml), owing to autoinduction (8, 24, 36, 37, 42, 51, 53). Only limited steady-state PK data exist for humans who receiving RIF doses of ≥15 mg/kg. Two studies of healthy volunteers receiving approximately 15 to 16 mg/kg reported AUC values of 101.6 to 164.5 μg · h/ml (24); these results were more consistent with the exposure we observed with 10 mg/kg in mice. Verbist reported average Cmax and AUC values of 18 μg/ml and 178 μg · h/ml, respectively, in Congolese patients administered 30 mg/kg (50). Therefore, while the murine doses for RPT in the current study corresponded well with similar doses administered to healthy volunteers, the RIF exposures observed in mice overestimated exposures observed at steady state in TB patients. These results indicate, despite the general similarities of the PK profiles for both drugs in mice and humans, that one cannot simply assume that a given dose (in mg/kg) will produce similar exposures in mice and in patients with TB. PK studies are a necessary component of animal models and clinical trials exploring new doses of these agents.
Given the close correspondence of rifamycin PK between mice and humans, the 4-fold-greater potency of RPT over RIF in experiment 1 would be expected to translate into improved clinical results with equivalent doses of RPT in place of RIF. However, the recently reported results of TB Trials Consortium Study 29 do not support this assumption. In that phase 2 trial, subjects with smear-positive pulmonary TB randomized to receive 10 mg/kg (up to 600 mg) of either RIF or RPT in combination with INH, PZA, and EMB for the first 8 weeks of treatment experienced similar rates of sputum conversion (10), although a trend toward superiority of RPT was observed among subjects with noncavitary disease in a post hoc subgroup analysis. The reason that the benefit of replacing RIF with RPT was not as great in Study 29 as it is in mice is not clear. A preliminary report of the RPT PK, based largely on African subjects, described median steady-state Cmax and AUC0–24 values of 12.6 μg/ml and 218 μg · h/ml, respectively (52)—values similar to those observed with a dose between P5 and P7.5 in our study. The RIF PK data from Study 29 were not provided in the preliminary report, but the mean RIF AUC0–24 at steady state among a similar population in 2 previous trials conducted by the same consortium was 42.3 μg · h/ml (51). Since P5 was superior to R10 (AUC, 142 μg · h/ml) in the present study, the lower-than-expected exposure observed with RPT at 10 mg/kg in Study 29 would not seem to be the only explanation for the apparent discrepancy between mouse and human results.
Another potential explanation involves pathological differences between murine and human TB. For example, the vast majority of tubercle bacilli reside intracellularly in conventional murine models, whereas the majority of bacteria in cavitary TB and, hence, in the sputum of TB patients, are extracellular (15). Since the ratio of intracellular to extracellular concentrations of RPT may be 6 times higher than that of RIF (30), the benefit of replacing RIF with RPT could be overestimated in BALB/c mice. Our experiment evaluated the influence of lung pathology on the relative activity of RIF and RPT by using two pathologically distinct murine models: BALB/c mice, in which no necrosis occurs and the vast majority of bacilli reside intracellullarly, and C3HeB/FeJ mice, characterized by formation of necrotic granulomas in which the majority of bacilli are extracellular and exposed to hypoxic conditions (7, 20). Remarkably, the relative potencies of RIF and RPT were similar between the 2 mouse strains and consistent with prior results. Moreover, the substitution of P10 for R10 in the first-line regimen produced similar significant improvements in the sterilizing activity of the regimen in both mouse strains, indicating that the presence of more necrotic granulomas per se did not influence the relative potencies of RPT and RIF. However, this result still leaves open the possibility that the high protein binding of RPT relative to RIF limits the diffusion of free drug into cavities and/or larger necrotic lesions. Some support for this hypothesis comes from a recent study with guinea pigs, in which necrotic granulomas were larger than those in C3HeB/FeJ mice (11). In that study, RPT was not more potent on a mg/kg basis than RIF when the two drugs were administered alone or in combination with INH and PZA. Further studies of lesion-specific RIF and RPT PK in a model characterized by caseous and cavitary lesions (e.g., guinea pigs, rabbits, or nonhuman primates) (27) would be useful in further evaluating this hypothesis, and this could have major importance for the evaluation of other highly protein-bound drugs currently in development, including TMC207, OPC-67683, and PA-824.
A final potential explanation for the apparent discrepancy between murine studies and Study 29 is that the primary end point of phase 2 trials like Study 29, sputum culture conversion at 2 months, may not be an adequate surrogate marker for relapse or the assessment of sterilizing activity (6, 10). Only properly designed phase 3 studies based on relapse as the primary outcome can address this issue.
Irrespective of the issues involved in translating results from animal models to clinical trials, the dose-dependent bactericidal and sterilizing effects of the rifamycins in this study, including the additional increase in sterilizing activity observed with the increased daily RPT dose from 10 to 20 mg/kg, support current efforts to define the maximum well-tolerated dose of both RPT and RIF in TB patients. Based on available preclinical and clinical data, there is no reason to expect that the activity of either rifamycin will plateau at any exposure level achievable in humans (8, 22, 23, 35, 38, 49). In the end, the choice of rifamycin may depend on which drug attains the higher exposure while maintaining an acceptable level of safety and tolerability. There is limited evidence that higher doses of RIF (e.g., 20 to 30 mg/kg) are well-tolerated when administered daily (8, 23, 28, 44, 45). Prior to TBTC Study 29, only a small number of patients had received RPT at 10 mg/kg daily for various periods of time without reported intolerance (25; Priftin package insert; Sanofi-Aventis, Kansas City, MO). However, 275 patients in Study 29 received RPT at 10 mg/kg daily with INH, PZA, and EMB for 8 weeks. Safety and tolerability of RPT were not different than for RIF at the same dose (10). In a follow-up study of the safety, tolerability, and PK of escalating doses of RPT in healthy volunteers, drug exposures comparable to P15 in mice were achieved in human subjects receiving 15 to 20 mg/kg, with food, and were well-tolerated (9).
In addition to supporting ongoing efforts to define the maximal well-tolerated doses of RIF and RPT, our study is the first to directly compare the bactericidal and sterilizing activities of the standard first-line regimen of RIF, INH, PZA, and EMB in C3HeB/FeJ mice and in a commonly used mouse strain. The similar efficacies of the first-line regimen in both strains and the selection of clinically relevant RIF-resistant mutants (14, 48) with monotherapy provide an important benchmark for future studies evaluating the potential value of C3HeB/FeJ mice as a model for evaluation of experimental chemotherapy for TB.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by the National Institutes of Health (N01-AI40007 and K08-AI58993) and the U.S. Food and Drug Administration (U18-FD004004).
Footnotes
Published ahead of print 4 June 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Almeida D, et al. 2009. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob.Agents Chemother. 53:4178–4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blumberg HM, et al. 2003. American Thoracic Society, Centers for Disease Control and Prevention, and Infectious Diseases Society of America: treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603–662 [DOI] [PubMed] [Google Scholar]
- 3. British Thoracic and Tuberculosis Association 1975. Short-course chemotherapy in pulmonary tuberculosis. Lancet i:119–124 [PubMed] [Google Scholar]
- 4. British Thoracic and Tuberculosis Association 1976. Short-course chemotherapy in pulmonary tuberculosis. Lancet ii:1102–1104 [PubMed] [Google Scholar]
- 5. Burman WJ, Gallicano K, Peloquin C. 2001. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin. Pharmacokinet. 40:327–341 [DOI] [PubMed] [Google Scholar]
- 6. Davies GR. 2010. Early clinical development of anti-tuberculosis drugs: science, statistics and sterilizing activity. Tuberculosis (Edinb.) 90:171–176 [DOI] [PubMed] [Google Scholar]
- 7. Davis SL, et al. 2009. Noninvasive pulmonary [18F]-2-fluoro-deoxy-D-glucose positron emission tomography correlates with bactericidal activity of tuberculosis drug treatment. Antimicrob. Agents Chemother. 53:4879–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diacon AH, et al. 2007. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob. Agents Chemother. 51:2994–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dooley KE, et al. 2012. Safety and pharmacokinetics of escalating daily doses of the antituberculosis drug rifapentine in healthy volunteers. Clin. Pharmacol. Ther. doi:10.1038/clpt.2011.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dorman S, et al. 2011. A phase II study of a rifapentine-containing regimen for intensive phase treatment of pulmonary tuberculosis: preliminary results for Tuberculosis Trials Consortium Study 29. Am. J. Respir. Crit. Care Med. 183:A6413 [Google Scholar]
- 11. Dutta NK, et al. 30 April 2012, posting date Rifapentine is not more active than rifampin against chronic tuberculosis in guinea pigs. Antimicrob. Agents Chemother. doi:10.1128/AAC.00500-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. East and Central African and British Medical Research Councils 1986. Controlled clinical trial of 4 short-course regimens of chemotherapy (three 6-month and one 8-month) for pulmonary tuberculosis: final report. Tubercle 67:5–15 [DOI] [PubMed] [Google Scholar]
- 13. East and Central African and British Medical Research Councils 1973. Controlled clinical trial of four short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis. Second report. Lancet i:1331–1338 [PubMed] [Google Scholar]
- 14. Gagneux S, et al. 2006. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science 312:1944–1946 [DOI] [PubMed] [Google Scholar]
- 15. Grosset J. 2003. Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrob. Agents Chemother. 47:833–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grosset J, et al. 1998. Once-weekly rifapentine-containing regimens for treatment of tuberculosis in mice. Am. J. Respir. Crit. Care Med. 157:1436–1440 [DOI] [PubMed] [Google Scholar]
- 17. Grosset JH, Rosenthal I, Zhang M, Williams K, Nuermberger EL. 2008. Low doses of rifapentine versus high doses of rifampin in the mouse model of tuberculosis. Int. J. Tuberc. Lung Dis. 12(11 Suppl. 2):S63 [Google Scholar]
- 18. Grosset JHE, Rosenthal IM, Zhang M, Nuermberger EL. 2008. Dose-ranging comparison of rifampin and rifapentine in combination with isoniazid and pyrazinamide in the mouse model of tuberculosis, abstr. A-1825. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 19. Gumbo T, et al. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 51:3781–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harper J, et al. 2012. Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions. J. Infect. Dis. 205:595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hunter RL, Jagannath C, Actor JK. 2007. Pathology of postprimary tuberculosis in humans and mice: contradiction of long-held beliefs. Tuberculosis (Edinb.) 87:267–278 [DOI] [PubMed] [Google Scholar]
- 22. Jayaram R, et al. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 47:2118–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jindani A, Aber VR, Edwards EA, Mitchison DA. 1980. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 121:939–949 [DOI] [PubMed] [Google Scholar]
- 24. Kenny MT, Strates B. 1981. Metabolism and pharmacokinetics of the antibiotic rifampin. Drug Metab. Rev. 12:159–218 [DOI] [PubMed] [Google Scholar]
- 25. Keung A, Eller MG, McKenzie KA, Weir SJ. 1999. Single and multiple dose pharmacokinetics of rifapentine in man: part II. Int. J. Tuberc. Lung Dis. 3:437–444 [PubMed] [Google Scholar]
- 26. Keung A, et al. 1999. Enzyme induction observed in healthy volunteers after repeated administration of rifapentine and its lack of effect on steady-state rifapentine pharmacokinetics: part I. Int. J. Tuberc. Lung Dis. 3:426–436 [PubMed] [Google Scholar]
- 27. Kjellsson MC, et al. 2012. Pharmacokinetic evaluation of the penetration of antituberculosis agents in rabbit pulmonary lesions. Antimicrob. Agents Chemother. 56:446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kochar DK, et al. 2006. A double blind, randomised placebo controlled trial of rifampicin with omeprazole in the treatment of human cutaneous leishmaniasis. J. Vector Borne. Dis. 43:161–167 [PubMed] [Google Scholar]
- 29. Lenaerts AM, Chase SE, Chmielewski AJ, Cynamon MH. 1999. Evaluation of rifapentine in long-term treatment regimens for tuberculosis in mice. Antimicrob. Agents Chemother. 43:2356–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mor N, Simon B, Mezo N, Heifets L. 1995. Comparison of activities of rifapentine and rifampin against Mycobacterium tuberculosis residing in human macrophages. Antimicrob. Agents Chemother. 39:2073–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nuermberger E. 2008. Using animal models to develop new treatments for tuberculosis. Semin. Respir. Crit. Care Med. 29:542–551 [DOI] [PubMed] [Google Scholar]
- 32. Nuermberger E, et al. 2006. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 50:2621–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nuermberger EL, et al. 2004. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am. J. Respir. Crit. Care Med. 170:1131–1134 [DOI] [PubMed] [Google Scholar]
- 34. Pan H, et al. 2005. Ipr1 gene mediates innate immunity to tuberculosis. Nature 434:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peloquin C. 2003. What is the ‘right’ dose of rifampin? Int. J. Tuberc. Lung Dis. 7:3–5 [PubMed] [Google Scholar]
- 36. Peloquin CA, et al. 1997. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob. Agents Chemother. 41:2670–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peloquin CA, Namdar R, Singleton MD, Nix DE. 1999. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest 115:12–18 [DOI] [PubMed] [Google Scholar]
- 38. Rosenthal IM, Zhang M, Grosset JH, Nuermberger EL. 2008. Rifapentine-containing regimens cure murine tuberculosis in weeks rather than months. Am. J. Respir. Crit. Care Med. 177:A789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosenthal IM, et al. 2006. Potent twice-weekly rifapentine-containing regimens in murine tuberculosis. Am. J. Respir. Crit. Care Med. 174:94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosenthal IM, Zhang M, Almeida D, Grosset JH, Nuermberger EL. 2008. Isoniazid or moxifloxacin in rifapentine-based regimens for experimental tuberculosis? Am. J. Respir. Crit. Care Med. 178:989–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosenthal IM, et al. 2007. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 4:e344 doi:10.1371/journal.pmed.0040344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ruslami R, et al. 2007. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob. Agents Chemother. 51:2546–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singapore Tuberculosis Service and British Medical Research Council 1981. Clinical trial of six-month and four-month regimens of chemotherapy in the treatment of pulmonary tuberculosis: the results up to 30 months. Tubercle 62:95–102 [DOI] [PubMed] [Google Scholar]
- 44. Solera J, et al. 1995. Doxycycline-rifampin versus doxycycline-streptomycin in treatment of human brucellosis due to Brucella melitensis. The GECMEI Group. Grupo de Estudio de Castilla-la Mancha de Enfermedades Infecciosas. Antimicrob. Agents Chemother. 39:2061–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steingart KR, et al. 2011. Higher-dose rifampin for the treatment of pulmonary tuberculosis: a systematic review. Int. J. Tuberc. Lung Dis. 15:305–316 [PubMed] [Google Scholar]
- 46. Tasneen R, et al. 2011. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob. Agents Chemother. 55:5485–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tasneen R, Peloquin C, Nuermberger E. 2011. Dose-ranging activity of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis, abstr. O_15. Progr. Abstr. Fourth Int. Workshop Clin. Pharmacol. Tuberc. Drugs, Chicago, IL Virology Education B.V., Utrecht, The Netherlands [Google Scholar]
- 48. Telenti A, et al. 1993. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet 341:647–650 [DOI] [PubMed] [Google Scholar]
- 49. Verbist L. 1969. Rifampicin activity “in vitro” and in established tuberculosis in mice. Acta Tuberc. Pneumol. Belg. 60:397–412 [PubMed] [Google Scholar]
- 50. Verbist L. 1971. Pharmacological study of rifampicin after repeated high dosage during intermittent combined therapy. I. Variation of the rifampicin serum levels (947 determinations). Respiration 28(Suppl.):7–16 [DOI] [PubMed] [Google Scholar]
- 51. Weiner M, et al. 2010. Effects of tuberculosis, race, and human gene SLCO1B1 polymorphisms on rifampin concentrations. Antimicrob. Agents Chemother. 54:4192–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weiner M, et al. 2011. Rifapentine exposure in a trial of daily rifapentine compared to rifampin during the intensive phase of TB treatment. Am. J. Respir. Crit. Care Med. 183:A6341 [Google Scholar]
- 53. Wilkins JJ, et al. 2008. Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption. Antimicrob. Agents Chemother. 52:2138–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Williams KN, et al. 2009. Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am. J. Respir. Crit. Care Med. 180:371–376 [DOI] [PubMed] [Google Scholar]
- 55. Zhang M, et al. 2011. Treatment of tuberculosis with rifamycin-containing regimens in immune-deficient mice. Am. J. Respir. Crit. Care Med. 183:1254–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang T, Li SY, Williams KN, Andries K, Nuermberger EL. 2011. Short-course chemotherapy with TMC207 and rifapentine in a murine model of latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 184:732–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.