Abstract
Lersivirine is a nonnucleoside reverse transcriptase inhibitor (NNRTI) with a unique resistance profile exhibiting potent antiviral activity against wild-type HIV and several clinically relevant NNRTI-resistant strains. Lersivirine, a weak inducer of the cytochrome P450 (CYP) enzyme CYP3A4, is metabolized by CYP3A4 and UDP glucuronosyltransferase 2B7 (UGT2B7). Two open, randomized, two-way (study 1; study A5271008) or three-way (study 2; study A5271043) crossover phase I studies were carried out under steady-state conditions in healthy subjects. Study 1 (n = 17) investigated the effect of oral rifampin on the pharmacokinetics (PKs) of lersivirine. Study 2 (n = 18) investigated the effect of oral rifabutin on the PKs of lersivirine and the effect of lersivirine on the PKs of rifabutin and its active metabolite, 25-O-desacetyl-rifabutin. Coadministration with rifampin decreased the profile of the lersivirine area under the plasma concentration-time curve from time zero to 24 h postdose (AUC24), maximum plasma concentration (Cmax), and plasma concentration observed at 24 h postdose (C24) by 85% (90% confidence interval [CI], 83, 87), 83% (90% CI, 79, 85), and 92% (90% CI, 89, 94), respectively, versus the values for lersivirine alone. Coadministration with rifabutin decreased the lersivirine AUC24, Cmax, and C24 by 34% (90% CI, 29, 39), 25% (90% CI, 16, 33), and 58% (90% CI, 52, 64), respectively, compared with the values for lersivirine alone. Neither the rifabutin concentration profile nor overall exposure was affected following coadministration with lersivirine. Lersivirine and rifabutin reduced the 25-O-desacetyl-rifabutin AUC24 by 27% (90% CI, 21, 32) and Cmax by 27% (90% CI, 19, 34). Lersivirine should not be coadministered with rifampin, which is a potent inducer of CYP3A4, UGT2B7, and P-glycoprotein activity and thus substantially lowers lersivirine exposure. No dose adjustment of rifabutin is necessary in the presence of lersivirine; an upward dose adjustment of lersivirine may be warranted when it is coadministered with rifabutin.
INTRODUCTION
Lersivirine is a nonnucleoside reverse transcriptase inhibitor (NNRTI) of human immunodeficiency virus type 1 (HIV-1) that displays potent antiviral activity against wild-type virus and also against certain clinically relevant NNRTI-resistant viruses (23).
X-ray crystallography has demonstrated that lersivirine binds to the reverse transcriptase in a novel way, which is thought to contribute to antiviral activity against key class resistant mutants, such as those with the K103N, Y181C, and G190A mutations (5, 28). However, the mechanism of lersivirine resilience against several drug-resistant virus strains is not yet fully understood. The antiviral activity and the safety and tolerability of lersivirine have been explored in a phase IIa study in treatment-naïve patients (12).
Lersivirine is metabolized by the cytochrome P450 (CYP) enzyme CYP3A4 and UDP glucuronosyltransferase 2B7 (UGT2B7); it is also a weak inhibitor of glucuronidation (10, 35). Lersivirine has been shown to be a weak inducer of CYP3A4 activity at clinical doses (9) and, on the basis of in vitro data, is an inhibitor and substrate for P-glycoprotein (P-gp) (Pfizer Inc., data on file). However, experiments in human hepatocytes have shown a concentration-dependent increase in induction with lersivirine. In general, lersivirine is considered unlikely to inhibit metabolism of other substances cleared by CYP enzymes at clinical doses.
Rifampin is a bacterial RNA polymerase inhibitor that is used to treat tuberculosis and nontuberculosis mycobacterial infections and is a potent CYP and P-glycoprotein inducer (17, 31). Rifampin is metabolized by B-esterases to 25-desacetyl-rifampin (16) and is an autoinducer of metabolism. The terminal half-life of rifampin is estimated to be approximately 3 h (range, 2 to 5 h) (1).
Rifabutin and its active metabolite, 25-O-desacetyl-rifabutin, have demonstrated in vitro activity against the Mycobacterium avium complex organism isolated from HIV-positive and -negative individuals. Rifabutin has also demonstrated in vitro activity and clinical efficacy against Mycobacterium tuberculosis (27).
Rifabutin is metabolized and eliminated mainly by the CYP3A pathway and is a moderate inducer of CYP3A4 activity (3, 18). Rifabutin has an apparent terminal half-life of 45 h (range, 16 to 69 h) (32). Due to the potent CYP induction liability of rifampin, rifabutin is used as an alternative to rifampin in individuals infected with HIV who are coinfected with Mycobacterium tuberculosis (3).
As an estimated one-third of individuals living with HIV infection are coinfected with M. tuberculosis (37), it is important that treatments in development be compatible for coadministration (11). This report describes the findings from two pharmacokinetic (PK) studies, of similar design, of the antituberculosis agents rifampin and rifabutin when each is coadministered with lersivirine.
The objective of study 1 (study A5271008) was to investigate the effects of rifampin on the steady-state PKs of lersivirine administered at 1,000 mg once daily (q.d.). The objective of study 2 (study A5271043) was to investigate the effects of lersivirine and rifabutin on each other's PKs and also the effect that lersivirine has on the PKs of the active metabolite of rifabutin (25-O-desacetyl-rifabutin). Both studies also investigated the safety and tolerability of lersivirine when it was coadministered with rifabutin or rifampin.
MATERIALS AND METHODS
Subjects.
Eligible subjects were healthy males aged 21 to 55 years (study 1) or healthy male or female subjects aged 18 to 55 years (study 2), who had a body mass index (BMI) of 18 to 30 kg/m2 (study 1) or 17.5 to 30.5 kg/m2 (study 2) and a total body weight of >45 kg (study 1) or >50 kg (study 2). Subjects with a history of significant disease, HIV infection, or hepatitis B or C were excluded. Prescription and nonprescription drugs, vitamins, and dietary supplement medications were not permitted during the study and had to be discontinued within 7 days or 5 half-lives (whichever was longer) prior to the first dose of study medication.
Study designs.
These were phase I, open, randomized, two-way crossover (study 1; study A5271008) or three-way crossover (study 2; study A5271043) studies conducted at the Pfizer Singapore Clinical Research Unit to investigate the effect of oral doses of rifampin (study 1) and rifabutin (study 2) on the steady-state PKs of lersivirine and the effect of lersivirine on the PKs of rifabutin and 25-O-desacetyl-rifabutin (the active metabolite of rifabutin) (study 2). The studies consisted of a screening visit up to 28 days before the start of dosing, two 14-day treatment periods (study 1; Table 1), or three 10-day treatment periods (study 2; Table 2) with a minimum washout period of 14 days between each.
Table 1.
Design for study 1a
| Randomized sequence group | Period 1 |
Period 2 |
||||
|---|---|---|---|---|---|---|
| Days 1–10 | Days 11–13 | Day 14 | Days 1–10 | Days 11–13 | Day 14 | |
| Sequence 1 | Treatment A | Treatment C | Treatment E | Treatment B | Treatment D | Treatment F |
| Sequence 2 | Treatment B | Treatment D | Treatment F | Treatment A | Treatment C | Treatment E |
| Day(s) of PK data collection | 1, 9, 10 | 11, 13 | 14 | 1, 9, 10 | 11, 13 | 14 |
A ≥14-day washout was used between periods 1 and 2. Treatment A, lersivirine at 1,000 mg q.d. plus rifampin at 600 mg q.d.; treatment B, lersivirine at 1,000 mg q.d. plus placebo; treatment C, lersivirine at 1,000 mg BID plus rifampin at 600 mg q.d.; treatment D, lersivirine at 1,000 mg BID plus placebo; treatment E, lersivirine at 1,000 mg plus rifampin at 600 mg q.d.; treatment F, lersivirine at 1,000 mg plus placebo.
Table 2.
Design for study 2a
| Randomized sequence group | Period 1 | Period 2 | Period 3 |
|---|---|---|---|
| Sequence 1 | Treatment A | Treatment B | Treatment C |
| Sequence 2 | Treatment B | Treatment C | Treatment A |
| Sequence 3 | Treatment C | Treatment A | Treatment B |
| Sequence 4 | Treatment B | Treatment A | Treatment C |
| Sequence 5 | Treatment A | Treatment C | Treatment B |
| Sequence 6 | Treatment C | Treatment B | Treatment A |
A ≥14-day washout was used between periods 1 and 2 and periods 2 and 3, and PK data were collected on day 10. All treatments are from days 1 to 10. Treatment A, lersivirine at 1,000 mg q.d.; treatment B, rifabutin at 300 mg q.d.; treatment C, lersivirine at 1,000 mg q.d. plus rifabutin at 300 mg q.d.
In study 1, subjects received lersivirine at 1,000 mg q.d. on days 1 to 10 and 1,000 mg twice a day (BID) on days 11 to 13, with a single dose given on the morning of day 14 during both treatment periods in the fasted state. On days 1 to 14, subjects received rifampin at 600 mg q.d. in one period and placebo q.d. in the other period, according to randomization, with the morning dose of lersivirine. Only the PK and adverse event (AE) data relating to lersivirine administered q.d. are presented in this report. In study 2, subjects were randomized to receive lersivirine at 1,000 mg q.d. alone, rifabutin at 300 mg q.d. alone, and lersivirine at 1,000 mg q.d. and rifabutin 300 mg q.d. combined, in a random order across the three treatment periods. In study 2, lersivirine and rifabutin were administered with a meal, as it has been shown that food may help increase rifabutin tolerability. Administration with food does not significantly affect the exposures of lersivirine or rifabutin (8, 24).
Investigator site personnel administered the study treatment during each period with water at ambient temperature to a total volume of 240 ml. Subjects were asked to swallow the whole tablet or capsule and not chew it prior to swallowing. In order to standardize the conditions on PK sampling days, all subjects were required to refrain from lying down (except when required for blood pressure, pulse rate, and electrocardiographic measurements), eating, and drinking beverages other than water during the first 4 h after dosing. The mouth of each subject was examined following dosing to ensure that the study medication was taken.
Sampling and analytical methods.
In study 1, blood samples were collected on days 1, 9, 11, and 13 at 0 h (predose), on day 10 at 0 h (predose) and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 18, and 24 h postdose, and on day 14 at 0 h (predose) and at 0.5, 1, 2, 3, 4, 6, 8, 10, and 12 h postdose. In study 2, blood samples were collected on day 10 at 0 h (postdose) and 0.5, 1, 2, 3, 4, 6, 8, 10, 12, and 24 h postdose.
In studies 1 and 2, blood samples of 5 ml and 4 ml, respectively, were taken to provide a minimum of 2 ml plasma for lersivirine PK analysis and transferred into appropriately labeled tubes containing lithium heparin. In addition, for study 2, blood samples of 6 ml were taken to provide a minimum of 2.5 ml plasma for rifabutin and 25-O-desacetyl-rifabutin PK analysis and were transferred into appropriately labeled tubes containing sodium heparin. All samples were centrifuged at approximately 1,700 × g for 10 min at 4°C. Plasma was stored in appropriately labeled screw-cap polypropylene tubes at approximately −20°C within 1 h of collection. Samples were analyzed using solid-phase extraction and a validated high-performance liquid chromatography/dual mass spectrometry (HPLC/MS/MS) assay.
The assay precision was 5.5% (coefficient of variance [CV]; study 1) and ≤8.5% (study 2) for lersivirine, ±69.9% (±10.3% without one potential outlier) for rifabutin (study 2), and ±9.3% for 25-O-desacetyl-rifabutin (study 2). The between-day assay accuracy (percent relative error [RE]) of the estimated quality control samples ranged from −5.6% to 2.4% (study 1) and −3.3% to 6.3% (study 2) for lersivirine, −6.5% to 21.7% (1.1% without one potential outlier) for rifabutin (study 2), and −4.8% to 5.7% for 25-O-desacetyl-rifabutin (study 2). Calibration ranges were 1.0 to 2,000 ng/ml for lersivirine (studies 1 and 2), 2.5 to 1,000 ng/ml for rifabutin (study 2), and 1.0 to 1,000 ng/ml for 25-O-desacetyl-rifabutin (study 2).
Safety.
Safety evaluations included AE monitoring, laboratory safety tests, liver function tests (study 1), and single 12-lead electrocardiogram, supine blood pressure, and pulse rate recording.
Sample size.
In study 1, a minimum sample size of 12 was required to provide 90% confidence intervals (CIs) for the difference between treatments of ±0.0539 and ±0.1525 on the natural log scale for area under the plasma concentration-time profile from time zero to infinity postdose (AUCτ) and maximum plasma concentration (Cmax), respectively, with 80% coverage probability. The above-described calculations assumed the estimated intrasubject standard deviations of the natural log of the area under the plasma concentration-time profile from time zero to 24 h postdose (AUC24) and Cmax to be 0.089 and 0.252, respectively, for lersivirine.
To estimate the effect of rifabutin on the PKs of lersivirine (study 2), the effect of lersivirine on the PKs of rifabutin, and the effect of lersivirine on the PKs of 25-O-desacetyl-rifabutin in study 2, 18 subjects were required to provide 90% CIs for the difference between treatments of ±0.101 and ±0.140, ±0.098 and ±0.159, and ±0.105 and ±0.152, respectively, on the natural log scale for AUC24 and Cmax, respectively, with 90% coverage probability. The calculations described above assumed the estimated intrasubject standard deviations of natural log AUC24 and Cmax to be 0.155 and 0.215, respectively, for lersivirine, 0.150 and 0.244, respectively, for rifabutin, and 0.161 and 0.234, respectively, for 25-O-desacetyl-rifabutin.
Statistical analyses.
The PK parameters AUC24, plasma concentration observed at 24 h postdose (C24), Cmax, and time to Cmax (Tmax) were summarized by treatment. An ad hoc analysis was performed to assess the elimination half-life (t1/2) for lersivirine in the presence of rifampin and rifabutin. AUC parameters were determined using the linear/log trapezoidal method, and Cmax, C24, and Tmax were determined by observation. Natural log-transformed data were analyzed using a mixed-effect model with sequence, period, and treatment as fixed effects and subject within sequence as a random effect. The adjusted geometric mean for each treatment was obtained by exponentiation of the mean estimate on the natural log scale obtained from the model. Estimates of the adjusted mean differences (test/reference) and the corresponding 90% CIs were obtained from the model. The adjusted mean differences and 90% CIs for the differences were exponentiated to provide estimates of the ratio of adjusted geometric means (test/reference), where the test was lersivirine plus rifampin (study 1) or lersivirine plus rifabutin (study 2) and the reference was lersivirine plus placebo (study 1), lersivirine alone (study 2), or rifabutin alone (study 2).
RESULTS
Subjects.
All the 17 subjects in study 1 were Asian males. The majority of subjects in study 2 were Asian (17/18) and male (16/18). The baseline demographic characteristics are shown in Table 3. In study 1, 13 out of the 17 randomized subjects completed the study. Four subjects discontinued as they were unwilling to participate further in the study. The subjects discontinued on days 1 (lersivirine plus placebo), 6 (lersivirine plus placebo), and 7 (lersivirine plus rifampin) of period 1 and day 3 (lersivirine plus rifampin) of period 2. Out of the 18 subjects randomized in study 2, 17 subjects completed the study; 1 subject permanently discontinued on day 6 of period 1 due to a treatment-related AE of moderate pyrexia, an adverse event historically associated with rifabutin (26), while receiving lersivirine coadministered with rifabutin.
Table 3.
Demographic characteristics (studies 1 and 2)a
| Characteristic | Study 1 (n = 17) | Study 2 (n = 18) |
|---|---|---|
| Age (yr) | 30.8 (5.7) | 32.0 (5.9) |
| Wt (kg) | 68.1 (10.7) | 67.0 (9.7) |
| BMI (kg/m2) | 23.0 (2.9) | 22.8 (2.2) |
| Ht (cm) | 171.9 (6.9) | 171.5 (9.0) |
Data represent means (standard deviations).
Pharmacokinetics. (i) Study 1.
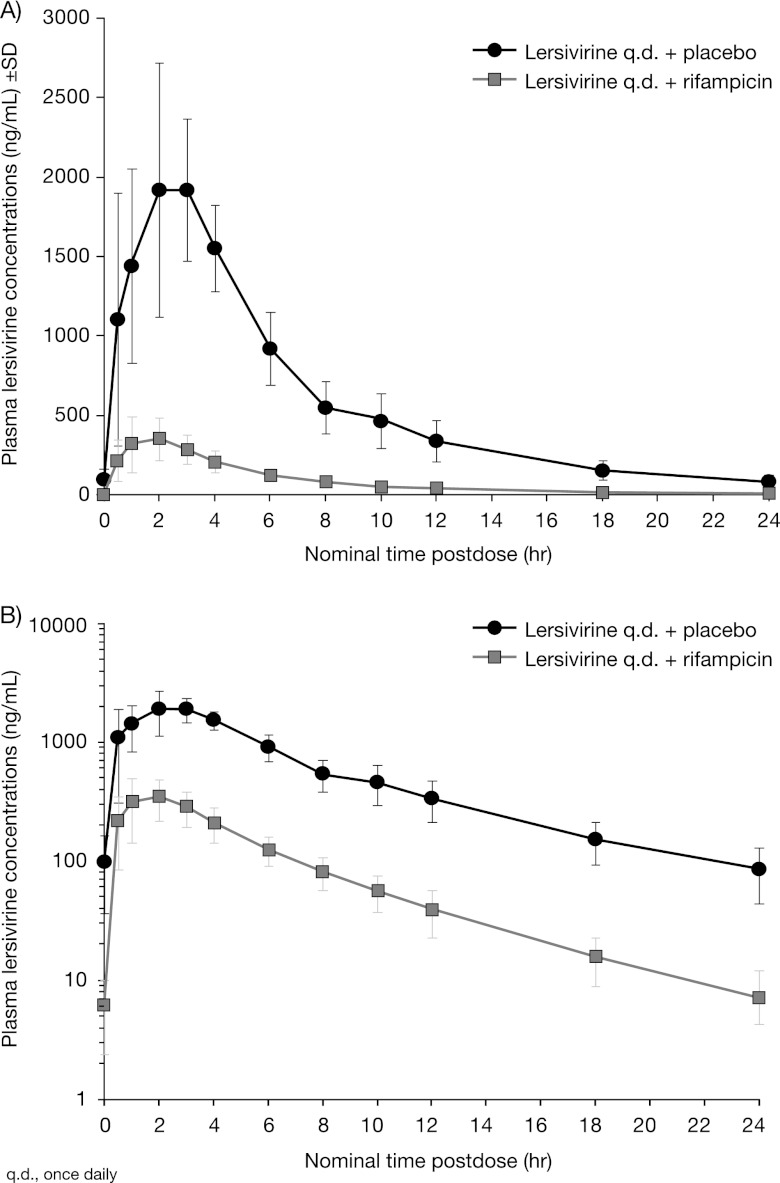
Mean lersivirine AUC24, Cmax, and C24 were substantially lower in the presence of rifampin with lersivirine (Table 4; Fig. 1). The mean t1/2 and median Tmax at day 10 were decreased for lersivirine plus rifampin compared with those for lersivirine plus placebo.
Table 4.
Lersivirine pharmacokinetics when coadministered with rifampin (study 1) or rifabutin (study 2)
| Parameter (units) | Study 1 (effect of rifampin on PKs of lersivirine) |
Study 2 (effect of rifabutin on PKs of lersivirine) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 (n = 13)a |
Treatment 2 (n = 14)b |
Ratio of adjusted geometric means (A/B) | 90% CI | Treatment 1 (n = 17)c |
Treatment 2 (n = 17)d |
Ratio of adjusted geometric means (A/B) | 90% CI | |||||
| Geometric mean (% CV) | Adjusted geometric mean (A)e | Geometric mean (% CV) | Adjusted geometric mean (B) | Geometric mean (% CV) | Adjusted geometric mean (A) | Geometric mean (% CV) | Adjusted geometric mean (B) | |||||
| AUC24 (ng · h/ml) | 2,023 (24) | 2,016 | 13,868 (22) | 13,691 | 0.15 | 0.13, 0.17 | 11,670 (18) | 11,700 | 17,870 (21) | 17,760 | 0.66 | 0.61, 0.71 |
| Cmax (ng/ml) | 375 (34) | 373 | 2,185 (25) | 2,145 | 0.17 | 0.15, 0.21 | 2,139 (24) | 2,127 | 2,880 (31) | 2,837 | 0.75 | 0.67, 0.84 |
| C24 (ng/ml) | 6 (65) | 6.2 | 78 (49) | 78.1 | 0.08 | 0.06, 0.11 | 48.5 (34) | 49 | 117.3 (42) | 117.6 | 0.42 | 0.36, 0.48 |
| t1/2 (h) | 4.7 (35) | 5.8 (23) | 4.9 (16) | 6.1 (30) | ||||||||
| Tmax (h)f | 2 (1–3) | 2.5 (0.5–4.0) | 3 (1–6) | 3 (1–6) | ||||||||
Treatment was with lersivirine at 1,000 mg q.d. plus rifampin at 600 mg q.d. Four subjects did not have samples for PK analysis collected due to discontinuations.
Treatment was with lersivirine at 1,000 mg q.d. plus placebo q.d. Three subjects did not have PK samples collected due to discontinuations. n = 13 for adjusted geometric means.
Treatment was with lersivirine at 1,000 mg q.d. plus rifabutin at 300 mg q.d. One subject discontinued due to an AE of pyrexia.
Treatment was with lersivirine at 1,000 mg q.d. One subject discontinued due to an AE of pyrexia.
The adjusted geometric mean was obtained by exponentiation of the mean estimate from the statistical analysis. Natural log-transformed data were analyzed using a mixed-effect model with sequence, period, and treatment as fixed effects and subject within sequence as a random effect.
Data represent median (range).
Fig 1.
Mean plasma lersivirine concentration following lersivirine or rifampin and lersivirine administration on linear (A) and semilog (B) scales (study 1).
(ii) Study 2.
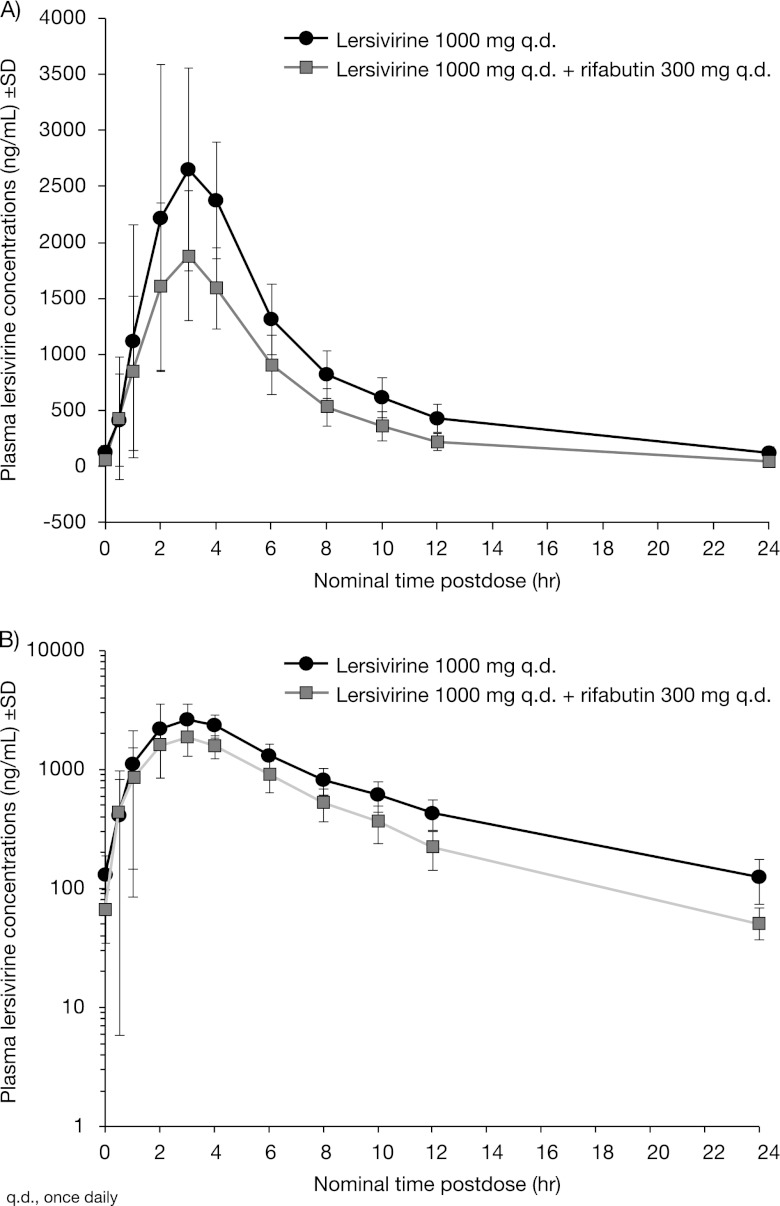
Coadministration with rifabutin lowered the steady-state plasma concentrations of lersivirine as well as the lersivirine t1/2 (Table 4; Fig. 2). No difference in lersivirine median Tmax was observed between when lersivirine was administered alone and when lersivirine was coadministered with rifabutin (Table 4).
Fig 2.
Mean plasma lersivirine concentration following lersivirine or rifabutin and lersivirine administration on linear (A) and semilog (B) scales (study 2).
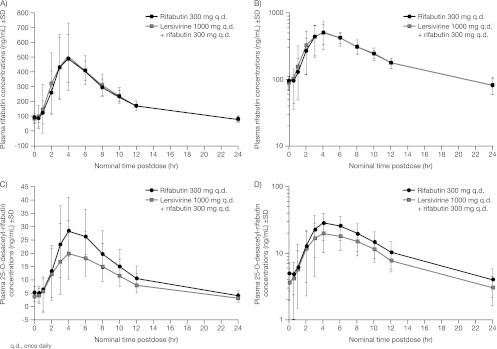
The rifabutin concentration profile or overall exposure did not change following coadministration with lersivirine (Table 5; Fig. 3). Following coadministration of lersivirine and rifabutin, lower exposure of the active rifabutin metabolite, 25-O-desacetyl-rifabutin, was observed (Table 5; Fig. 3).
Table 5.
Rifabutin and 25-O-desacetyl-rifabutin pharmacokinetics when coadministered with lersivirine (study 2)
| Effect studied and parameter (units) | Treatment 1 (n = 17)a |
Treatment 2 (n = 17)b |
Ratio of adjusted geometric means (A/B) | 90% CI | ||
|---|---|---|---|---|---|---|
| Geometric mean (% CV) | Adjusted geometric mean (A)c | Geometric mean (% CV) | Adjusted geometric mean (B) | |||
| Effect of lersivirine on PKs of rifabutin | ||||||
| AUC24 (ng · h/ml) | 5,146 (19) | 5,103 | 5,030 (18) | 5,010 | 1.02 | 0.96, 1.08 |
| Cmax (ng/ml) | 578 (33) | 572 | 522 (31) | 519 | 1.10 | 0.98, 1.24 |
| C24 (ng/ml) | 78.7 (26) | 78.0 | 78.9 (28) | 78.3 | 1.00 | 0.94, 1.06 |
| Tmax (h)d | 4 (1–8) | 4 (2–6) | ||||
| Effect of lersivirine on PKs of 25-O-desacetyl-rifabutin | ||||||
| AUC24 (ng · h/ml) | 203 (39) | 202 | 275 (40) | 275 | 0.73 | 0.68, 0.79 |
| Cmax (ng/ml) | 21.3 (41) | 21.0 | 28.9 (43) | 28.7 | 0.73 | 0.66, 0.81 |
| C24 (ng/ml) | 3.04 (37) | 3.04 | 3.60 (46) | 3.59 | 0.85 | 0.73, 0.98 |
| Tmax (h)d | 4 (1–8) | 4 (2–6) | ||||
Treatment was with lersivirine at 1,000 mg q.d. plus rifabutin at 300 mg q.d. One subject discontinued.
Treatment was with rifabutin at 300 mg q.d. One subject discontinued.
The adjusted geometric mean was obtained by exponentiation of the mean estimate from the statistical analysis. Natural log-transformed data were analyzed using a mixed-effect model with sequence, period, and treatment as fixed effects and subject within sequence as a random effect.
Data represent median (range).
Fig 3.
Mean plasma rifabutin (A and B) and 25-O-desacetyl-rifabutin (C and D) concentrations following rifabutin or lersivirine and rifabutin administration on linear (A and C) and semilog (B and D) scales (study 2).
Safety.
There were no deaths, serious AEs, severe AEs, dose reductions, or temporary discontinuations due to AEs in either study. AEs that occurred in more than two subjects are summarized by study and treatment group in Tables 6 and 7. One subject in study 2 discontinued due to an AE of pyrexia (day 6) and also presented with nausea (day 11), chills (day 6), decreased appetite (day 6), headache (day 6), and chromaturia (day 1), as well as laboratory abnormalities (reduced lymphocytes [absolute; day 10], increased lymphocytes [percent; day 12], and reduced total neutrophils [absolute; day 12]). No unexpected clinically significant AEs or laboratory abnormalities were observed in either study.
Table 6.
Incidence of all causality treatment-emergent adverse events that occurred in ≥2 subjects in any one treatment arm in study 1a
| Adverse event | No. (%) of subjects |
|||
|---|---|---|---|---|
| Lersivirine at 1,000 mg q.d. + rifampin at 600 mg q.d. (n = 15) |
Lersivirine at 1,000 mg q.d. + placebo q.d. (n = 16) |
|||
| Mild | Moderate | Mild | Moderate | |
| Chromaturia | 15 (100) | 0 | 0 | 0 |
| Dizziness | 8 (53) | 0 | 7 (44) | 1 (6) |
| Nausea | 2 (13) | 1 (7) | 7 (44) | 0 |
| Somnolence | 1 (7) | 0 | 2 (13) | 0 |
| Insomnia | 2 (13) | 0 | 1 (6) | 0 |
| Flatulence | 0 | 0 | 3 (19) | 0 |
| Headache | 1 (8) | 0 | 1 (6) | 1 (6) |
| Abdominal distension | 0 | 0 | 2 (13) | 0 |
| Diarrhea | 2 (13) | 0 | 1 (6) | 0 |
| Lip dry | 0 | 0 | 2 (12) | 0 |
No cases of severe treatment-emergent adverse events were recorded.
Table 7.
Incidence of all causality treatment-emergent adverse events that occurred in ≥2 subjects in any one treatment arm in study 2a
| Adverse event | No. (%) of subjects |
|||||
|---|---|---|---|---|---|---|
| Lersivirine at 1,000 mg q.d. (n = 17) |
Rifabutin at 300 mg q.d. (n = 17) |
Lersivirine at 1,000 mg q.d. + rifabutin at 300 mg q.d. (n = 18) |
||||
| Mild | Moderate | Mild | Moderate | Mild | Moderate | |
| Chromaturia | 0 | 0 | 14 (82) | 1 (6) | 18 (100) | 0 |
| Abdominal distension | 1 (6) | 0 | 0 | 0 | 2 (11) | 0 |
| Diarrhea | 2 (12) | 0 | 1 (6) | 0 | 1 (6) | 0 |
| Nausea | 6 (35) | 2 (12) | 0 | 0 | 5 (28) | 1 (6) |
| Vomiting | 1 (6) | 0 | 0 | 0 | 3 (17) | 0 |
| Chills | 0 | 0 | 1 (6) | 0 | 3 (17) | 0 |
| Pyrexia | 1 (6) | 0 | 4 (24) | 0 | 1 (6) | 1 (6) |
| Dizziness | 6 (35) | 0 | 0 | 0 | 3 (17) | 1 (6) |
| Head discomfort | 1 (6) | 0 | 0 | 0 | 2 (11) | 0 |
| Headache | 4 (24) | 1 (6) | 3 (18) | 1 (6) | 6 (33) | 1 (6) |
| Oropharyngeal pain | 0 | 0 | 2 (12) | 0 | 1 (6) | 0 |
No cases of severe treatment-emergent adverse events were recorded.
DISCUSSION
Coadministration of lersivirine with either rifampin or rifabutin decreased lersivirine exposure by 85% and 34% (as measured by AUC24), respectively, and decreased t1/2, which is representative of an increase in lersivirine clearance. The substantial decrease in exposure of lersivirine in the presence of rifampin is indicative of induction of both glucuronidation and CYP-mediated lersivirine clearance pathways. Lersivirine is a substrate for CYP3A4, as has been shown with recombinant CYP3A4, where the intrinsic clearance rate of lersivirine was 0.9 μl/pmol CYP/min, and to a far lesser extent with CYP3A5 (<0.08 μl/pmol CYP/min) (35). On the basis of in vitro data, lersivirine is also an inhibitor and substrate for P-gp (Pfizer Inc., data on file). UGT2B7 is also involved in the metabolism of lersivirine, such that with recombinant UGT2B7, glucuronide formation was linear with time up to 60 min and glucuronide at up to 1 mg/ml (35). The lersivirine plasma concentration was lowered to a greater extent with rifampin, a more potent inducer, than rifabutin.
Lersivirine did not affect the exposure of rifabutin but did reduce the exposure of 25-O-desacetyl-rifabutin. The decrease in exposure of the active metabolite of rifabutin may indicate that there is induction of the CYP3A4 pathway by lersivirine and/or autoinduction by rifabutin when rifabutin and lersivirine are coadministered. However, given that 25-O-desacetyl-rifabutin contributes up to 10% of the total antimicrobial activity (26) with rifabutin treatment, the decrease in metabolite exposure is not thought to be clinically relevant.
A limitation of the study was that all participants were Asian males. However, race has been shown to have only a marginal effect on lersivirine PKs, with Asian subjects having slightly lower lersivirine apparent oral clearance (CL/F), after adjusting for weight (36). A formal analysis comparing lersivirine PKs between men and women has not been conducted; however, preliminary data suggest that the difference between gender and age is not significant, after adjusting for weight and race. These data and the fact that genetic variants of both CYP3A and UGT2B7, enzymes predominantly responsible for lersivirine metabolism, have been shown not to be of clinical relevance (6, 13, 14, 25) suggest that the results of this study are generalizable to other populations.
Exposures of other NNRTIs, such as efavirenz, nevirapine, and rilpivirine, have been shown to change in the presence of rifampin and rifabutin (7, 15, 17, 19, 34). In one study, the change in efavirenz exposure following coadministration with rifampin ranged from a decrease of 65% to an increase of 37% (21), which suggests interindividual differences in enzyme activity. Furthermore, studies have shown that individuals with CYP2B6 516 TT genotypes are at risk of high efavirenz plasma exposures even in the presence of rifampin (20, 29). However, efavirenz trough concentrations, the best predictor of virological activity, have been shown to remain above the concentration necessary to suppress HIV in vitro in patients on concomitant rifampin (22). In contrast, efavirenz exposure is not significantly affected by rifabutin (2). A marked decrease (10 to 68%) in nevirapine concentrations is consistently observed in most PK studies in the presence of rifampin (33), and studies carried out in Indian (30) and African (4) patients coinfected with HIV and M. tuberculosis reported a significant proportion with subtherapeutic plasma nevirapine concentrations. Rifabutin has also been shown to induce a small decrease in the nevirapine exposure; however, the clinical significance is unclear. Two PK studies have investigated the effect of rifampin and rifabutin on rilpivirine exposure. When combined with rifampin, the rilpivirine AUC24 decreased by 80% and the Cmax and the minimum concentration in plasma (Cmin) decreased by 69% and 89%, respectively (34). When combined with rifabutin, the rilpivirine AUC24 decreased by 46% and the Cmax and Cmin decreased by 35% and 49%, respectively (7).
In conclusion, rifampin was shown to be a more potent inducer of lersivirine metabolism than rifabutin; therefore, lersivirine should not be coadministered with rifampin. No dose adjustment is necessary for rifabutin in the presence of lersivirine; however, an upward daily dose adjustment of lersivirine may be warranted when it is coadministered with rifabutin.
ACKNOWLEDGMENTS
Study A5271008 was funded by Pfizer Inc. Study A5271043 was conducted by Pfizer Inc. and funded by ViiV Healthcare.
Manoli Vourvahis, Rong Wang, Gary Layton, Heng Wee Choo, and Margaret Tawadrous are employees of Pfizer Inc. and hold stock options in Pfizer Inc. John Davis and Chew-Lan Chong were employed by Pfizer Inc. at the time of the study. Editorial support was provided by Karen Irving at Complete Medical Communications and was funded by ViiV Healthcare.
Footnotes
Published ahead of print 29 May 2012
REFERENCES
- 1. Back DJ, et al. 1980. The effect of rifampicin on the pharmacokinetics of ethynylestradiol in women. Contraception 21:135–143 [DOI] [PubMed] [Google Scholar]
- 2. Benedek IH, et al. 1998. Pharmacokinetic interaction between multiple doses of efavirenz (EFV) and rifabutin in healthy volunteers, abstr 461. Abstr. 36th Annu. Meeting Infect. Dis. Soc. Am., Denver, CO [Google Scholar]
- 3. Breen RA, Swaden L, Ballinger J, Lipman MC. 2006. Tuberculosis and HIV co-infection: a practical therapeutic approach. Drugs 66:2299–2308 [DOI] [PubMed] [Google Scholar]
- 4. Cohen K, et al. 2008. Effect of rifampicin-based antitubercular therapy on nevirapine plasma concentrations in South African adults with HIV-associated tuberculosis. J. Antimicrob. Chemother. 61:389–393 [DOI] [PubMed] [Google Scholar]
- 5. Corbau R, et al. 2010. Lersivirine, a nonnucleoside reverse transcriptase inhibitor with activity against drug-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 54:4451–4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Court MH, et al. 2003. Evaluation of 3′-azido-3′-deoxythymidine, morphine, and codeine as probe substrates for UDP-glucuronosyltransferase 2B7 (UGT2B7) in human liver microsomes: specificity and influence of the UGT2B7*2 polymorphism. Drug Metab. Dispos. 31:1125–1133 [DOI] [PubMed] [Google Scholar]
- 7. Crauwels HM, et al. 2008. The pharmacokinetic interaction between rifabutin and TMC278, an investigational NNRTI, poster TUPE0080. Abstr. XVII Int. AIDS Conf [Google Scholar]
- 8. Davis J, et al. 2007. Safety, toleration and pharmacokinetics of single and multiple oral doses of UK-453,061, a novel NNRTI, in healthy male subjects, poster 109. Abstr. 4th IAS Conf. HIV Pathogenesis, Treatment, Prevention [Google Scholar]
- 9. Davis J, et al. 22 April 2012. The effect of lersivirine (UK-453,061), a next-generation NNTRI, on the pharmacokinetics of midazolam and oral contraceptives in healthy subjects. J. Clin. Pharmacol. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 10. Davis J, et al. 2008. The effect of UK-453,061, a next-generation NNRTI, on the pharmacokinetics of zidovudine, midazolam, and contraceptive steroids, abstr P25. Abstr. 9th Int. Workshop Clin. Pharmacol. HIV Ther., New Orleans, LA [Google Scholar]
- 11. Diedrich CR, Flynn JL. 2011. HIV/Mycobacterium tuberculosis coinfection immunology: how does HIV exacerbate TB? Infect. Immun. 79:1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fätkenheuer G, et al. 2009. Activity, pharmacokinetics and safety of lersivirine (UK-453,061), a next-generation nonnucleoside reverse transcriptase inhibitor, during 7-day monotherapy in HIV-1 infected patients. AIDS 23:2115–2122 [DOI] [PubMed] [Google Scholar]
- 13. Floyd MD, et al. 2003. Genotype-phenotype associations for common CYP3A4 and CYP3A5 variants in the basal and induced metabolism of midazolam in European- and African-American men and women. Pharmacogenetics 13:595–606 [DOI] [PubMed] [Google Scholar]
- 14. He P, Court MH, Greenblatt DJ, von Moltke LL. 2005. Genotype-phenotype associations of cytochrome P450 3A4 and 3A5 polymorphism with midazolam clearance in vivo. Clin. Pharmacol. Ther. 77:373–387 [DOI] [PubMed] [Google Scholar]
- 15. Hsu O, Hill CJ, Kim M, Tan B, O'Brien JG. 2010. Decreased plasma efavirenz concentrations in a patient receiving rifabutin. Am. J. Health Syst. Pharm. 67:1611–1614 [DOI] [PubMed] [Google Scholar]
- 16. Jamis-Dow CA, Katki AG, Collins JM, Klecker RW. 1997. Rifampin and rifabutin and their metabolism by human liver esterases. Xenobiotica 27:1015–1024 [DOI] [PubMed] [Google Scholar]
- 17. Kakuda TN, Scholler-Gyure M, Hoetelmans RM. 2011. Pharmacokinetic interactions between etravirine and non-antiretroviral drugs. Clin. Pharmacokinet. 50:25–39 [DOI] [PubMed] [Google Scholar]
- 18. Kraft WK, et al. 2004. Indinavir and rifabutin drug interactions in healthy volunteers. J. Clin. Pharmacol. 44:305–313 [DOI] [PubMed] [Google Scholar]
- 19. Kwara A, Lartey M, Sagoe KW, Court MH. 2011. Paradoxically elevated efavirenz concentrations in HIV/tuberculosis-coinfected patients with CYP2B6 516TT genotype on rifampin-containing antituberculous therapy. AIDS 25:388–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kwara A, et al. 2008. Pharmacokinetics of efavirenz when co-administered with rifampin in TB/HIV co-infected patients: pharmacogenetic effect of CYP2B6 variation. J. Clin. Pharmacol. 48:1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lopez-Cortes LF, et al. 2002. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin. Pharmacokinet. 41:681–690 [DOI] [PubMed] [Google Scholar]
- 22. Manosuthi W, et al. 2005. Efavirenz levels and 24-week efficacy in HIV-infected patients with tuberculosis receiving highly active antiretroviral therapy and rifampicin. AIDS 19:1481–1486 [DOI] [PubMed] [Google Scholar]
- 23. Mori J, Thornberry A, Perros M, Westby M, Craig C. 2009. In vitro passage of HIV-1 in the presence of the next-generation NNRTI, lersivirine (UK-453,061): phenotypic and genotypic observations, poster 63. Abstr. European HIV Drug Resistance Workshop [Google Scholar]
- 24. Narang PK, Lewis RC, Bianchine JR. 1992. Rifabutin absorption in humans: relative bioavailability and food effect. Clin. Pharmacol. Ther. 52:335–341 [DOI] [PubMed] [Google Scholar]
- 25. Peterkin VC, et al. 2007. Limited influence of UGT1A1*28 and no effect of UGT2B7*2 polymorphisms on UGT1A1 or UGT2B7 activities and protein expression in human liver microsomes. Br. J. Clin. Pharmacol. 64:458–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pfizer Inc. US 2011. Mycobutin US physician prescribing information. Pfizer Inc. US, New York, NY [Google Scholar]
- 27. Pham PA, Flexner C. 2011. Emerging antiretroviral drug interactions. J. Antimicrob. Chemother. 66:235–239 [DOI] [PubMed] [Google Scholar]
- 28. Phillips C, Irving S, Ringrose H, Corbau R, Mowbray C. 2007. HIV-1 reverse transcriptase structure-based drug design: crystals to clinic, abstr. M505 03. Abstr. 24th European Crystallographic Meeting, Marrakech, Morocco [Google Scholar]
- 29. Ramachandran G, et al. 2009. CYP2B6 G516T polymorphism but not rifampin coadministration influences steady-state pharmacokinetics of efavirenz in human immunodeficiency virus-infected patients in South India. Antimicrob. Agents Chemother. 53:863–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramachandran G, et al. 2006. Increasing nevirapine dose can overcome reduced bioavailability due to rifampicin coadministration. J. Acquir. Immune Defic. Syndr. 42:36–41 [DOI] [PubMed] [Google Scholar]
- 31. Ren Y, et al. 2009. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J. Acquir. Immune Defic. Syndr. 50:439–443 [DOI] [PubMed] [Google Scholar]
- 32. Skinner MH, Blaschke TF. 1995. Clinical pharmacokinetics of rifabutin. Clin. Pharmacokinet. 28:115–125 [DOI] [PubMed] [Google Scholar]
- 33. Stohr W, et al. 2008. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir. Ther. 13:675–685 [PubMed] [Google Scholar]
- 34. Van Heeswijk RPG, et al. 2006. The effects of CYP34A modulation on the pharmacokinetics of TMC278, an investigational non-nucleoside reverse transcriptase inhibitor (NNRTI), poster 74. Abstr. 7th Int. Workshop Clin. Pharmacol. HIV Ther., Lisbon, Portugal [Google Scholar]
- 35. Vourvahis M, et al. 2010. Excretion and metabolism of lersivirine (5-([3,5-diethyl-1-(2-hydroxyethyl)(3,5-14C2)-1H-pyrazol-4-yl]oxy)benzene-1,3-dicarbonitrile), a next-generation non-nucleoside reverse transcriptase inhibitor, after administration of [14C]lersivirine to healthy volunteers. Drug Metab. Dispos. 38:789–800 [DOI] [PubMed] [Google Scholar]
- 36. Weatherley B, Vourvahis M, McFadyen L. 2011. Modeling of maraviroc pharmacokinetics in the presence of atazanavir/ritonavir in healthy volunteers and HIV-1-infected patients, abstr P-05. Abstr. 12th Int. Workshop Clin. Pharmacol. HIV Ther., Miami, Florida [Google Scholar]
- 37. World Health Organization 2007. Tuberculosis facts. World Health Organization, Geneva, Switzerland: www.who.int/tb/publications/2007/factsheet_2007.pdf [Google Scholar]