Abstract
While extended infusions of piperacillin-tazobactam (TZP) are increasingly used in practice, the effect of infusion on the pharmacokinetic (PK) profile of TZP has not been widely assessed. To assess its effect on the pharmacokinetic profile of TZP, seven serum samples were collected from 11 hospitalized patients who received 3.375 g TZP intravenously for 4 h every 8 h. Population pharmacokinetic models were fit to the PK data utilizing first-order, Michaelis-Menten (MM), and parallel first-order/MM clearance. A population PK model with first-order clearance was fit to the tazobactam PK data. Monte Carlo simulations (MCSs) were used to determine the most effective administration schedule to ensure that free piperacillin concentrations were above the MIC for at least 50% of the dosing interval (50% fT>MIC) and to quantify the extent of the nonlinear clearance. The model incorporating parallel linear/MM clearance best fit the piperacillin PK data. The MCSs demonstrated that approximately 50% of the administered piperacillin is cleared by the nonlinear clearance mechanism. The results of the MCSs also revealed that more intensive TZP extended infusion dosing schemes (3.375 to 4.5 g intravenously [3-h infusion] every 6 h) than those commonly used in clinical practice were needed to maximize the 50% fT>MIC for MICs of ≥8 mg/liter. This study suggests that extended infusion of TZP is the most effective method of administration for patients with nosocomial infections. Due to the hyperclearance nature of the hospitalized patient populations studied, more intensive TZP dosing regimens may be needed to maximize fT>MIC in certain hospitalized populations.
INTRODUCTION
Piperacillin-tazobactam (TZP) is a combination of an extended-spectrum β-lactam antibiotic and a β-lactamase inhibitor and is frequently used for nosocomial infections (3, 21). For β-lactam antibiotics, the pharmacodynamic index that best links drug exposure with the observed antibacterial effect is the fraction of the dosing interval in which free drug concentrations are above the MIC (9). Near-maximal effect is generally observed when free concentrations exceed the MIC for at least 50% of the dosing interval (fT>MIC 50%) (12). Although TZP is frequently administered as a rapid infusion, extended infusions of TZP are increasingly used in clinical practice because they facilitate the extension of the fT>MIC.
Although the method is more commonplace than rapid infusions, the impact of prolonging the infusion time of TZP on its pharmacokinetic (PK) profile has not been widely assessed. Here, we describe the population pharmacokinetics for both piperacillin and tazobactam among hospitalized patients receiving an extended infusion regimen. The goal was to identify the model that best explained the observed clearance of both piperacillin and tazobactam. When modeling piperacillin, it is important to consider linear, Michalis-Menten (MM), and parallel first-order/MM models. Piperacillin is cleared via a combination of renal tubular secretion and glomerular filtration (25). While glomerular filtration is a linear process, tubular secretion, via an anion transporter system (organic anion transporting polypeptide 1), is nonlinear (i.e., the transporter's activity is saturable and has a maximum rate). Understanding the optimal clearance mechanism of piperacillin has important implications for clinical practice. If piperacillin is found to have MM or parallel first-order/MM clearance, then increasing the piperacillin dose may lead to a disproportionate increase in plasma exposure and result in better probability of target attainment or, perhaps, toxicity (4, 22).
Monte Carlo simulation was also used to explore the potential clinical consequences of inherent pharmacokinetic variability in hospitalized patients. In particular, simulation studies were used to explore the potential pharmacodynamic benefits of using an extended infusion, as suggested by Lodise et al. (18), compared to the licensed schedule of administration for 30 min (14). Alternative TZP regimens were also explored in an effort to maximize the probability of target attainment (PTA) against a range of MICs at the higher end of the CLSI susceptibility range for nonfermentative Gram-negative pathogens. Finally, D-optimal design was used to identify the most informative sampling times to generate robust population pharmacokinetic parameter estimates for future studies (26).
MATERIALS AND METHODS
Pharmacokinetic study.
Patients who received extended infusions of TZP for suspected or documented nosocomial infections at the Albany Medical Center Hospital (Albany, New York) were eligible for enrollment. This study received approval from the Albany Medical Center Hospital institutional review board. Only patients that resided in the hospital for at least 48 h prior to starting TZP were considered. As standard hospital protocol, patients were administered 3 g piperacillin in combination with 0.375 g tazobactam intravenously during a 4-h infusion period every 8 h. Written informed consent was obtained from all patients participating in the study. Demographic data (including age, sex, race, height, and weight of the patient), cause of admission to the hospital, and underlying renal function were recorded.
For each patient, the length of infusion and infusion start and stop times were recorded for 24 h prior to the dose being studied. Seven blood samples were collected after the 3rd but before the 11th dose (i.e., at steady state). The times for the collection of samples for this study, 2, 4, 4.5, 5, 5.5, 6.5, and 7 h after the initiation of the infusion, were developed using D-optimal design and based on a previous population PK model (18). The exact time of each 3- to 5-ml sample acquisition was recorded. All blood samples were allowed to clot for 15 min at room temperature and then centrifuged at 2,400 rpm for 10 min. Sera from each sample were separated into three vials and stored at −80°C.
Piperacillin and tazobactam assay.
Total piperacillin and tazobactam concentrations were measured using a previously validated high-performance liquid chromatography (HPLC) method (15). Briefly, drug concentrations were measured using a gradient-controlled pump (model 626; Waters, Milford, MA), an autosampler (WISP 717 plus; Waters), and a UV detector (model sm 4000; LDC Analytical, Riviera Beach, FL). A reverse-phase HPLC column (Bondpak C18 Guard-pak precolumn; Phenomenex prodigy; 10-μm volume; 4.6 mm by 250 mm) was used. The injection volume was 5 μl. A standard curve encompassing 2 to 100 mg/liter and 1 to 50 mg/liter for piperacillin and tazobactam, respectively, was constructed from stock solutions. The internal standard was penicillin G. The following mobile phases were used: A, 90% (vol/vol) HPLC-grade acetonitrile and 10% (vol/vol) sodium phosphate buffer (0.01 M; pH 2.7); B, 100% HPLC methanol; C, 3% (vol/vol) HPLC-grade acetonitrile and 97% (vol/vol) sodium phosphate buffer (0.01 M; pH 2.7); and D, 100% water. The gradient-controlled pump was programmed as 5% of A and 95% of C at 0 to 10 min, 45% of A and 55% of C at 10 to 18 min, and 5% of A and 95% of C at 18 to 22 min. The mobile-phase flow rate was 1.2 ml/min. The wavelength of the UV detector was programmed as 218 nm at 0 to 10 min, 254 nm at 10 to 20 min, and 218 nm at 20 to 22 min. The intra- and interassay coefficient of variation was <5.8% for both compounds. The lower limit of quantification was 1 and 2 mg/liter for tazobactam and piperacillin, respectively.
Population pharmacokinetic modeling.
All data were analyzed using a population pharmacokinetic methodology. The nonparametric adaptive grid (NPAG) program Pmetrics within the R statistical environment was used (19, 20). The PK data were weighted by the inverse of the estimated assay variance for both piperacillin and tazobactam. A polynomial describing the assay variance, derived from regression of the measured drug concentrations and observed assay variances, was determined. An adaptive scalar (γ) was used which multiplies the polynomial described above and was determined with each cycle to obtain the best approximation to the homoscedastic assumption. The means, medians, and standard deviations of the population parameters were estimated. Bayesian estimates for the parameters (using the “population of one” utility in NPAG) for each patient were also obtained. Scatter plots of observed versus predicted piperacillin concentrations were examined for individual patients and for the population as a whole. The fit of each of the structural models to the data were assessed in the following way: (i) the log-likelihood value; (ii) the coefficients of determination (r2) from regression of the observed-predicted plots before and after the Bayesian step; and (iii) the Akaike information criterion (AIC) (1). Statistically significant differences between models were determined by assessing twice the difference in log-likelihood values against a χ2 distribution with the appropriate number of degrees of freedom (i.e., difference in parameter number for the respective models).
For piperacillin, three two-compartment structural mathematical models were evaluated: (i) elimination as a first-order process (equation 1); (ii) an MM process alone (equation 2); and (iii) an MM process with parallel first-order elimination (equation 3). The reason for the model choices is based on the known drug handling of piperacillin. The drug is known to be cleared by glomerular filtration, which is a linear process. It is also known to be tubularly secreted, which is an inherently MM process. The goal here is to determine the amount of drug clearance due to the MM process relative to the linear process. If the amount of MM clearance relative to the linear process is low, the linear system should suffice. If the amount of MM drug clearance is higher, then the MM or parallel first-order/MM model would be needed.
The ordinary differential equations for these models were the following:
| (1) |
| (2) |
| (3) |
| (4) |
X1 and X2 are the amounts of piperacillin (in milligrams) in the central compartment and peripheral compartment, respectively. R (1) represents the infusion of drug. SCL (liters per hour) is the clearance, and Vc is the volume of the central compartment (liters). Vmax is the maximum rate of enzyme activity (milligrams per hour), and Km is the concentration of piperacillin where enzyme activity is half maximal (milligrams per liter). Kcp and Kpc are the first-order intercompartmental rate constants.
For tazobactam, only a first-order elimination model was assessed. The ordinary differential equations for this model were the following:
| (5) |
| (6) |
where X1 and X2 are the amounts of tazobactam (in milligrams) in the central compartment and peripheral compartment, respectively.
Monte Carlo simulation.
Each Monte Carlo simulation was performed using a 5,000-subject simulation. The mean parameter vector and the full covariance matrix from the population PK analysis was embedded in subroutine PRIOR of the ADAPT 5 program (10, 11). Both normal and log-normal parameter distributions were explored in the simulations and distinguished on the ability to recapitulate the original parameter values and their dispersions.
As an additional method for assessing the overall fit of the models to the PK data, 5,000 subject simulations of 3 g of piperacillin administered for 4 h every 8 h was performed using each of the three mathematical models: (i) first-order process; (ii) an MM process alone; and (iii) an MM process with parallel first-order elimination. For each mathematical model, the median, 5th-percentile, and 95th-percentile concentrations for the population were identified for piperacillin at the beginning and end of drug administration and every 15 min throughout the ninth dosing interval (steady state). The observed patient data were simultaneously plotted with the simulated concentration-time curves. The fidelity by which the concentration-time curves mirrored the raw data were assessed by visual inspection.
To compare the amount of piperacillin removed during 24 h according to the clearance terms in the parallel linear/MM, 5,000-subject simulations were performed using 3 doses of 4 g of piperacillin administered during either 30 min or 4 h every 8 h for both models. The amount of piperacillin cleared by each of the clearance mechanisms was assessed using the differential equations below. Equations 7 and 8 were used to determine the amount of drug cleared by the MM and linear clearance mechanisms, respectively. Additionally, for the parallel linear/MM model, the time the piperacillin concentration was above the Michaelis-Menten constant during the third dosing interval was determined.
| (7) |
| (8) |
Finally, for the overall best-fitting model, 5,000-subject simulations were performed using 3 or 4 g of piperacillin administered for 30 min or for 4 h every 8 h and 3 or 4 g of piperacillin administered for 30 min or for 3 h every 6 h. For each regimen, the fraction of simulated subjects who achieved the pharmacodynamic target of 50% fT>MIC for a range of MICs from 0.5 to 128 mg/liter was determined.
Optimal sampling schedule.
The optimal sampling times, following the first dose and at steady state (9th dose), for an infusion of 4 g of piperacillin and 0.5 g of tazobactam administered for 30 min or for 4 h every 8 h were investigated. The multiple-model file from the output of NPAG was used. The parallel linear/MM PK model was used. Each of the parameter vectors was utilized in a D-optimal design analysis using the ADAPT 5 program (11). The optimal sampling points were then weighted by their probability and presented in a histogram as previously described by Tam et al. (26). Summation of the D-optimal design analysis for each support point allowed delineation of six optimal sampling times for the population. Each of the sampling times optimally identifies a parameter within the structural model. This process was performed for both the first dose and a steady-state sampling schedule for both piperacillin and tazobactam.
RESULTS
Pharmacokinetic study.
Between February and July 2005, 11 patients were enrolled with a mean age of 44.7 ± 12.5 years. Mean height and weight were 1.90 ± 0.23 m and 78.0 ± 22.1 kg, respectively. All patients were Caucasian, and 7 (64%) were male. Receipt of TZP during intensive care unit (ICU) stay occurred in 7 (58.33%) patients. Mean baseline creatinine clearance was 122 ± 35 ml/min. The demographics and clinical characteristics are displayed in Table 1. A total of 71 plasma concentrations were obtained after multiple dosing from 11 individuals.
Table 1.
Clinical characteristics of 11 patientsa
| Characteristic | Result |
||
|---|---|---|---|
| Mean | SD | Range | |
| Age (yr) | 44.7 | 12.5 | 20–58 |
| Male (no. [%]) | 7 (64) | ||
| Body wt (kg) | 78.0 | 22.1 | 38.1–122.5 |
| Ht (m) | 1.90 | 0.23 | 1.50–2.15 |
| ICU admission (no. [%]) | 7 (64) | ||
| Creatinine clearance (ml/min) | 122.0 | 35.0 | 77.4–169.1 |
All patients were white.
Population pharmacokinetic modeling.
The means, medians, and standard deviations of the population parameter estimates for the three models are shown in Table 2. The goodness of fit for each of the four models to the data was comparable. The log-likelihood values, Akaike information criterion, and outputs from the regression of observed versus predicted values after the Bayesian step, including the coefficients of determination (r2), are shown in Table 3.
Table 2.
Piperacillin population pharmacokinetic parameter estimates obtained by Pmetricsa
| Model and drug | Parameter and result (mean ± SD [median]) |
|||||
|---|---|---|---|---|---|---|
| Vmax (mg/h) | Km (mg/liter) | Vc (liter) | Kcp (h−1) | Kpc (h−1) | SCL (liter/h) | |
| Piperacillin | ||||||
| Linear | 18.90 ± 9.19 (16.41) | 2.62 ± 3.91 (0.57) | 28.38 ± 17.41 (39.62) | 17.44 ± 6.09 (15.14) | ||
| Michaelis-Menten | 1,634.68 ± 597.33 (1863.70) | 78.57 ± 52.51 (89.69) | 13.10 ± 7.13 (14.73) | 12.80 ± 9.97 (11.83) | 20.89 ± 14.72 (30.09) | |
| Parallel First-Order/MM | 898.91 ± 402.61 (808.31) | 90.13 ± 74.14 (77.43) | 13.67 ± 7.20 (15.78) | 9.19 ± 10.25 (3.79) | 20.95 ± 16.91 (28.58) | 6.62 ± 3.81 (6.89) |
| Tazobactam | ||||||
| Linear | 21.13 ± 10.75 (20.31) | 5.96 ± 11.77 (0.37) | 29.10 ± 17.14 (39.69) | 15.16 ± 4.63 (13.68) | ||
Vmax is the maximum elimination rate; Km is the Michaelis-Menten constant; Vc is the apparent volume of distribution of the central compartment; Kcp and Kpc are first-order intercompartmental transfer rate constants; SCL is clearance; MM is Michaelis-Menten.
Table 3.
Evaluation of the predictive performance of piperacillin and tazobactam population modelsa
| Model and drug | Log likelihood | Linear regression of observed-predicted for each patient |
AIC | χ2 compared to: |
|||
|---|---|---|---|---|---|---|---|
| Intercept | Slope | r2 | Linear model | MM model | |||
| Piperacillin | |||||||
| Linear | −169.41 | 0.653 | 1.010 | 0.918 | 184.41 | NA | |
| Michaelis-Menten | −156.86 | 0.945 | 0.992 | 0.943 | 177.86 | 3.96E−04 | NA |
| Parallel girst-order/MM | −154.54 | 0.822 | 1.000 | 0.939 | 182.54 | 5.90E−04 | 0.13 |
| Tazobactam | |||||||
| Linear | −51.78 | 0.009 | 1.030 | 0.908 | 66.78 | ||
r2 is the coefficient of determination for the best-fit linear regression for the predicted-observed plot after the maximal a posteriori probability (MAP) Bayesian step. AIC is the Akaike information criterion. MM is Michaelis-Menten. NA, not applicable.
For the linear model fitted to the piperacillin data, a linear regression of the observed-predicted plot for each patient after the Bayesian step revealed the following: observed = 1.01 × predicted + 0.653; r2 = 0.918. The following was revealed for the MM model: observed = 0.992 × predicted + 0.945; r2 = 0.94. For the parallel first-order/MM model, the regression line was the following: observed = 1.000 × predicted + 0.822; r2 = 0.939. The log-likelihood values were −169.41, −156.86, and −154.54 for the linear, MM, and parallel first-order/MM models, respectively. Evaluation of the log-likelihood values against a χ2 distribution, with the appropriate number of degrees of freedom, revealed P < 0.005 for the MM and parallel first-order/MM models compared to the linear model (i.e., differences in the log-likelihood values were statistically significant despite the larger number of parameters). The Akaike information criterion was 184.41, 177.86, and 182.54 for the linear, MM, and parallel first-order/MM models, respectively.
For tazobactam, linear regression of the observed-predicted plot for each patient after the Bayesian step revealed the best-fit regression line of observed = 1.03 × predicted − 0.009; r2 = 0.908. The log likelihood and AIC were −51.78 and 66.78, respectively. The population estimates for tazobactam clearance and volume of distribution were 15.16 liters/h and 21.13 liters, respectively.
Monte Carlo simulation. (i) Observed versus simulated concentration-time profiles.
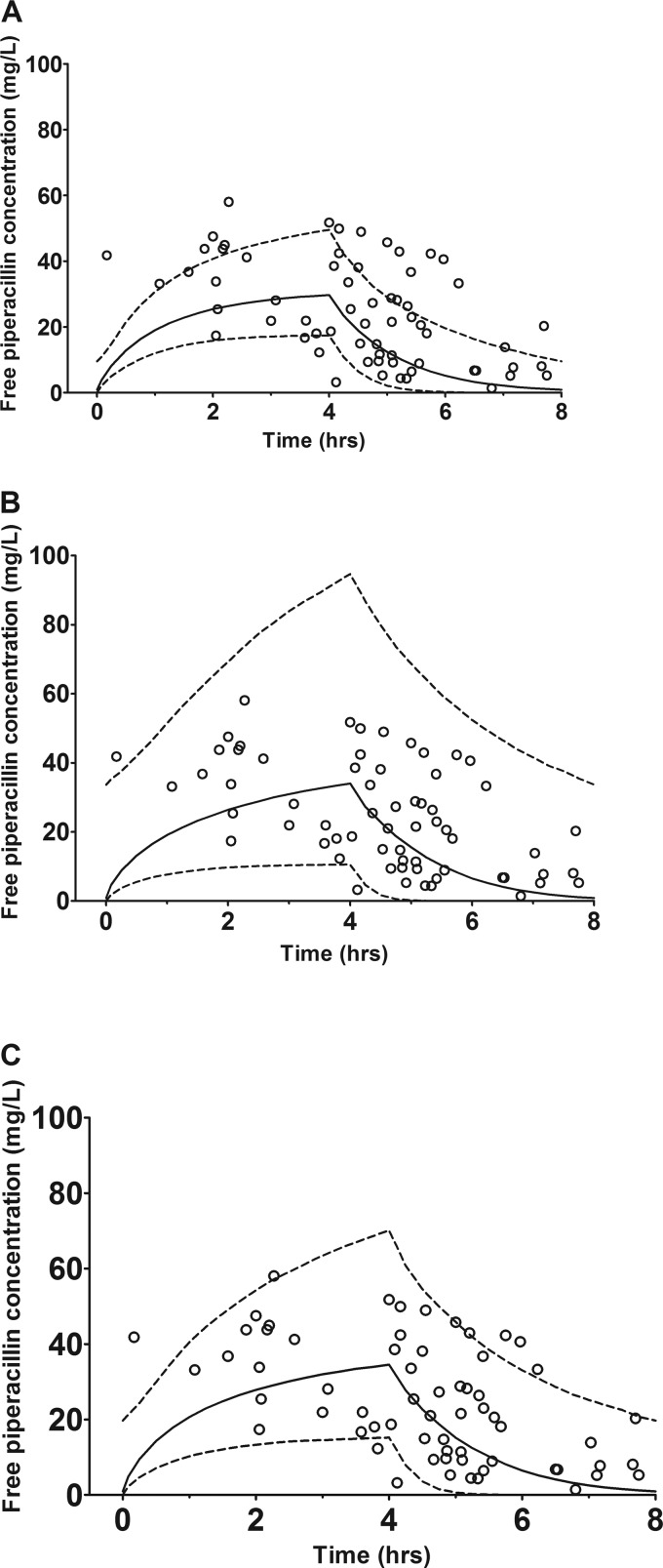
The three mathematical models, (i) a first-order process, (ii) an MM process alone, and (iii) an MM process with parallel first-order elimination, were compared using a 5,000-subject simulation of 3 g of piperacillin administered for 4 h every 8 h. The mean population parameter values and their SD for piperacillin, using a log-normal distribution, were readily recapitulated. Figure 1 shows the median, 5th-percentile, and 95th-percentile piperacillin concentrations for the population generated by each model alongside the observed patient data. Visual inspection shows the parallel linear/MM model most accurately represented the observed data.
Fig 1.
Three mathematical models evaluated for piperacillin. The lines represent the median, 5th-percentile, and 95th-percentile of unbound piperacillin concentrations at steady state for piperacillin (3 g) administered for 4 h. Open circles represent observed piperacillin plasma concentrations. (A) Linear model; (B) Michaelis-Menten model; and (C) parallel first-order/Michaelis-Menten model.
(ii) Comparison of amount of piperacillin removed by the clearance terms in the parallel linear/MM model.
Following administration of 3 doses of 4 g of piperacillin for 4 h every 8 h, a median (% of total; ±SD; range) of 6.13 g (53.25%; ±2.24 g; 0.35 to 11.52 g) was cleared by the MM mechanism, and a median (% of total; ±SD; range) of 5.39 g (46.75%; ± 2.17 g; 0.35 to 11.52 g) was cleared by the linear mechanism for the parallel linear/MM model. Following administration of 3 doses of 4 g of piperacillin for 30 min every 8 h, a median (%; ±SD; range) of 5.33 g (45.75%; ±2.10 g; 0.33 to 11.23 g) was cleared by the MM mechanism, and a median (%; ±SD; range) of 6.31 g (54.25%; ±2.14 g; 0.41 to 11.64 g) was cleared by the linear mechanism. The mean (±SD) portion of the third dosing interval in which the total piperacillin concentration was greater than the Michaelis-Menten constant was 18.84% ± 24.43% for the third dose of 4 g of piperacillin administered during 4 h and was 22.05% ± 16.67% for 4 g of piperacillin administered during 30 min. However, 2,674 and 625 patients administered 4 g of piperacillin for 4 h and 30 min, respectively, never had a piperacillin concentration above the Michaelis-Menten constant.
(iii) Probability of target attainment analyses.
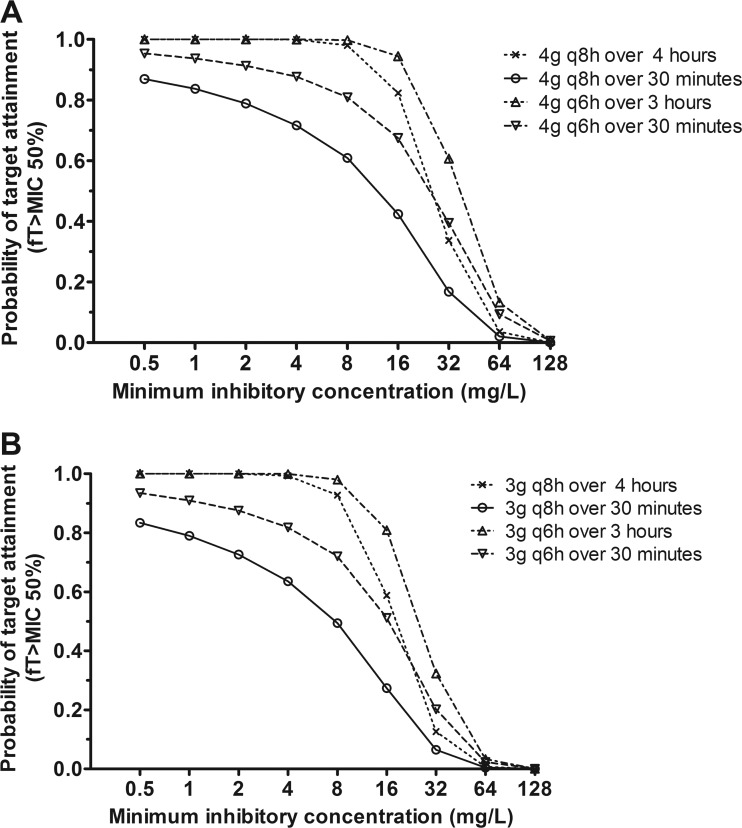
Although there were no significant differences in log-likelihood values and AIC between the MM and parallel linear/MM models, the parallel linear/MM models were used for the PTA analyses based on the results of the observed versus simulated concentration-time profile plots. Also, this model is best supported by the known physiology. The results of the PTA analyses for piperacillin are displayed in Fig. 2. Infusion of 4 g of piperacillin, during 3 or 4 h every 6 or 8 h, results in a PTA of more than 98% for organisms with MICs of ≤8 mg/liter. For pathogens with a MIC of 16 mg/liter, the PTA was 94% when piperacillin was administered for 3 h every 6 h and was 82% when piperacillin was administered for 4 h every 8 h. For MICs of >16 mg/liter, the PTA rapidly plummeted to zero for both regimens. Administration of 4 g of piperacillin for 30 min every 6 or 8 h results in a PTA of 95 and 87%, respectively, for a MIC of 0.5 mg/liter. A gradual decline in target attainment was then observed as the MIC increased, with a PTA of 81 and 61% for the 6- and 8-h regimens, respectively, for a MIC of 8 mg/liter. Administering 3 g of piperacillin for 3 or 4 h every 6 or 8 h results in a PTA of more than 98% for organisms with MICs of ≤8 mg/liter. For organisms with a MIC of 16 mg/liter, the PTAs were 81 and 59% for 3 g piperacillin administered for 3 or 4 h every 6 or 8 h, respectively, and 51 and 27% for 3 g piperacillin administered for 30 min every 6 or 8 h, respectively.
Fig 2.
Results of the Monte Carlo simulation with the fractional target attainments against a range of MICs were determined for the following regimens: 4 g piperacillin administered intravenously (i.v.) for either 30 min or 4 h every 8 h as well as for 30 min or 3 h every 6 h (A), and 3 g piperacillin administered i.v. for either 30 min or 4 h every 8 h as well as 4 g piperacillin administered i.v. for 30 min or 3 h every 6 h (B).
Optimal sampling schedule for future studies.
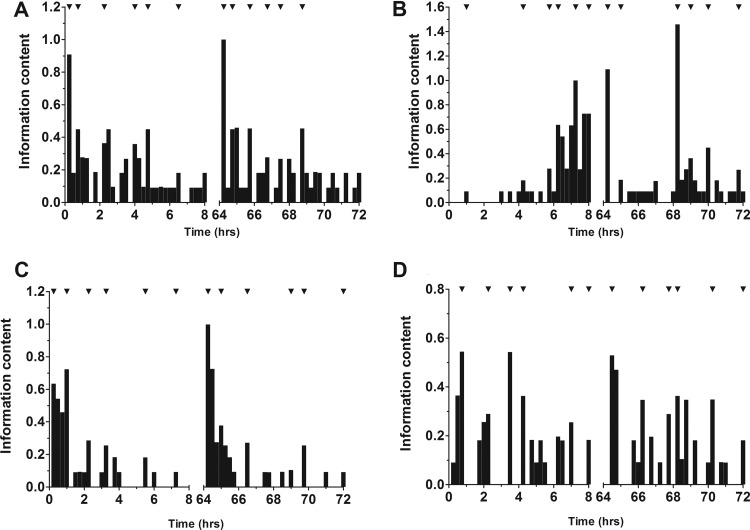
Summation of the D-optimal design analysis for each support point, following weighting by their probability, allowed identification of 6 sample time points (Fig. 3). For 4 g piperacillin/0.5 g tazobactam administered for 4 h every 8 h, the sampling time points should be 1.0, 3.25, 4.5, 5.25, 7.25, and 8 h after the initiation of the first dose and 0.25, 1.75, 4.0, 4.75, 6.0, and 7.75 h after initiation of the dose at steady state (Table 4). For 4 g piperacillin–0.5 g tazobactam administered for 30 min every 8 h, the sampling time points should be 0.25, 1.0, 2.25, 3.25, 5.50, and 7.25 h after initiation of the first dose and 0.25, 0.75, 2.0, 3.75, 4.5, and 6.25 h after initiation of the dose at steady state.
Fig 3.
Histograms showing the results of the D-optimal design analysis. (A) Piperacillin (4 g) administered for 30 min; (B) piperacillin (4 g) administered for 4 h; (C) tazobactam (0.5 g) administered for 30 min; and (D) tazobactam (0.375 g) administered for 4 h. The timings identified are during both the first-dose and the steady-state interval.
Table 4.
Optimal timing after the first dose and a steady-state dose as identified by D-optimal design
| Drug regimen | Optimal timing (h after dosage initiation) ofa: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First dose | Steady-state dose | |||||||||||
| Piperacillin (4 g) administered for 30 min | 0.25 | 0.75 | 2.25 | 4.00 | 4.75 | 6.50 | 0.25 | 0.75 | 1.75 | 2.75 | 3.50 | 4.75 |
| Tazobactam (500 mg) administered for 30 min | 0.25 | 1.00 | 2.25 | 3.25 | 5.50 | 7.25 | 0.25 | 1.00 | 2.50 | 5.00 | 5.75 | 8.00 |
| Piperacillin (4 g)-tazobactam (500 mg) administered for 30 min | 0.25 | 1.00 | 2.25 | 3.75 | 5.25 | 6.75 | 0.25 | 0.75 | 2.00 | 3.75 | 4.50 | 6.25 |
| Piperacillin (4 g) administered for 4 h | 1.00 | 4.25 | 5.75 | 6.25 | 7.25 | 8.00 | 0.25 | 1.00 | 4.25 | 5.00 | 6.00 | 7.75 |
| Tazobactam (500 mg) administered for 4 h | 0.75 | 2.25 | 3.50 | 4.25 | 7.00 | 8.00 | 0.50 | 2.25 | 3.75 | 4.25 | 6.25 | 8.00 |
| Piperacillin (4 g)-tazobactam (500 mg) administered for 4 h | 1.00 | 3.25 | 4.5 | 5.25 | 7.25 | 8.00 | 0.25 | 1.75 | 4.00 | 4.75 | 6.00 | 7.75 |
Values in bold represent a composite time point for sampling combinations of piperacillin at 4 g and tazobactam at 500 mg.
DISCUSSION
Analysis of this cohort of hospitalized patients receiving extended infusions of TZP found that use of a population model for piperacillin with a parallel linear/MM clearance term best described the observed data. After the Bayesian step, the predictive performances of the MM and parallel linear/MM model were statistically superior to the linear model. Although there were no significant differences in log-likelihood values and AIC between the MM and parallel linear/MM models, the results of the observed versus simulated concentration-time profile plots (Fig. 1) clearly demonstrated that the parallel linear/MM model best fit the data. For these reasons, the parallel linear/MM model was used for the PTA simulations and the D-optimal design. The superiority of a nonlinear clearance structural model for piperacillin is consistent with the findings of several previous studies and contradictory to others (2, 5, 6, 18, 23, 28). While there is support in the literature for both clearance structures, the parallel linear/MM model is more physiologically plausible, as piperacillin is cleared via both linear glomerular filtration and nonlinear tubular secretion. Quantification of the nonlinear clearance demonstrated that, on average, just under half of the administered dose is cleared by the MM mechanism with both extended and intermittent dosing. Furthermore, the results of the simulations indicated that, on average, concentrations exceeded the Km for about 20% of the dosing interval with both infusion methodologies. While these findings do not represent a major concern for the TZP dosing schemes currently used in clinical practice, the presence of a saturable clearance mechanism for piperacillin will be an important consideration if more intensive TZP dosing schemes (>16 g of piperacillin/day) are contemplated (4, 22). Further study of TZP pharmacokinetics following dosage escalation and in different study populations is required to confirm these observations.
With regard to the parallel linear/MM model, the population estimates for the volume of distribution and clearance in our study were similar to those observed in patients with sepsis by Roberts et al. (23) but higher than those of other population analyses (2, 5, 18). The enhanced or hyperdynamic clearance conditions observed in our study were largely driven by the high average creatinine clearance observed among the patients included in our study. This is not the first time this hyperclearance phenomenon among hospitalized patients has been described (7, 8, 13, 27). In keeping with other pharmacokinetic studies of critically ill patients, we observed significant variability, with a coefficient of variation of around 50% for the population pharmacokinetic parameters (24).
The consequences of the enhanced clearance or hyperclearance of piperacillin observed in our hospitalized patient study cohort were readily apparent in the Monte Carlo simulations. The simulation suggests that for a pathogen with a MIC of ≤8 mg/liter, an extended infusion of 4 g of piperacillin administered for 6 or 8 h reaches an acceptable probability of target attainment rate of >98%. Extended infusions, administered every 6 or 8 h, reach a satisfactory target attainment of 94 and 82%, respectively, for a MIC of 16 mg/liter. However, intermittent administration of 4 g piperacillin, for 6 or 8 h, only reaches satisfactory target attainments for the most sensitive of organisms. Collectively, the results of the Monte Carlo simulations suggest that changing medical practice from bolus dosing to an extended infusion would improve target attainment rates dramatically for organisms with a MIC of ≤16 mg/liter. More importantly, the results of the simulations suggest that more intensive extended-infusion TZP dosing regimens (3.375 to 4.5 g intravenously [3-h infusion] every 6 h) than those commonly used in clinical practice (3.375 to 4.5 g intravenously [4-h infusion] every 8 h) are required to maximize fT>MIC for higher MICs, especially in patients with augmented clearance of piperacillin. While it is customary to make dose reductions in patients with renal impairment, there are no such recommendations for individuals with enhanced glomerular filtration rates. Further pharmacokinetic studies are sorely needed to determine the most optimal dosage regimens in patients who present with augmented renal clearance. When designing these PK studies in patients with augmented renal clearance, the models should include a term to account for the potential nonlinear clearance of piperacillin.
Based on the best available data to date, the results of tazobactam simulations show that dosing every 8 h provides reasonable concentrations against β-lactamase-producing bacteria. Although data are very limited, in vitro experiments suggest that the antibacterial activity of piperacillin-tazobactam is lost when the inhibitor concentrations fall below a critical threshold of 4 mg/liter (16, 17). While daily areas under the curves were similar between intermittent and extended dosing schemes, the Monte Carlo simulations demonstrated that the period of time the tazobactam concentration is above 4 mg/liter is longer when an extended infusion is used as the method of administration (data not shown). While these results are reassuring, the pharmacodynamic target and its relationship to tazobactam pharmacokinetics are not well understood and require further elucidation.
In conclusion, we have shown that a population model based on parallel linear/MM clearance best describes the observed data. The population estimates are consistent with previous studies but show large interpatient variability. Monte Carlo simulation suggests that TZP at 4.5 g administered for 3 h every 6 h can be used to successfully treat organisms with a MIC of ≤16 mg/liter. However, there remain unanswered questions regarding the pharmacodynamic target of β-lactamase inhibitors and the impact that using extended infusion may have on β-lactamase-β-lactamase inhibitor interactions. Further clinical trials and in vitro experiments are required to answer these questions. By providing the optimal sampling schedule from our D-optimal design analysis, we hope that future pharmacokinetic clinical trials are designed to capture the most accurate population estimates for both piperacillin and tazobactam with the minimal number of sampling time points.
ACKNOWLEDGMENTS
Timothy Felton is supported by a Medical Research Council Clinical Research Training Fellowship. William Hope is supported by a National Institutes of Health Research (NIHR) Clinician Scientist Fellowship.
Footnotes
Published ahead of print 14 May 2012
REFERENCES
- 1. Akaike H. 1979. A Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika 66:237 [Google Scholar]
- 2. Auclair B, Ducharme MP. 1999. Piperacillin and tazobactam exhibit linear pharmacokinetics after multiple standard clinical doses. Antimicrob. Agents Chemother. 43:1465–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergan T, Williams JD. 1982. Dose dependence of piperacillin pharmacokinetics. Chemotherapy 28:153–159 [DOI] [PubMed] [Google Scholar]
- 4. Blondiaux N, et al. 2010. Daily serum piperacillin monitoring is advisable in critically ill patients. Int. J. Antimicrob. Agents 35:500–503 [DOI] [PubMed] [Google Scholar]
- 5. Bulitta JB, et al. 2007. Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 51:2497–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bulitta JB, et al. 2010. Nonlinear pharmacokinetics of piperacillin in healthy volunteers–implications for optimal dosage regimens. Br. J. Clin. Pharmacol. 70:682–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conil J-M, et al. 2006. Influence of renal function on trough serum concentrations of piperacillin in intensive care unit patients. Intensive Care Med. 32:2063–2066 [DOI] [PubMed] [Google Scholar]
- 8. Cox HJ, Bhandari S, Rigby AS, Kilpatrick ES. 2008. Mortality at low and high estimated glomerular filtration rate values: a “U” shaped curve. Nephron. Clin. Practice 110:c67–c72 [DOI] [PubMed] [Google Scholar]
- 9. Craig W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10 [DOI] [PubMed] [Google Scholar]
- 10. D'Argenio DZ, Schumitzky A. 1979. A program package for simulation and parameter estimation in pharmacokinetic systems. Computer Programs Biomed. 9:115–134 [DOI] [PubMed] [Google Scholar]
- 11. D'Argenio DZ, Schumitzky A, Xiaoning W. 2009. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles, CA [Google Scholar]
- 12. Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of “bug and drug.” Nat. Rev. Microbiol. 2:289–300 [DOI] [PubMed] [Google Scholar]
- 13. Fuster-Lluch O, Gerónimo-Pardo M, PeyrÓ-García R, Lizán-García M. 2008. Glomerular hyperfiltration and albuminuria in critically ill patients. Anaesth. Intensive Care 36:674–680 [DOI] [PubMed] [Google Scholar]
- 14. Hayashi Y, Ja Roberts Paterson DL, Lipman J. 2010. Pharmacokinetic evaluation of piperacillin-tazobactam. Expert Opin. Drug Metab. Toxicol. 6:1017–1031 [DOI] [PubMed] [Google Scholar]
- 15. Kim M-K, et al. 2002. Pharmacokinetic and pharmacodynamic evaluation of two dosing regimens for piperacillin-tazobactam. Pharmacotherapy 22:569–577 [DOI] [PubMed] [Google Scholar]
- 16. Kuck NA, Jacobus NV, Petersen PJ, Weiss WJ, Testa RT. 1989. Comparative in vitro and in vivo activities of piperacillin combined with the beta-lactamase inhibitors tazobactam, clavulanic acid, and sulbactam. Antimicrob. Agents Chemother. 33:1964–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lister PD, Prevan M, Sanders CC. 1997. Importance of beta-lactamase inhibitor pharmacokinetics in the pharmacodynamics of inhibitor-drug combinations: studies with piperacillin-tazobactam and piperacillin-sulbactam. Antimicrob. Agents Chemother. 41:721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lodise TP, Jr, Lomaestro B, Rodvold KAA, Danziger LH, Drusano GL. 2004. Pharmacodynamic profiling of piperacillin in the presence of tazobactam in patients through the use of population pharmacokinetic models and Monte Carlo simulation. Antimicrobial agents and chemotherapy. Am. Soc. Microbiol. 48:4718–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neely MN, van Guilder M, Yamada W, Schumitzky A, Jelliffe R. Accurate detection of outliers and subpopulations with Pmetrics: a non-parametric and parametric pharmacometric modeling and simulation package for R. Ther. Drug Monit., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. R Development Core Team 2011. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 21. Rello J, et al. 2010. Determinants of choice and prescription patterns in empiric antibiotic therapy for HAP/VAP. J. Eur. Resp. Soc. 37:1332–1339 [Google Scholar]
- 22. Roberts JA, Hope WW, Lipman J. 2010. Therapeutic drug monitoring of beta-lactams for critically ill patients: unwarranted or essential? Int. J. Antimicrob. Agents 35:419–420 [DOI] [PubMed] [Google Scholar]
- 23. Roberts JA, Kirkpatrick CMJ, Roberts MS, Dalley AJ, Lipman J. 2010. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int. J. Antimicrob. Agents 35:156–163 [DOI] [PubMed] [Google Scholar]
- 24. Roberts JA, Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 37:840–851 [DOI] [PubMed] [Google Scholar]
- 25. Sörgel F, Kinzig M. 1993. The chemistry, pharmacokinetics and tissue distribution of piperacillin/tazobactam. J. Antimicrob. Chemother. 31(Suppl. A):39–60 [DOI] [PubMed] [Google Scholar]
- 26. Tam VH, Preston SL, Drusano GL. 2003. Optimal sampling schedule design for populations of patients. Antimicrob. Agents Chemother. 47:2888–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Udy Aa, et al. 2011. Sub-therapeutic initial β-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest doi:10.1378/chest.11-1671. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 28. Vinks AA, den Hollander JG, Overbeek SE, Jelliffe RW, Mouton JW. 2003. Population pharmacokinetic analysis of nonlinear behavior of piperacillin during intermittent or continuous infusion in patients with cystic fibrosis. Antimicrob. Agents Chemother. 47:541–547 [DOI] [PMC free article] [PubMed] [Google Scholar]